Abstract
Immunization of pregnant women can be an efficient strategy to induce early protection in infants in developing countries. Pneumococcal protein-based vaccines may have the capacity to induce pneumococcal serotype-independent protection. To understand the potential of maternal pneumococcal protein-specific antibodies in infants in high-risk areas, we studied the placental transfer of naturally acquired antibodies to pneumolysin (Ply) and pneumococcal surface protein A family 1 and 2 (PspA1 and PspA2) in relation to onset of pneumococcal nasopharyngeal carriage in infants in Papua New Guinea (PNG). In this study, 76% of the infants carried Streptococcus pneumoniae in the upper respiratory tract within the first month of life, at a median age of 19 days. Maternal and cord blood antibody titers to Ply (ρ = 0.824, P < 0.001), PspA1 (ρ = 0.746, P < 0.001), and PspA2 (ρ = 0.631, P < 0.001) were strongly correlated. Maternal pneumococcal carriage (hazard ratio [HR], 2.60; 95% confidence interval [CI], 1.25 to 5.39) and younger maternal age (HR, 0.74; 95% CI, 0.54 to 1.00) were independent risk factors for early carriage, while higher cord Ply-specific antibody titers predicted a significantly delayed onset (HR, 0.71; 95% CI, 0.52 to 1.00) and cord PspA1-specific antibodies a significantly younger onset of carriage in PNG infants (HR, 1.57; 95% CI, 1.03 to 2.40). Maternal vaccination with a pneumococcal protein-based vaccine should be considered as a strategy to protect high-risk infants against pneumococcal disease by reducing carriage risks in both mothers and infants.
Every year approximately 1 million children under 5 years of age die of pneumococcal pneumonia, meningitis, or sepsis, mostly in developing countries (4). Despite the efficacy of pneumococcal conjugate vaccines, Streptococcus pneumoniae remains an important cause of serious morbidity and mortality in young infants in developing countries (5, 8, 44), where the age of onset of disease is often younger than the recommended vaccination age of 6 weeks old and many of the serotypes causing serious disease are not included in currently available conjugate vaccines. Alternative vaccines and vaccine strategies are therefore needed to induce the earliest protection possible in high-risk infants.
Early onset of pneumococcal colonization and prolonged carriage in the upper respiratory tract are believed to play important roles in the high incidence and early onset of pneumococcal diseases in children in developing countries (10, 25). In the highlands of Papua New Guinea (PNG), where this study was performed, all infants carry pneumococci in the upper respiratory tract by the age of 3 months old, and 60% of them are already carriers during the neonatal period at a median age of 17 days old (14). This is in contrast to high-income countries, where less than half of the children experience pneumococcal colonization within the first year of life (3, 10, 43). Besides children, pneumococcal carriage rates remain higher in adulthood in developing countries, including PNG, where approximately half of the adults carry pneumococci in the upper respiratory tract (17, 39), compared to 1 to 13% of adults in low-risk countries (12, 15, 20). Consequently, maternal pneumococcal carriage may be an important risk factor for early colonization in infants in high-risk areas, in particular considering the frequent and close contact between mother and child in the critical early period of life when infants are highly susceptible.
In the first few months of life, when the human immune system is still highly immature (27), infants largely depend on passively acquired maternal immunoglobulin G (IgG) antibodies to protect themselves against invading pathogens. Immunization of pregnant women is a strategy that has been proven to reduce infection risks in both mothers and infants (9, 13, 45). This includes the potential to reduce acute lower respiratory illnesses in infants in high-risk areas, as shown with maternal immunization with the 23-valent pneumococcal polysaccharide vaccine (36, 37). However, the efficacy of pneumococcal polysaccharide vaccines on reducing nasopharyngeal colonization is limited, whereas the protective effect of pneumococcal conjugate vaccines is restricted by the number of pneumococcal serotypes that can be included. On the other hand, novel vaccines based on conserved pneumococcal proteins may offer better, serotype-independent protection against pneumococcal carriage and disease. This may include maternal immunization strategies, as supported by findings in mice (21).
Pneumolysin (Ply) and pneumococcal surface protein A (PspA) are two conserved proteins that are expressed by virtually all S. pneumoniae isolates and that are being considered as vaccine candidates. Pneumolysin is the thiol-activated cytolysin produced by S. pneumoniae that enables the bacterium to penetrate the host's physical defenses through its cytotoxic effect on epithelial cells, thus facilitating carriage and disease (28). PspA is a cell wall-associated protein that plays a role in inhibiting complement-mediated opsonization (7, 33) and can prevent lactoferrin-mediated clearance (19). In contrast to Ply, PspA shows structural diversity between pneumococcal strains and has been classified into three families based on the sequence variability of the most C-terminal 100 amino acids of the N-terminal domain of PspA. Although S. pneumoniae strains expressing family 1 or 2 PspA proteins account for 98% of clinical isolates, protective PspA-specific IgG antibodies binding to this highly variable region are family dependent (7).
