Abstract
Diagnosis of celiac disease frequently depends upon serology assays. We set out to prospectively assess the diagnostic value of five serology tests: an enzyme-linked immunosorbent assay (ELISA) for tissue transglutaminase (tTG)-immunoglobulin A (IgA) and tTG-IgG, a chemiluminescence assay for tTG-IgA, an ELISA for deamidated gliadin peptide (DGP) IgG and IgA screening, and detection of endomysial antibodies (Abs) by indirect immunofluorescence. One hundred sixteen children at high risk for developing celiac disease were evaluated clinically and underwent small bowel biopsies and blood serology tests. We examined differences between younger and older children in terms of clinical presentation, test performance, and the ability of high Ab levels to correctly predict diagnosis of celiac disease. Celiac disease was diagnosed for 85 (73%) children. No significant clinical differences were observed between the biopsy-positive and biopsy-negative groups. Children ≤3 years of age revealed higher concentrations of tTG-IgA and DGP Abs than children >3 years old (P = 0.017 and 0.007, respectively). High Ab concentrations were predictive of villous atrophies, with sensitivities ranging from 92.8% to 97.9%, depending on the assay and the cutoff points applied. Sensitivities, specificities, positive predictive values, and negative predictive values varied among assays and improved after correction for best cutoff points. Assay specificities obtained in the clinical setting were lower than expected. The new tTG-IgA chemiluminescence assay demonstrated high throughput but low specificity (74.2%). The tTG-IgA ELISA exhibited the highest test efficiency, and the tTG-IgA chemiluminescence assay was suitable for large-scale screening, with reduced specificity. High concentrations of celiac disease-specific Abs bring into question the need for performance of biopsies on children at high risk.
Celiac disease (CD) is a common autoimmune enteropathy that occurs in genetically predisposed children and adults upon ingestion of gluten or related proteins (19). The diverse presentation of CD includes classical clinical symptoms, such as diarrhea, weight loss, failure to thrive, malabsorption, and anemia, and atypical manifestations, such as nonspecific abdominal pain, esophageal reflux, osteoporosis, hypertransaminasemia, and neurological symptoms (15, 25). Population studies have shown that the incidences of CD in Europe and North America are 0.5 to 1% (10). Even though the rate of diagnosis has increased in recent years, according to the accepted iceberg concept (11), the majority of affected individuals are still undiagnosed (10, 18).
According to the latest consensus report on CD, small bowel biopsies are considered the gold standard and are mandatory for diagnosis (15). Obtaining a biopsy specimen is an invasive procedure and at times may miss patchy mucosal changes. Poor orientation of the removed tissue may lead to difficulties in interpretation. On the other hand, serology testing for CD-specific antibodies (Abs) is easy to perform and a wide range of commercial kits are now available. The serology tests are sensitive and specific and are becoming the obligatory tool for correctly referring patients for biopsies. Immunoglobulin A (IgA) against the tissue transglutaminase (tTG) antigen is accepted as the best serology screening tool performed by the enzyme-linked immunosorbent assay (ELISA) method (15). Recently, a new human recombinant tTG-IgA chemiluminescence assay was developed for use with the Immulite 2000 analyzer. This platform enables large-scale testing at a high throughput, an advantage which should be taken into account due to the increasing requests for serology testing. In many clinical laboratories, the fluorescence endomysial Ab (EMA) assay is used for confirming the presence of tTG-IgA. The EMA assay is known for its high sensitivity and specificity for diagnosing CD but requires much technologist labor and yet suffers from interobserver variability in interpretation. Abs to deamidated gliadin peptides (DGP) were shown to be of diagnostic value, and DGP Ab kits are being extensively evaluated (2, 28, 29, 32, 36). A DGP assay recognizing both IgA and IgG Abs, known as the DGP (IgA+IgG) screen, is intended for detecting both IgA-deficient and IgA-sufficient CD patients. Thus, the need for measuring total IgA for all tested subjects is eliminated. IgA deficiency affects approximately 1/500 of the general population and is a 10-fold-increased risk factor for CD (8). Performance of the DGP (IgA+IgG) screen could reduce test costs by eliminating the need for IgA screening.
tTG-IgA Ab titer was shown to correlate well with severity of biopsy result in adults and pediatric populations (14, 33). This positive correlation has raised the possibility of avoiding small bowel biopsies, when tTG-IgA Ab concentrations are especially high, for diagnosing high-risk populations (3, 13). This concept is not thoroughly studied with the various tTG-IgA commercial kits or other CD Ab specificities. The majority of studies regarding the diagnostic value of CD serology were conducted in research settings. A few publications raised the possibility that serology assays may be less accurate when used in clinical settings (1, 21). We therefore examined a group of children presenting clinical suspicion for developing CD in our community clinical setting. The high prevalence of biopsy-proven CD children in this population enabled us to examine the diagnostic value of several serology kits by comparing the results for two age groups. We also calculated the correlations between Ab titer and severity of biopsy result and assessed the possibility that high Ab titers have predictive value for biopsy results.
MATERIALS AND METHODS
Population study.
One hundred sixteen children referred from December 2006 until March 2008 to the Pediatric Gastroenterology Unit at the Edmond and Lily Safra Children's Hospital, Sheba Medical Center, Ramat-Gan, Israel, participated in this study. The ethics committee at Sheba Medical Center approved this study protocol. All parents of participating children received oral and written explanation and signed an informed consent according to the Declaration of Helsinki requirements.
The selection criteria for the children participating in the study were the presence of clinical signs and symptoms of CD in the children and/or the existence of known CD patients among the children's relatives. These children underwent small bowel biopsies and blood sample collection. Histology evaluation was carried out in the hospital's pathology unit, and the results were considered the gold standard for our study. Serology tests were carried out at the Central Laboratory-Immunology Unit of Maccabi-Health Services, Rechovot, Israel.
