Abstract
In a previous study, an apathogenic strain of bovine herpesvirus 4 (BoHV-4) cloned as a bacterial artificial chromosome and expressing a chimeric peptide (gE2/gD) as a secreted form was described. Recombinant virus-inoculated animals produced antibodies against bovine viral diarrhea virus (BVDV) gE2 and BoHV-1 gD. However, neutralizing antibodies were produced only against BVDV, not against BoHV-1. In the present work a recombinant BoHV-4 expressing a membrane-linked form of gE2/gD chimeric peptide was constructed, and inoculated rabbits produced serum-neutralizing antibodies against both BVDV and BoHV-1. Protein cell sorting and targeting are a very important issue when immunodominant antigens are engineered for recombinant virus vaccine development.
Bovine viral diarrhea virus (BVDV) is a member of the family Flaviviridae and is one of the three viruses in the Pestivirus genus of importance in veterinary medicine, including hog cholera virus and border disease virus. BVDV is a virus frequently associated with several manifestations in cattle ranging from unapparent infections to disease of high mortality (4). Bovine herpesvirus 1 (BoHV-1) is another very important herpesvirus pathogen for the cattle industry and causes significant economic losses worldwide (2). Infection is accompanied by various clinical manifestations, such as infectious bovine rhinotracheitis, infectious pustular vulvovaginitis, balanoposthitis, abortion, and generalized systemic infection. BoHV-1 is known to play an important role in the bovine respiratory disease complex, commonly referred to as shipping fever (2). Inflammation and necrosis of respiratory epithelia and immunosuppression often lead to increased susceptibility to secondary viral and bacterial infections, resulting in severe clinical disease. For both pathogens, vaccination remains the most important tool in terms of prevention, and novel vaccines are desired. The use of recombinant viral vaccines, although still far from reality, seems to be the most promising in terms of their safety and efficacy, and bovine herpesvirus 4 (BoHV-4), due to its biological characteristics, has been suggested to be a good candidate (8, 16).
In a previous work (8), an apathogenic Movar-like strain of BoHV-4 was isolated from the cell milk fraction of a healthy cow and its genome was cloned as a bacterial artificial chromosome (BAC) and manipulated to express as a secreted form a chimeric peptide (gE2/gD) made by the fusion of BVDV gE2 and the BoHV-1 gD immunodominant ectodomain. Infected rabbits produced antibodies against both BVDV and BoHV-1, but the serum-neutralizing fraction of such antibodies was detected only for BVDV. Because the cellular location of antigens expressed by DNA-based vaccines has been shown to modulate the immune response (17), in the present work a membrane-linked version of the chimeric peptide (gE2/gD-TM) expressed by a BoHV-4-based vector was constructed and compared with the secreted one. Inoculated rabbits successfully produced serum-neutralizing antibodies against BoHV-1 and BVDV.
MATERIALS AND METHODS
Cells.
Madin-Darby bovine kidney (MDBK; ATCC CCL-22), bovine embryo kidney (BEK; from M. Ferrari, Istituto Zooprofilattico Sperimentale, Brescia, Italy), rabbit kidney (RK-13; ATCC CCL37), and human embryo kidney (HEK 293T; ATCC CRL-11268) cell lines were cultured in Dulbecco's modified essential medium (Sigma) containing 10% fetal bovine serum (FBS), 2 mM of l-glutamine, 100 IU/ml of penicillin (Sigma), 100 μg/ml of streptomycin (Sigma), and 2.5 μg/ml of amphotericin B. Plates or flasks were incubated at 37°C in a humidified atmosphere of 95% air-5% CO2. Rabbit bone marrow stromal cells (RBMSC) were isolated and cultured according to a previously reported method (15) with some modification. Briefly, bone marrow was harvested from a New Zealand White rabbit weighing 1.5 to 2.0 kg, by means of suction with a 20-ml sterile syringe. Five milliliters of heparin (100 IU/ml) was used to anticoagulate the sample. The sample was recovered after centrifugation at 900 × g. Up to 4 × 105 nucleated cells were loaded onto 3 ml Percoll (Sigma) of a density of 1.089 g/ml in a 15-ml conical tube. Cell separation was accomplished by centrifugation at 1,100 × g for 20 min at 20°C. The cells were cultured in flasks at 2 × 105 cells/ml with Dulbecco's modified Eagle's medium F-12 (DMEM-F-12; Gibco) containing 15% FBS (HyClone) at 37°C and 5% CO2. Medium was replaced every third day. Freshly dissociated rabbit aortic endothelial cells (RAEC) were obtained from the aorta by procedures previously described (23). Briefly, several pieces of endothelium were incubated at 37°C for 35 min in dispersal solution containing 0.9 mg ml−1 papain and 0.8 mg ml−1 dithiothreitol. After the enzymatic digestions, tissue fragments were washed with enzyme-free, Dulbecco's phosphate-buffered saline (PBS) and then filtered and centrifuged. Supernatant containing the cells was cultured in flasks at 2 × 105 cells/ml with DMEM-F-12 (Gibco) containing 15% FBS (HyClone) at 37°C and 5% CO2. Medium was replaced every third day.