Both Ply and PspA have been shown to be highly immunogenic and to protect mice against disease and colonization following pneumococcal challenge (2, 6, 7, 11, 34). There is evidence that in humans naturally acquired IgA and IgG antibodies to PspA and Ply can mediate protection against subsequent pneumococcal carriage and disease (22, 24, 30, 31, 38, 46). Moreover, naturally acquired antibodies to Ply and PspA have been shown to be transferred from mother to child and to protect against early pneumococcal carriage and infection, at least in populations in low-risk areas (18, 38). It is not known whether these findings hold true for areas of high endemicity, where infants are at a considerably higher risk for early carriage and disease.
In order to understand the role of maternal antibodies to Ply and PspA in protecting high-risk infants against early carriage, we studied antibody titers in paired maternal and cord blood samples in relation to the infant's age of first pneumococcal nasopharyngeal carriage. We hypothesized that, compared to lower-risk settings, maternal Ply- and PspA-specific antibody titers would be higher and would be associated with a delay in the age of first pneumococcal carriage in the offspring.
MATERIALS AND METHODS
Study population.
Study mothers and newborns were participants in a neonatal pneumococcal conjugate vaccination trial performed in the Asaro Valley in the Eastern Highlands Province of PNG (42). Pregnant women were recruited in villages located within an hour's drive from Goroka town, the provincial capital, or the antenatal clinic of Goroka General Hospital, the only tertiary hospital in the province. Inclusion criteria for newborns were the intention of the family to remain in the study area for at least 2 years, a birth weight of at least 2,000 g, no acute neonatal infection, and no severe congenital abnormality. While there was no routine human immunodeficiency virus testing, antenatal testing is recommended and children of mothers known to be human immunodeficiency virus positive were excluded.
After birth, umbilical cord blood samples (25 to 50 ml) were collected in sterile tubes containing an equal volume of RPMI 1640 (Invitrogen-Life Technologies, Melbourne, Australia) and preservative-free heparin (20 IU/ml). The total volume of cord blood collected was recorded to correct for the plasma dilution factor. Venous blood samples (10 ml) were collected from mothers 1 month postpartum in 100 IU/ml preservative-free heparin. Since cord blood collections were started only later in the trial, paired maternal and cord blood plasma samples were available for only 89 of the 313 newborns enrolled in the vaccination study. Pernasal swabs (PNS) were collected from the infants at 1, 2, 3, and 4 weeks of age and from the mothers at the time of delivery.
Apart from two sets of twins that were born by caesarean section, all 89 babies (58% boys) were born by natural delivery at an overall mean gestation age of 39.5 weeks (standard deviation [SD], 1.4) with a mean birth weight of 3,300 g (SD, 540). Thirty-eight percent of the pregnancies were primigravidae. At the time of delivery, the 87 PNG study mothers had a mean age of 26 years (SD, 6). As part of the vaccination trial, one-third of the newborns had been randomized to receive one dose of a 7-valent pneumococcal conjugate vaccine (Prevnar; Wyeth) at birth (34 of the 89 newborns with paired maternal samples). All study children received bacillus Calmette-Guérin vaccine, a dose of oral polio vaccine, and a dose of hepatitis B vaccine at birth.
As a maternal control group from a low-risk area, serum samples collected 1 month postpartum from 50 women participating in a neonatal allergy study in Perth, Western Australia, were included.
In PNG, assent was sought from women and their partners at the time of recruitment, and written informed consent was obtained shortly after delivery. In Australia, written informed consent was obtained from the pregnant women at the time of recruitment. Ethical approval for this study was obtained from the PNG Medical Research Advisory Committee and the Princess Margaret Hospital Ethics Committee in Perth, Australia.
Pneumococcal protein antigens.
The pneumolysin toxoid used in this study (PdB) was genetically engineered from a serotype 2 pneumococcus and kindly provided by James Paton (School of Molecular and Biomedical Science, The University of Adelaide, Adelaide, Australia) (2). The toxoid, which carries a Trp-433 →Phe substitution, was purified from Escherichia coli JM109(pJCP202) and stored in 50% glycerol.
PspA1 (family 1, clade 2) was derived from the recombinant PspA/Rx1AA1.0.302 protein, which comprised 302 N-terminal amino acids of the pneumococcal strain Rx1 PspA, and PspA2 (family 2, clade 3) from PspA/V-24AA1.0.410, consisting of amino acids 1 to 410 of the wild-type mature PspA of S. pneumoniae Taiwan19F-14. Both recombinant PspA expression constructs were transformed into competent E. coli BL21 Star(DE3)pLysS cells in the presence of 100 μg/ml ampicillin and 34 μg/ml chloramphenicol (Invitrogen Corp., Carlsbad, CA). Recombinant protein was expressed with a C-terminal hexahistidine fusion tag, and soluble recombinant proteins were purified using Ni2+-nitrilotriacetic acid agarose chromatography in the presence of 600 mM NaCl (Qiagen GmbH, Germany). Fractions containing the relevant proteins were pooled, dialyzed into 10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 2 mM EDTA, and applied to a Bio-Rad Macro-prep High Q anion exchange support (Bio-Rad, Hercules, CA). Elution was achieved with a linear gradient of 100 to 500 mM NaCl in Tris-HCl, pH 7.4, 2 mM EDTA. Fractions containing the relevant protein were pooled and further purified using size exclusion chromatography by applying the samples to a HiPrep HR S200 26/60 column. A single peak was obtained for each of the PspA proteins. Finally, the proteins were sterilized and endotoxin removed using 0.2-μm Mustang E filters (Pall Life Sciences, Portsmouth, United Kingdom). The purities of both PspA1 (∼40 kDa) and PspAs (∼55 kDa) were checked on a 12.5% sodium dodecyl sulfate-polyacrylamide gel by the method of Laemmli (23), and the concentrations were determined using the optical density at 280 nm (OD280) measurements and extinction coefficients (PspA1 ɛ, 10,430; PspA2 ɛ, 13,410).