Histopathology.
Biopsy specimens from the distal duodenum (with a minimum of five conventional forceps samples per patient) were obtained by upper duodenoscopy. Samples were fixed in buffered formalin and embedded in paraffin wax. Standard sections were obtained and stained with hematoxylin and eosin. The histopathology slides were examined without any knowledge of serology results at the hospital's pathology unit. The results of villous atrophy were categorized according to the modified Marsh criteria (27). Briefly, Marsh 0 represents normal mucosa, Marsh 1 represents normal mucosa architecture with increased intraepithelial lymphocytes (>30 intraepithelial lymphocytes/100 enterocytes), Marsh 2 represents additional crypt hyperplasia, Marsh 3a represents partial villous atrophy, Marsh 3b represents subtotal villous atrophy, and Marsh 3c represents total villous atrophy.
Serum analysis.
All serology tests were performed in a community-based laboratory among the hundreds of routine samples arriving daily. The laboratory personnel had no previous knowledge of clinical diagnosis or biopsy findings. Five serology tests were performed using the following methods via commercial kits in accordance with the manufacturers' instructions: (i) tTG-IgA Celikey ELISA (intra-assay coefficient of variation [CV], 4.9 to 8.7%; manufacturer-recommended cutoffs, <5 U/ml for negative results, 5 to 8 U/ml for borderline results, and >8 U/ml for positive results; Phadia, Freiburg, Germany), (ii) tTG-IgA Immulite 2000 (CV, 3.9 to 6.1%; manufacturer-recommended cutoffs, <4 U/ml for negative results and >4 U/ml for positive results; Siemens, Deerfield, IL), (iii) tTG-IgG Celikey ELISA (CV, 3.6 to 7.2%; manufacturer-recommended cutoffs, <7 U/ml for negative results, 7 to 10 U/ml for borderline results, and >10 U/ml for positive results; Phadia, Freiburg, Germany), (iv) DGP (IgG+IgA) screen ELISA (Quanta Lite; CV, 0.5 to 4.7%; manufacturer-recommended cutoffs, <20 U for negative results, 20 to 30 U for weak-positive results, and >30 U for moderate-to-strong-positive results; Inova Diagnostics, San Diego, CA), and (v) EMA immunofluorescence using primate smooth muscle slides and a dual anti-IgG/anti-IgA conjugate (manufacturer-recommended cutoffs, <1/5 for negative results and >1/5 for positive results; Immco Diagnostics, Buffalo, NY). The initial sample dilution was 1/5. Positive samples were titrated by serial dilutions up to 1/160. All slides were examined by two independent observers.
Serum IgA levels were determined for all specimens to rule out IgA deficiency. IgA levels were measured by nephelometry (BNII, Siemens, Deerfield, IL), using Dade Behring IFCC calibrators and reagents. Age-dependent IgA reference ranges are as described by Bienvenu et al. (4).
When a discrepancy was found between serology results and biopsy findings, human leukocyte antigen type DQ2/DQ8 (HLA-DQB1) typing was performed on extracted DNA by PCR methods based on sequence-specific oligonucleotide probing/sequence-specific priming.
Statistical analysis.
The data were analyzed using BMDP statistical software (W. J. Dixon, University of California Press, Los Angeles). Discrete variables were compared, by group, using Fisher's exact test. Values for the continuous variable, age, were compared using Student's t test. When comparing the serological assays by age groups, we used the Mann-Whitney nonparametric U test and presented the results as median concentrations. Since the Marsh scores are not continuous variables, Spearman nonparametric correlations between the Marsh scores and the results for all the serology assays were calculated. Logistic regression analysis was applied for each assay separately in order to derive the best cutoff point for predicting Marsh scores of ≥1. Since the EMA assay has a logarithmic distribution, we applied the following transformation: log5(reciprocal of end point titer)/5 + 1. Using the cutoff values recommended by the manufacturer and the best cutoff points derived from the logistic regressions, we dichotomized the results of the assays and were thus able to produce two-by-two contingency tables which enabled us to calculate sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and test efficiency.
RESULTS
Demographic, clinical, and histological findings.
The mean age of the participating children was 6.7 years (range, 1 to 17 years), with 52 (45%) male and 64 (55%) female subjects. All children were referred for small intestinal biopsy at the hospital's pediatric gastroenterology unit as a result of CD-suggestive clinical symptoms or being a relative of a CD patient. Final diagnosis of CD was determined according to histological criteria (early or mild mucosal changes [Marsh 1, 2, and 3a] or severe mucosal changes [Marsh 3b and 3c], as accepted) (10, 26, 27). Table 1 shows the demographic data, histology findings, and clinical reasons for referral, sorted according to biopsy-positive and biopsy-negative children. No significant differences in gender, age, or clinical symptoms were observed between the two groups, besides anemia being slightly more common in the biopsy-positive group (P = 0.075). Histology analysis revealed that 90.5% of the children diagnosed with CD had partial or severe villous atrophy (Marsh 3a, 3b, and 3c) at the time of diagnosis. Surprisingly, failure to thrive was more pronounced among children in the biopsy-negative group, even though this difference was not significant (P = 0.35). Fourteen children were referred for biopsy testing due to other clinical symptoms, as listed in Table 1.
TABLE 1.