Viruses.
BoHV-4-A, BAC-BoHV-4-A, BAC-BoHV-4-A-CMV-IgK-gE2gD-TM, BoHV-4-EFGPΔTK, BoHV-1 (strain Oregon), and BVDV (strain NADL) were propagated by infecting confluent monolayers of MDBK or BEK cells at a multiplicity of infection (MOI) of 0.5 50% tissue culture infectious doses (TCID50) per cell and maintained in minimal essential medium (MEM; Sigma) with 2% FBS for 2 h. The medium was then removed and replaced with fresh MEM containing 10% FBS. When approximately 90% of the cell monolayer exhibited cytopathic effect (CPE) (72 h postinfection), the virus was prepared by freezing and thawing cells three times and pelleting the virions through 30% sucrose, as described previously (3). Virus pellets were resuspended in cold MEM without FBS. TCID50 were determined with MDBK cells by limiting dilution.
Constructs.
pCMV-IgK-gE2gD-TM was obtained by subcloning an 853-bp SalI/MluI terminal fragment containing the poly(A) site from pCMVgD (5) into the C-terminal SalI/MluI sites of pIgKgE2gD-11 (8), which was obtained by subcloning the SalI fragment from pCMVgD into the C-terminal XhoI/SalI sites of pIgKgE2. The XhoI site of E2 and the SalI site of gD are compatible and in frame, although the two sites were eliminated after the ligation. The first targeting vector, pTK-KanaGalK-TK, was generated by subcloning the galactokinase prokaryotic expression cassette (GalK), along with the kanamycin resistance expression cassette, into the pINT2 shuttle vector (9). The second targeting vector, pTK-CMV-IgK-gE2gD-TM-TK, was generated by subcloning the AseI/MluI fragment (cut and blunt ended) from pCMV-IgKgE2gD-TM into the SmaI site of pINT2 (9).
BAC BoHV-4-A recombineering and selection.
Recombineering was performed as previously described (8) with some modifications. SW102 bacteria containing KanaGalK targeted into the BoHV-4-A thymidine kinase (TK) locus were grown, heat induced, and electroporated with a gel-purified fragment (TK-CMV-IgK-gE2gD-TM-TK) obtained by cutting pTK-CMV-IgK-gE2gD-TM-TK with XhoI/ClaI (Roche). For the counterselection step, the bacteria were recovered in 10 ml LB in a 50-ml baffled conical flask and incubated for 4.5 h in a 32°C shaking water bath. Bacterial serial dilutions were plated on M63 minimal medium plates containing 15 g/liter agar, 0.2% glycerol (Sigma), 1 mg/liter d-biotin, 45 mg/liter l-leucine, 0.2% 2-deoxygalactose (Sigma), and 25 μg/ml chloramphenicol. Plates were incubated for 3 to 5 days at 32°C. Several selected colonies were picked up, streaked on MacConkey agar indicator plates (Difco, BD Biosciences) containing 20 μg/ml of chloramphenicol, and incubated at 32°C for 3 days until white colonies appeared. White colonies were grown in duplicate for 5 to 8 h in 1 ml of LB containing 50 μg/ml of kanamycin or LB containing 20 μg/ml of chloramphenicol. Only those colonies growing on chloramphenicol and not on kanamycin were kept and grown overnight in 5 ml of LB containing 20 μg/ml of chloramphenicol. BAC-BoHV-4-A was purified and analyzed through HindIII restriction enzyme digestion for pTK-CMV-IgK-gE2gD-TM-TK fragment targeted integration. Original detailed protocols for recombineering can also be found at the recombineering website (http://recombineering.ncifcrf.gov).