Pneumococcal protein enzyme-linked immunosorbent assay (ELISA).
Plasma was separated from umbilical cord blood samples within 18 h of collection by spinning for 30 min at 400 × g, while maternal venous blood samples were spun for 10 min at 700 × g within 2 h of collection. Samples were stored at −20°C until shipment of aliquots (1 to 1.5 ml) to Perth for further analysis.
Microtiter ELISA plates (96-well flat-bottomed Polysorp; Nunc, Denmark) were coated with Ply (1.25 μg/ml), PspA1 (2.5 μg/ml), or PspA2 (2.5 μg/ml) in coating buffer (sodium carbonate buffer, pH 9.6) and incubated overnight at 4°C for Ply and at 37°C in 5% CO2 for PspA. Plates were washed four times with phosphate-buffered saline (PBS)-0.05% Tween before blocking with PBS-0.05% Tween plus 5% skim milk powder at 37°C, 5% CO2 for 1 h to prevent nonspecific binding. In-house reference and quality control (high and low) sera standards (obtained from healthy laboratory volunteers) and test samples (maternal and cord blood plasma) were diluted in assay buffer solution (1× PBS-0.05% Tween plus 5% skim milk powder) at predetermined starting dilutions (for cord blood, 1/50; PNG maternal, 1/400; Australian maternal, 1/25) and serially diluted (twofold) before being added to the plates. After discarding the block buffer, in-house reference sera, high and low quality control and test sera were added and incubated for 2 h at room temperature on a plate shaker. After four washes, an alkaline phosphatase-conjugated goat anti-human IgG diluted 1/2,000 (Biosource) was added and plates were incubated for 1.5 h at room temperature on a plate shaker before washing five times (three times with wash buffer and two times with distilled water) and incubating with p-nitrophenyl phosphate (tablets; Sigma) substrate buffer for 1.5 h at 37°C and 5% CO2. Substrate color development was stopped by adding a solution of 3 M sodium hydroxide. Absorbance levels were measured as ODs by using an automated microtiter plate reader (Sunrise; Tecan Austria GmbH, Austria) at a reading wavelength of 405 nm and reference wavelength of 620 nm. The OD readings were then converted into arbitrary ELISA units (AEU).
Bacteriology.
PNS samples were stored in 1 ml of skim milk-glucose-glycerine broth at −70°C until further processing at the Papua New Guinea Institute for Medical Research to determine pneumococcal carriage, using standard bacteriological culturing, isolation, and pneumococcal identification methods (25, 32). In summary, after thawing and vortexing, 10 μl of the PNS sample suspension (primary inocula) was transferred onto culture plates containing either plain horse blood agar, chocolate agar, gentamicin (5 μg/ml) blood agar, or bacitracin (300 μg/ml) chocolate agar (Oxoid, Australia), using sterile disposable inoculating loops (3 mm), and incubated at 36°C in 5% CO2 for 24 h and a further 24 h if extra bacterial growth was required. Presumptive pneumococcal colonies were then cultured with an optochin disc and confirmed to be S. pneumoniae based on their susceptibility.
Statistical analyses.
All statistical analyses were performed using the statistical package SPSS 15.0 (SPSS Inc.). IgG antibody levels were log transformed into geometric mean titers (GMT). Differences between groups were tested using the nonparametric Mann-Whitney U test, and correlations were studied using the Spearman's rank correlation method. Logistic regression was used to analyze associations between antibody responses and risk for infant carriage within the first month of life, whereas the Cox regression was used to study associations between antibody titers and age of first pneumococcal carriage of the infants within the first month of life. In the regression models, antibody responses were studied as Z-scores, which were calculated based on the following equation: (log antibody titer − mean log antibody titer)/standard deviation of log antibody titer. Where indicated, regression models were adjusted for confounding by maternal pneumococcal carriage, maternal age, and pneumococcal conjugate vaccination. An association was considered to be significant at a P level of <0.05.
RESULTS
Pneumococcal carriage in PNG mothers and infants.
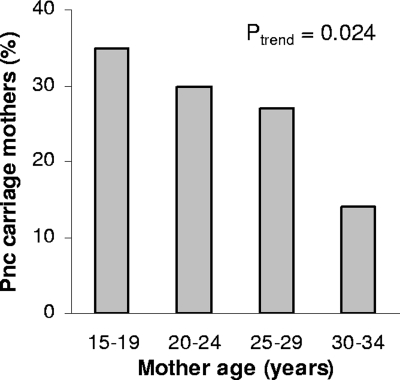
At the time of delivery, 30% of the PNG study mothers carried pneumococci in the upper respiratory tract, with higher carriage rates being observed in younger compared to older mothers (Fig. 1). Of the newborns that had completed all four weekly visits, 76% (55/72) carried S. pneumoniae at least once during the first month of life, with a median age of onset of 19 days (interquartile range, 12 to 28). Maternal pneumococcal carriage at the time of delivery (hazard ratio [HR], 1.97; 95% confidence interval [CI], 1.01 to 3.85; P = 0.046) (Fig. 2) and younger maternal age (HR, 0.76; 95% CI, 0.57 to 1.01, for every 5 years of age; P = 0.062) were found to be independent risk factors for earlier onset of pneumococcal carriage in infancy.