Demographic, histological, and clinical descriptions of children (n = 116) enrolled
| Parameter | Value for indicated group |
P | |
|---|---|---|---|
| Biopsy positive | Biopsy negative | ||
| No. of children | 85 | 31 | |
| Male | 37 | 15 | 0.68 |
| Female | 48 | 16 | 0.68 |
| Male/female ratio | 1:1.3 | 1:1.1 | |
| Mean age (yr) (range) | 6.2 (1-16) | 7.2 (1-17) | 0.24 |
| No. (%) with Marsh score of: | |||
| 0 | 0 | 31 (100) | |
| 1 or 2 | 8 (9.4) | 0 | |
| 3a | 34 (40) | 0 | |
| 3b | 14 (16.5) | 0 | |
| 3c | 29 (34.1) | 0 | |
| No. (%) with: | |||
| Failure to thrive | 22 (26) | 11 (35) | 0.35 |
| Anemia | 22 (26) | 3 (10) | 0.075 |
| First-degree relative with CD | 19 (22) | 4 (13) | 0.30 |
| Abdominal pain | 15 (18) | 5 (16) | 1.00 |
| Gastrointestinal manifestations | 13 (15) | 5 (16) | 1.00 |
| Other conditiona | 7 (8) | 7 (22.5) | 0.05 |
Attention deficit hyperactivity disorder, diabetes mellitus type 1, IgA deficiency, hypertransaminasemia, appetite loss, alopecia, or idiopathic purpura.
IgA deficiency.
Five patients (4.3%) in our study were IgA deficient (<6 mg/dl). Three of them were negative for CD by both criteria (a Marsh score of 0 and negative results for serology assays). The fourth patient scored Marsh 0 with a weak-positive DGP screen result (28.9 U). This 4-year-old girl suffered from diarrhea and anemia at the time of biopsy testing and continually suffered from a wide range of infections, such as acute tonsillitis, chronic otitis media, and urinary tract infections, thus reflecting classical immunodeficiency disease patterns. The fifth IgA-deficient patient was diagnosed with full-blown CD with total villous atrophy (Marsh 3c). As expected, the results for all IgA-based serology tests were negative for this patient, while those for both IgG-based tests (the DGP screen and the tTG-IgG assay) were strong positive (153 U and 150 U/ml, respectively).
CD serology-specific assays.
All sera were tested using five different serology assays to identify those tests which associate best with biopsy findings, considered the gold standard for diagnosis of CD. All assays detected CD Abs in the biopsy-negative group to a greater or lesser extent (Table 2). HLA-DQ2/DQ8 levels in these discrepant cases were determined, and 4 of the 14 patients were DQ2/DQ8 negative, thus clearly identifying false-positive serology results in the biopsy-negative group. Three of these four patients tested positive by the DGP (IgA+IgG) screen. Furthermore, the DGP (IgA+IgG) screen stood out, with 11 (35%) positive cases out of 31 biopsy-negative children, with a mean concentration of 34.8 U/ml. The tTG-IgA Celikey assay detected four positive cases in the biopsy-negative group (three of them with borderline levels). The EMA assay resulted in eight positives (five of them with borderline titers), and the tTG-IgA Immulite 2000 assay resulted in eight positives, twice as many as the tTG-IgA Celikey assay, even though both assays use human recombinant antigen. The tTG-IgG Celikey assay resulted in the lowest number of positives in the biopsy-negative group (2/31) but, similarly, displayed the lowest number of true positives (55/85) in the biopsy-positive group. One of the biopsy-negative subjects (no. 14 [Table 2]) tested positive in all CD IgA-serology assays and positive for the CD-associated *0302 allele. This child presented low body weight and short stature. Since clinical, serological, and genetic results were suggestive of CD, this result could imply a rare case of latent CD. The child was referred to undergo a repeated biopsy.
TABLE 2.
Concentrations of positive Abs measured in the biopsy- negative group
| Patient | Result for indicated assaya |
|||||
|---|---|---|---|---|---|---|
| tTG-IgA Celikey (U/ml) | EMA (titer) | tTG-IgG Celikey (U/ml) | DGP (IgG+IgA) screen (U) | tTG-IgA Immulite 2000 (U/ml) | HLA-DQ2/DQ8 | |
| 1 | − | − | − | 29 (w+) | − | − |
| 2 | − | − | − | 34 (+) | − | − |
| 3 | − | − | 13 (+) | 35 (+) | − | *02 |
| 4 | − | − | − | 47 (+) | − | *02/*0302 |
| 5 | − | − | − | 21 (w+) | − | *0302/*0302 |
| 6 | − | − | − | − | 7.6 (+) | − |
| 7 | − | 1/5 (BL) | − | 34 (+) | 4 | − |
| 8 | − | 1/5 (BL) | − | 32 (+) | 19 (+) | *0302 |
| 9 | − | 1/5 (BL) | − | − | − | *02/*0302 |
| 10 | 5.4 (BL) | 1/5 (BL) | − | 31 (+) | 6.3 (+) | *02 |
| 11 | 5.4 (BL) | 1/5 (BL) | − | 40 (+) | 11 (+) | *02/*02 |
| 12 | − | 1/10 (+) | − | 29 (w+) | 13 (+) | *0302/*0302 |
| 13 | 6.5 (BL) | 1/10 (+) | − | − | 9 (+) | *02/*02 |
| 14 | 19 (+) | 1/10 (+) | 7.6 (BL) | 51.4 (+) | 17 (+) | *0302 |
−, negative; BL, borderline; w+, weak positive; +, positive.
Those children who were positive for CD were further divided into ≤3-year-olds and >3-year-olds. This age cutoff point was chosen to obtain a large enough group of younger children for statistical analysis. The median concentrations of Abs measured for these groups are shown in Table 3. Total IgA measurements were higher among the older children, as expected, but all the CD-specific Abs revealed higher concentrations for the younger age group. The younger children showed somewhat more gastrointestinal manifestations (24.2%) and failure to thrive (39.4%) than the older children (12% and 24%, respectively), but these differences did not differ significantly (P = 0.15 and P = 0.11, respectively).
TABLE 3.
Median concentrations of CD-specific Abs and differences between younger (≤3-year-old) and older (>3-year-old) children among the biopsy-positive group
| Parameter | Result for indicated group |
P | |
|---|---|---|---|
| ≤3-yr-old | >3-yr-old | ||
| No. of children | 26 | 59 | |
| IgA concn (mg/dl) | 86.8 | 121.0 | 0.045 |
| Result for: | |||
| tTG-IgA Celikey assay (IU/ml) | 119.1 | 29.9 | 0.017 |
| EMA (reciprocal of endpoint titer) | 160 | 160 | 0.102 |
| tTG-IgG Celikey assay (IU/ml) | 28.0 | 8.5 | 0.007 |
| DGP (IgG+IgA) screen (U) | 95.9 | 68.1 | 0.007 |
| tTG-IgA Immulite 2000 assay (IU/ml) | 71.6 | 42.5 | 0.398 |
The results of the logistic regression analyses are shown in Table 4. The areas under the receiver operating characteristic curve (AUC) did not differ notably between the assays (they were all between 0.95 and 0.96), with the exception of the tTG-IgG assay (AUC, 0.87). A statistical evaluation of the assays using the manufacturer's cutoff points and the calculated best cutoff points is shown in Table 5. For all calculations, borderline Ab concentrations were considered positive. According to the manufacturer's cutoff points, the EMA and DGP (IgG+IgA) screen assays presented the best sensitivities (95.3%) but the worst specificities (74.2 and 64.5%, respectively). The tTG-IgA Celikey assay was found to be the most efficient test (91.4%), followed by the EMA (89.6%) and tTG-IgA Immulite 2000 (87.9%) assays. The tTG-IgG Celikey assay exhibited poor sensitivity (67.7%), thus leading to an extremely low NPV (49.2%) and inferior test efficiency (72.4%). For all assays, the best-fit cutoff points for our high-risk population (Table 5) showed increased specificity, with the exception of the tTG-IgG Celikey assay, where the cutoff point was lowered, thus improving sensitivity. According to the suggested cutoff points, the EMA and the tTG-IgA Celikey assays exhibit the same predictive values and are equally efficient.
TABLE 4.
Logistic regression analysis for prediction of positive biopsy results
| Assay | Odds ratioa | 95% CI | AUC |
|---|---|---|---|
| tTG-IgA Celikey | 1.35 | 1.17-1.57 | 0.96 |
| tTG-IgA Immulite 2000 | 1.22 | 1.11-1.34 | 0.95 |
| tTG-IgG Celikey | 1.34 | 1.14-1.56 | 0.87 |
| DGP IgG+IgA Screen | 1.10 | 1.06-1.15 | 0.95 |
| EMAb | 3.33 | 2.05-5.43 | 0.96 |
The odds ratio is a way of determining whether the probability of a certain event is the same for two groups and represents the change in the estimated odds of the outcome resulting from an increase in the continuous variable by 1 unit.
Using the formula log5(reciprocal of endpoint titer)/5 + 1.
TABLE 5.
Statistical performance of serology assays using cutoff values recommended by the manufacturer or determined by logistic regression analysisa
| Parameter | Value(s) for indicated assay |
||||
|---|---|---|---|---|---|
| EMA (titer) | tTG-IgA Celikey (U/ml) | tTG-IgA Immulite 2000 (U/ml) | DGP IgA+IgG screen (U) | tTG-IgG Celikey (U/ml) | |
| Cutoff points recommended by the manufacturer | |||||
| Cutoff value | <1/5 (−), ≥1/5 (+) | <5 (−), 5-8 (BL), >8 (+) | <4 (−), ≥4 (+) | <20 (−), 20-30 (w+), > 30 (s+) | <7 (−), 7-10 (BL), >10 (+) |
| Sensitivity (%) | 95.3 | 94.1 | 92.9 | 95.3 | 67.7 |
| Specificity (%) | 74.2 | 87.1 | 74.2 | 64.5 | 93.5 |
| PPV (%) | 91.0 | 95.2 | 90.8 | 83.3 | 96.5 |
| NPV (%) | 85.2 | 84.4 | 79.3 | 88 | 49.2 |
| Test efficiency (%) | 89.6 | 91.4 | 87.9 | 87 | 72.4 |
| Cutoff points determined by logic regression analysis | |||||
| Cutoff value | <1/10 (−), ≥1/10 (+) | <7 (−), ≥7 (+) | <10 (−), ≥10 (+) | <34 (−), ≥34 (+) | <3.6 (−), ≥3.6 (+) |
| Sensitivity (%) | 90.6 | 90.6 | 90.6 | 89.4 | 81.2 |
| Specificity (%) | 96.8 | 96.8 | 87.1 | 83.9 | 83.9 |
| PPV (%) | 98.7 | 98.7 | 95.1 | 93.8 | 93.2 |
| NPV (%) | 78.9 | 78.9 | 77.1 | 74.3 | 61.9 |
| Test efficiency (%) | 92.2 | 92.2 | 89.7 | 87.9 | 81.9 |
−, negative; +, positive; w+, weak positive; s+, strong positive; BL, borderline. Borderline and weak-positive results were considered positive for calculating sensitivity, specificity, PPV, NPV, and test efficiency.