FIG. 1.
Pneumococcal nasopharyngeal carriage in PNG mothers according to their age.
FIG. 2.
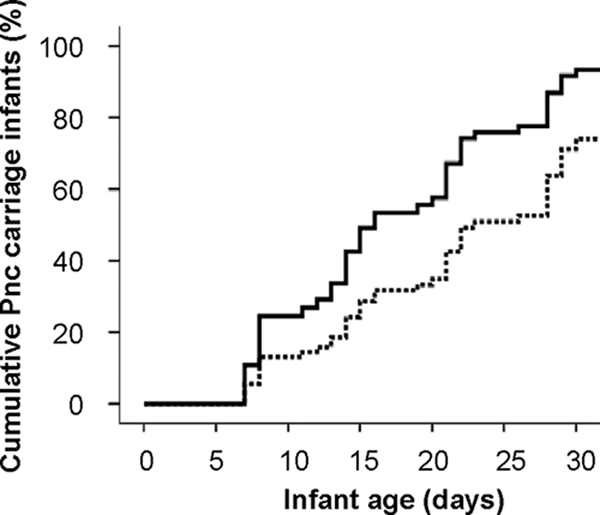
Maternal pneumococcal carriage as a predictor of age of first pneumococcal carriage in PNG infants. The solid line represents the group of PNG infants whose mothers carried pneumococci at the time of delivery (n = 20), and the broken line represents infants whose mothers were noncarriers (n = 47).
Antibody responses to Ply and PspA in mothers and newborns.
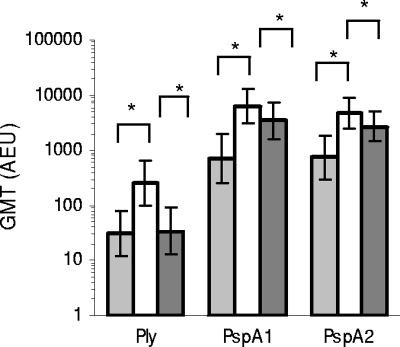
Compared to plasma samples from Australian (AUS) mothers, geometric mean IgG antibody titers to Ply, PspA1, and PspA2 were significantly higher in plasma samples of PNG mothers (Fig. 3). Antibody titers in paired PNG mothers and newborn cord blood samples were strongly correlated for all three studied pneumococcal proteins (Spearman correlation [ρ] for Ply = 0.824, P < 0.001; for PspA1, ρ = 0.746, P < 0.001; for PspA2, ρ = 0.631, P < 0.001), but cord antibody titers to Ply were on average 7.2-fold (median) lower compared to titers in paired maternal samples, whereas PspA1 and Pspa2 titers were, respectively, 1.8-fold and 1.7-fold lower (Fig. 3). IgG antibody titers specific for PspA1 and PspA2 were strongly correlated in sera of PNG mothers (ρ = 0.652, P < 0.001), PNG newborns (ρ = 0.677, P < 0.001), and AUS mothers (ρ = 0.620, P < 0.001), whereas such a correlation was not found between antibody titers to PspA1 and Ply (PNG mothers, ρ = 0.261, P = 0.015; PNG newborns, ρ = 0.178, P = 0.096; AUS mothers, ρ = 0.160, P = 0.266), or PspA2 and Ply (PNG mothers, ρ = 0.137, P = 0.207; PNG newborns, ρ = 0.142, P = 0.184; AUS mothers, ρ = 0.193, P = 0.178).
FIG. 3.
Antibody titers to pneumococcal proteins in PNG mothers and newborns and AUS mothers. Data shown are GMTs (and 95% confidence intervals) of antibodies to Ply, PspA1, and PspA2 in plasma samples of Australian mothers (light gray bars; n = 50) and of Papua New Guinean mothers (white bars; n = 87) and their newborns (dark gray bars; n = 89), with * indicating significant differences (P < 0.05) between groups.
Antibody titers to Ply decreased significantly with increasing age of PNG mothers in maternal sera (linear regression coefficient [β], −0.30 standard deviation (SD)/5 years of age; 95% CI, −0.48 to −0.12; P = 0.002) as well as in cord blood samples (β, −0.23 SD/5 years of age; 95% CI, −0.43 to −0.05; P = 0.015). In contrast, antibody titers to Ply increased with age in sera of AUS mothers (β, 0.34 SD/5 years of age; 95% CI, −0.14 to 0.69; P = 0.060). No age-related changes were found for antibodies to PspA1 and PspA2 in either PNG or AUS mothers (data not shown). No associations were found between pneumococcal carriage in PNG mothers at the time of delivery and antibody titers to Ply, PspA1, or PspA2 (Table 1).
TABLE 1.