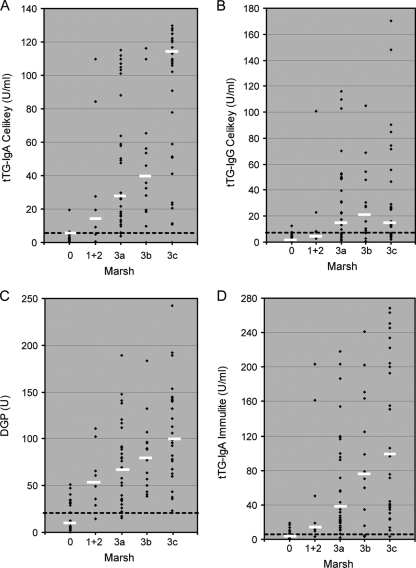
We evaluated the association between Ab concentration and severity of biopsy findings, as depicted in Fig. 1. In general, higher Ab values were measured for children with Marsh scores of 3a, 3b, and 3c. This association is well presented by the median Ab levels increasing with severity of histology Marsh grading. The correlations (r) between Ab level and severity of histology findings were as follows: for the EMA assay [using log5(reciprocal of endpoint titer)/5+1], 0.73; for the DGP (IgG+IGA) screen, 0.71; for the tTG-IgA Celikey assay, 0.72; for the tTG-IgA Immulite 2000 assay, 0.68; and for the tTG-IgG Celikey assay, 0.56. All correlation coefficients were highly significant (P < 0.001), considering the size of our study. In light of these positive correlations, we tested the capability of high Ab concentrations for correctly pointing out positive biopsy results. High Ab cutoff points were assigned to each assay as shown in Table 6, and the resulting numbers of patients with partial, subtotal, and total villous atrophy levels (Marsh 3a, 3b, and 3c) were determined. Sensitivities were calculated. The high Ab cutoff points were 10 times the manufacturer-recommended cutoff values for the tTG-IgA Celikey assay and the tTG-IgA Immulite 2000 assay, were 3 times the manufacturer-recommended cutoff values for the DGP (IgG+IgA) screen assay, represented titers of ≥1/160 for the EMA test, and were equal to the manufacturer-recommended cutoff point (10 U/ml) for the tTG-IgG Celikey assay. As observed in Table 6, high Ab levels could be used as a predicting tool for partial, subtotal, and total villous atrophy (Marsh 3a, 3b, and 3c, accordingly).
FIG. 1.
Individual values for the tTg-IgA Celikey assay (A), the tTG-IgG Celikey assay (B), the DGP (IgA+IgG) screen (C), and the tTG-IgA Immulite 2000 assay (D), stratified according to the Marsh grading of biopsy findings. Dashed lines represent assay cutoff values, and bars represent median Ab levels.
TABLE 6.
Sensitivities of high Ab levels for detecting positive biopsy results
| Assay | High Ab cutoff | No. of cases | No. of true positivesa | Sensitivity (%) |
|---|---|---|---|---|
| tTG-IgA Celikey | 50 U/ml | 42 | 40 | 95.2 |
| tTG-IgA Immulite 2000 | 40 U/ml | 48 | 45 | 93.7 |
| EMA | 1/160 | 57 | 54 | 94.7 |
| DGP (IgG+IgA) screen | 60 U | 56 | 52 | 92.8 |
| tTG-IgG Celikey | 10 U/ml | 48 | 47 | 97.9 |
True positives for partial, subtotal, and total villous atrophy.
DISCUSSION
The children enrolled in this study were at high risk for developing CD due to clinical symptoms or relationship to known CD patients and do not represent the general pediatric population in our country. This explains the high prevalence (73.2%) of CD in our study and the 1:1.1 male/female ratio found in the CD group. Fasano et al. (16), McGowan et al. (24), and West et al. (38) reported equal percentages of male and female EMA-positive, biopsy-proven CD individuals in North American and European studies.
The participating children were divided into CD positives and negatives according to biopsy results, which are considered the gold standard for diagnosis of CD (15, 17, 20, 26, 33, 35, 37). Several of the clinical indications listed were more noticeable in the biopsy-negative group, namely, failure to thrive and other high-risk characteristics that might imply CD, such as diabetes mellitus type 1, hypertransaminasemia, and IgA deficiency. All these clinical symptoms may indeed suggest CD (12), but their higher occurrence within the biopsy-negative group intensifies the concept that CD is mainly asymptomatic and the majority of cases remain undiagnosed (7, 9, 10, 16, 30). Lurz et al. (23) similarly presented failure to thrive as a more pronounced characteristic in the disease control group of high-risk children. They suggested that these children are often more aggressively investigated than those with milder symptoms.
Comparison of the CD-positive children in two age groups revealed that the children ≤3 years old demonstrated clinical symptoms such as gastrointestinal manifestations and failure to thrive more frequently than the >3-year-old children. These findings correlate well with the more-severe villous atrophy and higher titers of CD-specific Abs in this group, as measured by almost all assays. Similar differences in histological features, clinical findings, and Ab titers between younger (≤2-year-old) and older (>2-year-old) children were recently reported by Vivas et al. (35). Our findings are in agreement with those of that group and others (14). Furthermore, it has been suggested in the past that pediatric CD patients (≤2 years old) may have normal IgA-tTG and EMA levels (5, 6). We, as others, did not observe any lack of Ab sensitivity in this age group (3, 23, 35).
Five CD-specific serology assays were assessed in this study to inspect their value in CD diagnosis. One of the strengths of our study is the prospective testing of all serology assays with the same test tube on the same day, concurrently with the biopsy procedure. In our clinical setting, the EMA assay and the DGP (IgA+IgG) screen were the most sensitive assays and the DGP (IgA+IgG) screen was the least specific. With both the manufacturer-recommended cutoff and the best-fit cutoff based on logistic regression analysis, the DGP (IgA+IgG) screen assay clearly had more false positives than the other assays.