IgG antibody titers in maternal and cord samples in relation to maternal pneumococcal carriage
| Antigen | Geometric mean IgG titera (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Maternal venous blood from mother who was: |
Cord blood from mother who was: |
|||||
| Noncarrier | Carrier | P value | Noncarrier | Carrier | P value | |
| Ply | 223 (115-433) | 311 (123-789) | 0.143 | 30 (12-75) | 36 (10-130) | 0.459 |
| PspA1 | 6,654 (2,484-17,824) | 7,219 (3,677-14,174) | 0.696 | 3,572 (1,958-6,515) | 3,536 (1,855-6,741) | 0.671 |
| PspA2 | 4,866 (2,569-9,219) | 4,440 (1,651-11,941) | 0.435 | 2,610 (1,362-5,000) | 2,776 (1,360-5,667) | 0.826 |
Values are geometric mean concentrations (and 95% confidence intervals) of IgG antibodies to Ply, PspA1, and Pspa2 for venous blood samples of PNG mothers 1 month postpartum (n = 87) and paired cord blood samples (n = 89) in relation to nasopharyngeal pneumococcal carriage of the mothers at the time of delivery.
Protective effects of maternally derived Ply- and PspA-specific antibodies on early carriage.
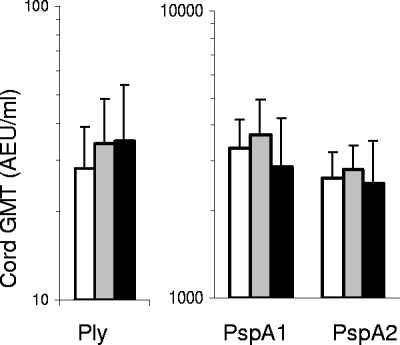
Cord IgG antibody titers to Ply tended to be lower in children that carried pneumococci within the first 2 weeks of life than in those that carried for the first time between 2 and 4 weeks of age or that did not carry within the first month, but this association was not significant (P = 0.321) (Fig. 4). In contrast, antibody titers for PspA1 or PspA2 tended to be the lowest in children that remained free of pneumococcal carriage within the first month of life (PspA1, P = 0.166; PspA2, P = 0.677). To study associations in relation to age of first pneumococcal carriage, cord and maternal antibody titers to Ply, PspA1, and PspA2 were studied in three different Cox regression models (Table 2). No significant associations were found when antibody responses were studied in univariate models (model I) or together in a multivariate Cox regression model (model II). However, IgG antibody titers to Ply were associated with a significantly delayed onset of first pneumococcal carriage and higher antibody titers to PspA1 with a significantly earlier onset of carriage in a multivariate regression model (model III) adjusting for maternal age (HRadjusted, 0.74; 95% CI, 0.54 to 1.00 for every 5 years of age; P = 0.048) and maternal pneumococcal carriage at the time of delivery (HRadjusted, 2.60; 95% CI, 1.25 to 5.39; P = 0.010). Findings were similar whether cord blood or maternal antibody titers to Ply and PspA1 were studied. Associations remained unchanged when models were coadjusted for 7-valent pneumococcal conjugate vaccine immunization at birth (data not shown).
FIG. 4.
Cord antibody titers to Ply, PspA1, and PspA2 in relation to early pneumococcal carriage. Data shown are GMTs (and 95% confidence intervals) of antibodies to Ply, PspA1, and PspA2 in cord samples of PNG newborns that carried pneumococci within the first 2 weeks of life (white bars; n = 27), within 2 to 4 weeks of life (gray bars; n = 28), or did not carry within the first 4 weeks of life (black bars; n = 17).
TABLE 2.
Cox regression for risk of early pneumococcal carriage in relation to cord and maternal IgG antibody titera
| Sample and antigen | Model I |
Model II |
Model III |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Cord blood | ||||||
| Ply | 0.90 (0.68-1.19) | 0.455 | 0.86 (0.64-1.14) | 0.294 | 0.69 (0.49-0.97) | 0.033 |
| PspA1 | 1.19 (0.91-1.57) | 0.201 | 1.42 (0.98-2.05) | 0.064 | 1.67 (1.03-2.71) | 0.037 |
| PspA2 | 1.01 (0.77-1.31) | 0.961 | 0.81 (0.57-1.15) | 0.232 | 0.89 (0.63-1.25) | 0.494 |
| Maternal blood | ||||||
| Ply | 0.92 (0.70-1.19) | 0.507 | 0.87 (0.66-1.13) | 0.291 | 0.68 (0.48-0.98) | 0.036 |
| PspA1 | 1.17 (0.88-1.55) | 0.292 | 1.46 (0.96-2.22) | 0.079 | 1.62 (0.97-2.69) | 0.065 |
| PspA2 | 0.99 (0.75-1.31) | 0.959 | 0.75 (0.50-1.12) | 0.164 | 0.83 (0.55-1.25) | 0.377 |
Cox regression analysis was used to study associations between age at onset of pneumococcal carriage and Z-scores of cord and maternal antibody titers to Ply, PspA1, and PspA2, using a univariate model(model I), a multivariate model (model II), and a multivariate model adjusting for maternal pneumococcal carriage and maternal age at the time of delivery in cord blood and maternal venous blood.
DISCUSSION
IgG antibodies to Ply that are transferred from mother to child were associated with delaying the age of first pneumococcal carriage in high-risk infants in PNG. This is in line with earlier findings for infants living in a relatively lower-risk area in the Philippines (18). Maternal antibodies to PspA family 1 did not protect, but in fact were associated with a significantly younger age of first carriage in the PNG infants. This is in contrast with an experimental human adult carriage study that reported a protective effect of PspA-specific antibodies (29, 30), but the findings are in line with a study in the Philippines that found no association between maternally derived PspA1-specific antibodies and protection against early carriage in infants (18). No associations were found between maternal antibodies to PspA family 2 and risk for early carriage in PNG infants. To our knowledge no other studies in humans have previously reported a possible protective effect of PspA2-specific antibodies.
In contrast to Ply, which is homogeneously expressed by virtually all pneumococcal strains, PspA has been classified into three families, with limited cross-protection between antibodies recognizing different PspA families (7). Low-affinity binding of antibodies from different PspA families may, however, hinder recognition and clearance by specific antibodies. This could explain the positive association between PspA antibodies and risk for early carriage observed in the PNG infants. To test this hypothesis and confirm a potential cross-inhibiting effect of PspA family-specific antibodies in young infants, functional antibody assays will have to be developed. In addition, more information will be needed regarding the geographical distribution and possible preferential nasopharyngeal colonization of young infants compared to adults by different PspA families to understand why findings can vary for different study populations.
In contrast to the Philippines study, we found that antibody titers were not equivalent in mother-newborn pairs (18) but were sevenfold and twofold lower in PNG newborns compared to their mothers for Ply-specific and PspA-specific antibodies, respectively. A difference between the two studies is that in the Philippines study, mothers were bled during the second to third trimester of pregnancy, when IgG antibodies are known to be decreased (1), whereas in our study maternal blood samples were collected 1 month postpartum, when titers have already started to increase. Alternatively, a reduced transfer efficiency of higher maternal IgG titers due to competition for receptor binding (16) may explain the lower antibody titers in PNG newborns compared to their mothers, although this does not offer an explanation for the relative lower transfer of Ply-specific compared with PspA-specific antibodies. Nevertheless, maternal and cord blood antibody titers gave very similar results in relation to early carriage risk, which indicates that the transfer of maternal antibodies was optimal in our study children. Moreover, this observation implies that maternal blood samples, even when collected 1 month postpartum, can be used as a surrogate for cord blood antibodies when cord samples are not available.
Maternal pneumococcal carriage and younger maternal age at the time of delivery were independent risk factors for early onset of pneumococcal carriage in PNG infants. It is not unexpected that mothers, in addition to siblings and other family members (34, 39), play an important role in transmission of S. pneumoniae in a high-risk area such as PNG, considering the high rates of pneumococcal carriage reported for adults compared with that of adults in low-risk areas (12, 15, 17, 20, 39). Serotyping of pneumococci isolated from nasopharyngeal swabs collected from mother-child pairs could be applied to further confirm the role of mother-to-child transmission, but due to lack of power this was not feasible for the current study. We suggest that behavioral changes may explain the protective role of older maternal age on early pneumococcal carriage, but as yet we have no insight into what these changes may be. Importantly, our observation reconfirms that prevention of maternal pneumococcal carriage through maternal immunization and/or reducing transmission risks through introducing measures such as improved hand washing practices (35, 40) will contribute to reducing early carriage risks in infants in high-risk areas such as in PNG.
In addition to antibodies transferred in utero, antibodies transmitted through breast milk can mediate protection against acute lower respiratory infections in young infants (26). Although breastfeeding is practiced by most Papua New Guinea women (41), this was not recorded on an individual basis for the mother-child pairs in our study. We acknowledge that the potential protective effect of breastfeeding is a relevant issue to take into account in future studies, as will be studying correlations between Ply and PspA antibody titers in breast milk compared to serum.
Finally, it is important to recognize that the protective effect of maternal Ply-specific antibodies was limited, since young infants in the highlands of PNG remain at high risk of early pneumococcal carriage. Comparable to findings from a study in the same area more than 20 years ago (14), nearly 80% of the study infants were found to carry S. pneumoniae in the upper respiratory tract within the first month of life, at a median age of 19 days. It remains to be shown that new vaccines such as pneumococcal protein-based vaccines can overcome early pneumococcal carriage and disease in young infants in developing countries, but vaccination strategies involving maternal immunization to enhance passive immunity and potentially reduce maternal pneumococcal carriage, in combination with neonatal vaccination to induce early memory T-cell responses (42), should be considered.
Acknowledgments
This work was supported by International Collaborative Research Grant Scheme, 071613/Z/03/Z, from the Wellcome Trust and Australian National Health and Medical Research Council (NHMRC). D. Lehmann is supported by an NHMRC program grant (353514), and A. van den Biggelaar is supported by an NHMRC R. Douglas Wright Biomedical Career Development grant (458780).
We thank J. Paton for providing pneumolysin antigen, the parents and guardians of the study children for their participation and ongoing support, and all staff of the Papua New Guinea Neonatal Pneumococcal Conjugate Vaccine Trial Team (in particular, G. Saleu, C. Opa, T. Orami, P. Namuigi, A. Javati, A. Sie, B. Nivio, J. Totave, R. Sehuko, L. Pui, N. Fufu, M. Dreyum, G. Inapero, and J. Reeder) and village reporters in the Asaro Valley for their contributions to this work.