The tTG-IgA Celikey and tTG-IgA Immulite 2000 assays revealed lower specificities (87% and 74%, respectively) than those reported in the literature (reviewed by Rostom et al. [31]). The reduced Ab specificity that we have found may have several explanations. On the one hand, the presence of DGP-positive children in the biopsy-negative group may actually indicate that these children will develop CD later in life. There are reports documenting DGP Abs preceding tTG-IgA (22). Our study does not include follow-up. Improvement of clinical symptoms and decline of Ab concentrations during a gluten-free diet would assist in final diagnosis for the serology-positive/biopsy-negative group. Nevertheless, these children are negative for CD on the basis of the current diagnostic criteria, regarding the biopsy findings as the gold standard (15). Only three biopsy-negative patients exhibited positive serology results with four of the five assays, with tTG-IgA and EMA detectable at borderline concentrations. A single patient, who probably had latent CD, revealed positive serology results by all assays. On the other hand, these positive Abs may indeed be false positive. Agardh reported false-positive DGP/tTG (IgG+IgA) Abs and DGP-IgG Abs in a disease control group of children (2). Similar seropositive results with negative biopsy findings were documented in other clinical studies (23, 24). Abrams et al. have shown in a recent paper that sensitivities in clinical practice are not as high as those reported in research laboratories (1). The same may apply for specificities. All biopsy-negative children in our clinical study were symptomatic and could be referred to as a “disease control” group, exhibiting lower specificities than blood donors, which usually serve as negative controls. Nevertheless, the majority of false-positive EMA results in our study were at or slightly above the cutoff point. The DGP (IgG+IgA) screen's false positives had a wider range of results. When the best-fit cutoff points based on logistic regression analysis were applied, the specificity was increased without too much effect on sensitivity. It should be stressed, however, that these best-fit cutoff points are suitable for high-risk children and may not apply for the general low-risk population.
In terms of overall test efficiency, the tTG-IgA Celikey assay displayed the best performance. The tTG-IgA assay is usually performed with an automated ELISA instrument and is therefore suitable for large-scale screening. The EMA assay is a manual, time-consuming, and subjective assay, used for confirmation of positive tTG-IgA samples. According to our results, confirmation with the EMA assay is unnecessary, since there were no tTG-IgA-positive/EMA-negative samples. The same conclusion was reached in a comparative study of 10 different tTG IgA/IgG assays (33).
The tTG-IgA Immulite 2000 assay utilizes a human recombinant tTG antigen on a random access platform, with the advantages of a sensitive chemiluminescence signal and high throughput. To the best of our knowledge, there are no previous publications evaluating this new immunoassay. According to our data, overall performance was comparable to that of the other serology assays, but test efficiency was lower than that of the tTG-IgA Celikey ELISA. The tTG-IgA Immulite 2000 assay may seem attractive as a front-line screening kit, though one must keep in mind that the reduced specificity may lead to the performance of too many confirmatory tests or unnecessary biopsies.
The tTG-IgG assay and the DGP (IgG+IgA) screen were initially performed for detecting positive CD among the IgA deficient. Since only one biopsy confirmed that a CD-positive patient was IgA deficient in our study, no conclusive results may be reached. On the other hand, the tTG-IgG Celikey assay is not suitable for testing the IgA-sufficient samples. The extremely low sensitivity obtained may be due to the facts that the IgA Abs have higher avidity to the tTG antigen and therefore that subsequent IgG Ab binding is reduced (34). The DGP (IgG+IgA) screen has been reported by others as an excellent substitute for the previous nonspecific Gliadin assays (2, 26, 28, 29, 36). In our study, the sensitivity of the DGP (IgG+IgA) screen was higher and the specificity was lower than those reported in two recent studies (29, 36). We have concentrated on children at high risk who were defined as CD positive according to biopsy grades Marsh 1 to Marsh 3c. The above-cited studies examined adults, where only Marsh scores of 3b to 3c (29) or Marsh scores of 3a to 3c (36) were considered positive. Nevertheless, other studies have shown the DGP assays to be as sensitive and specific as the tTG-IgA and EMA assays (22, 26, 28, 32). In conclusion, the reported sensitivities and specificities of the DGP assays vary significantly.
In light of the positive association between Ab concentration and Marsh grading, we set out to examine the proposal of Barker et al., where very high positive tTG-IgA levels would be enough for CD diagnosis of symptomatic patients, thus eliminating the need for small bowel biopsy (3). Since different kits use different cutoff points and there is no standardization, the proposal of Barker et al. should be examined with caution regarding other tTG-IgA assays. Our data confirm that for high-risk children, strong Ab levels could predict villous atrophy (Marsh 3a to Marsh 3c) with high sensitivity (92.8 to 97.9%, depending on the kit and cutoff used). For the remaining 2.1 to 7.2% cases of strong Ab titer, Marsh 2 grading was observed, strongly suggestive of CD. These results are in agreement with those of an additional study regarding pediatric and adult patients, recently published (13).
In conclusion, our data reveal that biopsy-proven CD was found in a large proportion of children with a wide range of classical and atypical symptoms. Younger children exhibited severe biopsy findings together with intense clinical indications and higher Ab concentrations more frequently. The five serology assays varied in their performance levels and appeared to exhibit lower specificities in the clinical setting than those previously reported. The tTG-IgA Celikey kit demonstrated the best test efficiency for the studied population.
Acknowledgments
We are grateful to the Pediatric Gastroenterology Unit staff for the collection of specimen and clinical data used in this study. We thank the Immunology Unit staff at Maccabi Health Services Central Laboratory for their assistance with the serology testing. We are thankful to Pearl Lilos for the statistical analysis and drafting of the article.
Footnotes
Published ahead of print on 23 September 2009.