Footnotes
Published ahead of print on 23 September 2009.
REFERENCES
- 1.Ailus, K. T. 1994. A follow-up study of immunoglobulin levels and autoantibodies in an unselected pregnant population. Am. J. Reprod. Immunol. 31:189-196. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J. E., R. A. Lock, C. C. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aniansson, G., B. Alm, B. Andersson, P. Larsson, O. Nylen, H. Peterson, P. Rigner, M. Svanborg, and C. Svanborg. 1992. Nasopharyngeal colonization during the first year of life. J. Infect. Dis. 165(Suppl. 1):S38-S42. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 2003. The world's forgotten children. Lancet 361:1. [PubMed] [Google Scholar]
- 5.Black, R. E., S. S. Morris, and J. Bryce. 2003. Where and why are 10 million children dying every year? Lancet 361:2226-2234. [DOI] [PubMed] [Google Scholar]
- 6.Campos, I. B., M. Darrieux, D. M. Ferreira, E. N. Miyaji, D. A. Silva, A. P. Areas, K. A. Aires, L. C. Leite, P. L. Ho, and M. L. Oliveira. 2008. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein A: induction of antibodies, complement deposition and partial protection against Streptococcus pneumoniae challenge. Microbes Infect. 10:481-488. [DOI] [PubMed] [Google Scholar]
- 7.Darrieux, M., E. N. Miyaji, D. M. Ferreira, L. M. Lopes, A. P. Lopes, B. Ren, D. E. Briles, S. K. Hollingshead, and L. C. Leite. 2007. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke, T. 2005. Neonatal pneumonia in developing countries. Arch. Dis. Child. Fetal Neonatal 90:F211-F219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englund, J. A. 2007. The influence of maternal immunization on infant immune responses. J. Comp. Pathol. 137(Suppl. 1):S16-S19. [DOI] [PubMed] [Google Scholar]
- 10.Faden, H., L. Duffy, R. Wasielewski, J. Wolf, D. Krystofik, Y. Tung, et al. 1997. Relationship between nasopharyngeal colonization and the development of otitis media in children. J. Infect. Dis. 175:1440-1445. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira, D. M., M. Darrieux, D. A. Silva, L. C. Leite, J. M. Ferreira, Jr., P. L. Ho, E. N. Miyaji, and M. L. Oliveira. 2009. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface proteins PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 16:636-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givon-Lavi, N., R. Dagan, D. Fraser, P. Yagupsky, and N. Porat. 1999. Marked differences in pneumococcal carriage and resistance patterns between day care centers located within a small area. Clin. Infect. Dis. 29:1274-1280. [DOI] [PubMed] [Google Scholar]
- 13.Glezen, W. P., and M. Alpers. 1999. Maternal immunization. Clin. Infect. Dis. 28:219-224. [DOI] [PubMed] [Google Scholar]
- 14.Gratten, M., H. Gratten, A. Poli, E. Carrad, M. Raymer, and G. Koki. 1986. Colonisation of Haemophilus influenzae and Streptococcus pneumoniae in the upper respiratory tract of neonates in Papua New Guinea: primary acquisition, duration of carriage, and relationship to carriage in mothers. Biol. Neonate 50:114-120. [DOI] [PubMed] [Google Scholar]
- 15.Gunnarsson, R. K., S. E. Holm, and M. Soderstrom. 1998. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from healthy children and adults. Scand. J. Prim. Health Care 16:13-17. [DOI] [PubMed] [Google Scholar]
- 16.Hartter, H. K., O. I. Oyedele, K. Dietz, S. Kreis, J. P. Hoffman, and C. P. Muller. 2000. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr. Infect. Dis. J. 19:635-641. [DOI] [PubMed] [Google Scholar]
- 17.Hill, P. C., A. Akisanya, K. Sankareh, Y. B. Cheung, M. Saaka, G. Lahai, B. M. Greenwood, and R. A. Adegbola. 2006. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin. Infect. Dis. 43:673-679. [DOI] [PubMed] [Google Scholar]
- 18.Holmlund, E., B. Quiambao, J. Ollgren, H. Nohynek, and H. Kayhty. 2006. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 24:57-65. [DOI] [PubMed] [Google Scholar]
- 19.Jedrzejas, M. J. 2007. Unveiling molecular mechanisms of bacterial surface proteins: Streptococcus pneumoniae as a model organism for structural studies. Cell. Mol. Life Sci. 64:2799-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jousimies-Somer, H. R., S. Savolainen, and J. S. Ylikoski. 1989. Comparison of the nasal bacterial floras in two groups of healthy subjects and in patients with acute maxillary sinusitis. J. Clin. Microbiol. 27:2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsurahara, T., M. Hotomi, K. Yamauchi, D. S. Billal, and N. Yamanaka. 2008. Protection against systemic fatal pneumococcal infection by maternal intranasal immunization with pneumococcal surface protein A (PspA). J. Infect. Chemother. 14:393-398. [DOI] [PubMed] [Google Scholar]