REFERENCES
- 1.Abrams, J. A., P. Brar, B. Diamond, H. Rotterdam, and P. H. Green. 2006. Utility in clinical practice of immunoglobulin A anti tissue transglutaminase antibody for the diagnosis of celiac disease. Clin. Gastroenterol. Hepatol. 4:726-730. [DOI] [PubMed] [Google Scholar]
- 2.Agardh, D. 2007. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin. Gastroenterol. Hepatol. 5:1276-1281. [DOI] [PubMed] [Google Scholar]
- 3.Barker, C. C., C. Mitton, G. Jevon, and T. Mock. 2005. Can. tissue transglutaminase antibody titers replace small bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics 115:1341-1346. [DOI] [PubMed] [Google Scholar]
- 4.Bienvenu, J., J. Whicher, B. Chir, and F. Aguzzi. 1996. Immunoglobulins, p. 11.01-13. In R. F. Ritchie (ed.), Serum proteins in clinical medicine, vol. I: laboratory section. Foundation of Blood Research, Scarborough, ME. [Google Scholar]
- 5.Bürgin-Wolff, A., I. Dahlbom, F. Hadziselimovic, and C. J. Petersson. 2002. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand. J. Gastroenterol. 37:685-691. [DOI] [PubMed] [Google Scholar]
- 6.Bürgin-Wolff, A., H. Gaze, F. Hadziselimovic, H. Huber, M. J. Lentze, D. Nusslé, and C. Reymond-Berthet. 1991. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch. Dis. Child. 66:941-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson, A. K., I. E. Axelsson, S. K. Borulf, A. C. Bredberg, and S. A. Ivarsson. 2001. Serological screening for celiac disease in healthy 2.5-year-old children in Sweden. Pediatrics 107:42-45. [DOI] [PubMed] [Google Scholar]
- 8.Cataldo, F., V. Marino, A. Ventura, G. Bottaro, and G. R. Corazza. 1998. Prevalence and clinical features of selective immunoglobulin A deficiency in celiac disease: an Italian multicentre study. Gut 42:362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catassi, C., E. Fabiani, I. M. Ratch, G. V. Coppa, P. L. Giorgi, R. Pierdomenico, S. Alessandrini, G. Iwanejko, R. Domenici, E. Mei, A. Miano, M. Marani, G. Bottaro, M. Spina, M. Dotti, A. Montanelli, M. Barbato, F. Viola, R. Lazzari, M. Vallini, G. Guariso, M. Plebani, F. Cataldo, G. Traverso, A. Ventura, et al. 1996. The coeliac iceberg in Italy: a multicentre antigliadin antibody screening for coeliac disease in school-age subjects. Acta Paediatr. Suppl. 412:29-35. [DOI] [PubMed] [Google Scholar]
- 10.Catassi, C., D. Kryszak, O. Louis-Jacques, D. R. Duerksen, I. Hill, S. E. Crowe, A. R. Brown, N. J. Procaccini, B. A. Wonderly, P. Hartley, J. Moreci, N. Bennett, K. Horvath, M. Burk, and A. Fasano. 2007. Detection of celiac disease in primary care: a multicenter case-finding study in North America. Am. J. Gastroenterol. 102:1454-1460. [DOI] [PubMed] [Google Scholar]
- 11.Catassi C., I. M. Ratsch, E. Fabiani, M. Rossini, F. Bordicchia, F. Candela, G. V. Coppa, and P. L. Giorgi. 1994. Coeliac disease in the year 2000: exploring the iceberg. Lancet 343:200-203. [DOI] [PubMed] [Google Scholar]
- 12.Chand, N., and A. A. Mihas. 2006. Celiac disease, current concepts in diagnosis and treatment. J. Clin. Gastroenterol. 40:3-14. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson, M. R., L. S. Book, K. M. Leiferman, J. J. Zone, and S. L. Neuhausen. 2008. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J. Clin. Gastroenterol. 42:256-260. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson, M. R., S. D. Firth, H. Wimpee, K. M. Leiferman, J. J. Zone, W. Horsley, M. A. O'Gorman, W. D. Jackson, S. L. Neuhausen, C. M. Hull, and L. S. Book. 2007. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin. Gastroenterol. Hepatol. 5:567-573. [DOI] [PubMed] [Google Scholar]
- 15.Fasano, A., M. Araya, S. Bhatnagar, D. Cameron, C. Catassi, M. Dirks, M. L. Mearin, L. Ortigosa, A. Phillips, et al. 2008. Federation of international societies of pediatric gastroenterology, hepatology and nutrition consensus report on celiac disease. J. Pediatr. Gastroenterol. Nutr. 47:214-219. [DOI] [PubMed] [Google Scholar]
- 16.Fasano, A., I. Berti, T. Geraduzzi, T. Not, R. B. Colletti, S. Drago, Y. Elitsur, P. H. Green, S. Guandalini, I. D. Hill, M. Pietzak, A. Ventura, M. Thorpe, D. Kryszak, F. Fornaroli, S. S. Wasserman, J. A. Murray, and K. Horvath. 2003. Prevalence of celiac disease in at-risk and not at-risk groups in the United States: a large multicenter study. Arch. Intern. Med. 163:286-292. [DOI] [PubMed] [Google Scholar]
- 17.Green, P. H. 2007. Where are all those patients with celiac disease? Am. J. Gastroenterol. 102:1461-1463. [DOI] [PubMed] [Google Scholar]
- 18.Green, P. H., and C. Cellier. 2007. Celiac disease. N. Engl. J. Med. 357:1731-1743. [DOI] [PubMed] [Google Scholar]
- 19.Green, P. H., and B. Jabri. 2006. Celiac disease. Annu. Rev. Med. 57:207-221. [DOI] [PubMed] [Google Scholar]
- 20.Hadithi, M., B. M. von Blomberg, J. B. Crusius, E. Bloemena, P. J. Kostense, J. W. Meijer, C. J. Mulder, C. D. Stehouwer, and A. S. Peña. 2007. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 147:294-302. [DOI] [PubMed] [Google Scholar]
- 21.Hill, I. D. 2005. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology 128(Suppl.):S25-S28. [DOI] [PubMed] [Google Scholar]
- 22.Liu, E., M. Li, L. Emery, I. Taki, K. Barriga, C. Tiberti, G. S. Eisenbarth, M. J. Rewers, and E. J. Hoffenberg. 2007. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 45:293-300. [DOI] [PubMed] [Google Scholar]
- 23.Lurz, E., U. Scheidegger, J. Spalinger, M. Schöni, and S. Schibli. 2009. Clinical presentation of celiac disease and the diagnostic accuracy of serologic markers in children. Eur. J. Pediatr. 168:839-845. [DOI] [PubMed] [Google Scholar]
- 24.McGowan, K. E., M. E. Lyon, S. D. Loken, and J. D. Butzner. 2007. Celiac disease: are endomysial antibody test results being used appropriately? Clin. Chem. 53:1775-1781. [DOI] [PubMed] [Google Scholar]
- 25.Mearin, M. L. 2007. Celiac disease among children and adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 37:86-105. [DOI] [PubMed] [Google Scholar]
- 26.Niveloni, S., E. Sugai, A. Cabanne, H. Vazquez, J. Argonz, E. Smecuol, M. L. Moreno, F. Nachman, R. Mazure, Z. Kogan, J. C. Gomez, E. Mauriño, and J. C. Bai. 2007. Antibodies against synthetic deamidated gliadin peptides as predictors of celiac disease: prospective assessment in an adult population with a high pretest probability of disease. Clin. Chem. 53:2186-2192. [DOI] [PubMed] [Google Scholar]
- 27.Oberhuber, G., G. Granditsch, and H. Vogelsang. 1999. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 11:1185-1194. [DOI] [PubMed] [Google Scholar]
- 28.Prince, H. E. 2006. Evaluation of the INOVA diagnostics enzyme-linked immunosorbent assay kits for measuring serum immunoglobulin G (IgG) and IgA to deamidated gliadin peptides. Clin. Vaccine Immunol. 13:150-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashtak, S., M. W. Ettore, H. Homburger, and J. A. Murray. 2008. Comparative usefulness of deamidated gliadin antibodies in the diagnosis of celiac disease. Clin. Gastroenterol. Hepatol. 6:426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravikumara, M., V. K. T. Nootigattu, and B. K. Sandhu. 2007. Ninety percent of celiac disease is being missed. J. Pediatr. Gastroenterol. Nutr. 45:497-499. [DOI] [PubMed] [Google Scholar]
- 31.Rostom, A., C. Dubé, A. Cranney, N. Saloojee, R. Sy, C. Garritty, M. Sampson, L. Zhang, F. Yazdi, V. Mamaladze, I. Pan, J. MacNeil, D. Mack, D. Patel, and D. Moher. 2005. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology 128(Suppl.):S38-S46. [DOI] [PubMed] [Google Scholar]
- 32.Sugai, E., H. Vázquez, F. Nachman, M. L. Moreno, R. Mazure, E. Smecuol, S. Niveloni, A. Cabanne, Z. Kogan, J. C. Gómez, E. Mauriño, and J. C. Bai. 2006. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin. Gastroenterol. Hepatol. 4:1112-1117. [DOI] [PubMed] [Google Scholar]
- 33.Van Meensel, B., M. Hiele, I. Hoffman, S. Vermeire, P. Rutgeerts, K. Geboes, and X. Bossuyt. 2004. Diagnostic accuracy of ten second-generation (human) tissue transglutaminase antibody assays in celiac disease. Clin. Chem. 50:2125-2135. [DOI] [PubMed] [Google Scholar]
- 34.Villalta, D., M. G. Alessio, M. Tampoia, E. Tonutti, I. Brusca, M. Bagnasco, G. Pesce, S. Stella, and N. Bizzaro. 2007. Testing for IgG class antibodies in celiac disease patients with selective IgA deficiency. A comparison of the diagnostic accuracy of 9 IgG anti-tissue transglutaminase, 1 IgG anti-gliadin and 1 IgG anti-deaminated gliadin peptide antibody assays. Clin. Chim. Acta 382:95-99. [DOI] [PubMed] [Google Scholar]
- 35.Vivas, S., J. M. Ruiz de Morales, M. Fernandez, M. Hernando, B. Herrero, J. Casqueiro, and S. Gutierrez. 2008. Age-related clinical, serological, and histopathological features of celiac disease. Am. J. Gastroenterol. 103:2360-2365. [DOI] [PubMed] [Google Scholar]
- 36.Volta, U., A. Granito, E. Fiorini, C. Parisi, M. Piscaglia, G. Pappas, P. Muratori, and F. B. Bianchi. 2008. Usefulness of antibodies to deamidated gliadin peptides in celiac disease diagnosis and follow-up. Dig. Dis. Sci. 53:1582-1588. [DOI] [PubMed] [Google Scholar]
- 37.Walker-Smith, J. A., S. Guandalini, J. Schmitzs, D. H. Shmerling, and J. K. Visakorpi. 1990. Revised criteria for the diagnosis of celiac disease. Report of the working group of European Society of Pediatric Gastroenterology and Nutrition. Arch. Dis. Child. 65:909-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West, J., R. F. Logan, P. G. Hill, A. Lloyd, S. Lewis, R. Hubbard, R. Reader, G. K. Holmes, and K. T. Khaw. 2003. Seroprevalence, correlates and characteristics of undetected celiac disease in England. Gut 52:960-965. [DOI] [PMC free article] [PubMed] [Google Scholar]