- 22.Kohler, C., A. A. Adegnika, R. Van der Linden, S. T. Agnandji, S. K. Chai, A. J. Luty, Z. Szepfalusi, P. G. Kremsner, and M. Yazdanbakhsh. 2008. Comparison of immunological status of African and European cord blood mononuclear cells. Pediatr. Res. 64:631-636. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Laine, C., T. Mwangi, C. M. Thompson, J. Obiero, M. Lipsitch, and J. A. Scott. 2004. Age-specific immunoglobulin g (IgG) and IgA to pneumococcal protein antigens in a population in coastal Kenya. Infect. Immun. 72:3331-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach, A. J., J. B. Boswell, V. Asche, T. G. Nienhuys, and J. D. Mathews. 1994. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatr. Infect. Dis. J. 13:983-989. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann, D., W. S. Pomat, I. D. Riley, and M. P. Alpers. 2003. Studies of maternal immunisation with pneumococcal polysaccharide vaccine in Papua New Guinea. Vaccine 21:3446-3450. [DOI] [PubMed] [Google Scholar]
- 27.Levy, O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7:379-390. [DOI] [PubMed] [Google Scholar]
- 28.Marriott, H. M., T. J. Mitchell, and D. H. Dockrell. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 8:497-509. [DOI] [PubMed] [Google Scholar]
- 29.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCool, T. L., T. R. Cate, E. I. Tuomanen, P. Adrian, T. J. Mitchell, and J. N. Weiser. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melin, M. M., S. K. Hollingshead, D. E. Briles, M. I. Lahdenkari, T. M. Kilpi, and H. M. Kayhty. 2008. Development of antibodies to PspA families 1 and 2 in children after exposure to Streptococcus pneumoniae. Clin. Vaccine Immunol. 15:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery, J. M., D. Lehmann, T. Smith, A. Michael, B. Joseph, T. Lupiwa, C. Coakley, V. Spooner, B. Best, I. D. Riley, et al. 1990. Bacterial colonization of the upper respiratory tract and its association with acute lower respiratory tract infections in Highland children of Papua New Guinea. Rev. Infect. Dis. 12(Suppl. 8):S1006-S1016. [DOI] [PubMed] [Google Scholar]
- 33.Ochs, M. M., W. Bartlett, D. E. Briles, B. Hicks, A. Jurkuvenas, P. Lau, B. Ren, and A. Millar. 2008. Vaccine-induced human antibodies to PspA augment complement C3 deposition on Streptococcus pneumoniae. Microb. Pathog. 44:204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogunniyi, A. D., M. Grabowicz, D. E. Briles, J. Cook, and J. C. Paton. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickering, H., and G. Rose. 1988. Nasal and hand carriage of Streptococcus pneumoniae in children and mothers in the Tari Basin of Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 82:911-913. [DOI] [PubMed] [Google Scholar]
- 36.Quiambao, B. P., H. Nohynek, H. Kayhty, J. Ollgren, L. Gozum, C. P. Gepanayao, V. Soriano, and P. H. Makela. 2003. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 21:3451-3454. [DOI] [PubMed] [Google Scholar]
- 37.Quiambao, B. P., H. M. Nohynek, H. Kayhty, J. P. Ollgren, L. S. Gozum, C. P. Gepanayao, V. C. Soriano, and P. H. Makela. 2007. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 25:4470-4477. [DOI] [PubMed] [Google Scholar]
- 38.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 39.Riley, I. D. 1979. Pneumonia in Papua New Guinea: a study of the effects of Western medicine upon disease in a developing country. University of Sydney, Sydney, Australia.
- 40.Stubbs, E., K. Hare, C. Wilson, P. Morris, and A. J. Leach. 2005. Streptococcus pneumoniae and noncapsular Haemophilus influenzae nasal carriage and hand contamination in children: a comparison of two populations at risk of otitis media. Pediatr. Infect. Dis. J. 24:423-428. [DOI] [PubMed] [Google Scholar]
- 41.Tracer, D. P. 2009. Breastfeeding structure as a test of parental investment theory in Papua New Guinea. Am. J. Hum Biol. 21:635-642. [DOI] [PubMed] [Google Scholar]
- 42.van den Biggelaar, A. H., P. C. Richmond, W. S. Pomat, S. Phuanukoonnon, M. A. Nadal-Sims, C. J. Devitt, P. M. Siba, D. Lehmann, and P. G. Holt. 2009. Neonatal pneumococcal conjugate vaccine immunization primes T cells for preferential Th2 cytokine expression: A randomized controlled trial in Papua New Guinea. Vaccine 27:1340-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson, K., K. Carville, J. Bowman, P. Jacoby, T. V. Riley, A. J. Leach, and D. Lehmann. 2006. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr. Infect. Dis. J. 25:782-790. [DOI] [PubMed] [Google Scholar]
- 44.WHO Young Infants Study Group. 1999. Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. Pediatr. Infect. Dis. J. 18:S17-S22. [DOI] [PubMed] [Google Scholar]
- 45.Zaman, K., E. Roy, S. E. Arifeen, M. Rahman, R. Raqib, E. Wilson, S. B. Omer, N. S. Shahid, R. F. Breiman, and M. C. Steinhoff. 2008. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 359:1555-1564. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Q., J. Bernatoniene, L. Bagrade, A. J. Pollard, T. J. Mitchell, J. C. Paton, and A. Finn. 2006. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur. J. Immunol. 36:46-57. [DOI] [PubMed] [Google Scholar]