Recombinant virus characterization.
pBAC-BoHV-4-A and BoHV-4-A-CMV-IgK-gE2gD-TM recombinants were characterized as previously described (8). Fifteen microliters of DNA prepared from bacteria containing pBAC-BoHV-4-A and derivatives was restriction enzyme digested, separated by electrophoresis overnight in a 1% agarose gel, stained with ethidium bromide, capillary transferred to a positively charged nylon membrane (Roche), and cross-linked by UV irradiation by standard procedures. The membrane was prehybridized in 50 ml of hybridization solution (7% sodium dodecyl sulfate, 0.5 M phosphate, pH 7.2, 1 mM EDTA) for 2 h at 65°C in a rotating hybridization oven (Techna Instruments). Probe preparation and digoxigenin nonisotopic labeling were performed by PCR. Five microliters of the probe was added to 100 μl of double-distilled water in a screw-cap tube, denatured in boiling water for 5 min, and cooled down on ice for another 2 min. Denatured probe was added to the hybridization solution, and the membrane was hybridized overnight at 65°C in a rotating hybridization oven (Techna Instruments). Following hybridization, the membrane was washed and blocked in 25 ml of blocking solution. Antidigoxigenin Fab fragment (150 U/200 μl [Roche]), diluted 1:15,000 in 25 ml of blocking solution, was applied to the membrane, and detection was performed following equilibration of the membrane in detection buffer for 2 min at room temperature. Chemiluminescent substrate (Roche) was added by scattering the drops over the surface of the membrane. After placement of the membrane between two plastic sheets, any bubbles present under the sheet were eliminated with a damp lab tissue to create a liquid seal around the membrane. Signal detection was obtained by exposing the membrane to X-ray film. The exposure time was adjusted with the intensity of the signal.
Cell culture electroporation and recombinant virus reconstitution.
HEK 293T cells were cultured and transfected as previously described (5). Thirty micrograms of pCMV-IgKE2gD-TM was electroporated in HEK 293T cells from a confluent 75-cm2 flask, and a plasmid expressing green fluorescent protein (GFP) under the control of the cytomegalovirus (CMV) promoter (pEGFP-C1) was identically electroporated (Equibio apparatus; 186 V, 960 μF, 4-mm-gap cuvettes) as a negative control and to monitor the efficiency of transfection through the number of green cells. Following electroporation, cells were recovered in a new 75-cm2 flask with 20 ml of DMEM and 10% FBS to allow cells to attach. Forty-eight hours postelectroporation, cells were extracted for Western immunoblotting. MDBK and BEK cells were maintained as a monolayer, subcultured to a fresh culture vessel, and electroporated when growth reached 70 to 90% confluence. BAC plasmid DNA (5 μg) in 500 μl DMEM without serum was electroporated (Equibio apparatus; 270 V, 960 μF, 4-mm-gap cuvettes) into BEK cells from a confluent 25-cm2 flask. Electroporated cells were returned to the flask, fed the next day, and split 1:2 when they reached confluence at 2 days postelectroporation. Cells were left to grow until CPE appeared. Recombinant viruses were propagated by infecting confluent monolayers of MDBK cells at an MOI of 0.5 TCID50 per cell and maintaining them in MEM with 10% FBS for 2 h. The medium was then removed and replaced with fresh MEM containing 10% FBS. When approximately 90% of the cell monolayer exhibited CPE (approximately 72 h postinfection), the virus was prepared by freezing and thawing cells three times and pelleting virions through 30% sucrose, as described previously (3). Virus pellets were resuspended in cold MEM without FBS. TCID50 were determined on MDBK cells by limiting dilution.
Western immunoblotting.
Cell extracts containing 50 μg of total protein were electrophoresed through 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to nylon membranes by electroblotting. Membranes were incubated with monoclonal anti-BVDV-gE2 (clone 157; VRMD, Inc., Pullman, WA) or anti-BoHV-1-gD (clone 1B8-F11; VRMD, Inc., Pullman, WA) antibody, probed with horseradish peroxidase-labeled anti-mouse immunoglobulin antibody (Sigma), and visualized by enhanced chemiluminescence (ECL kit; Pierce, Rockford, IL).
Flow cytometry.
Forty-eight hours posttransfection, HEK 293T cells with pCMV-IgKgE2/gD-TM were detached from the flask with 0.2 mM of EDTA in PBS. Five hundred thousand cells were stained with 2-μl volumes of each primary anti-BVDV-gE2 (clone 157; VRMD, Inc., Pullman, WA) or anti-BoHV-1-gD (clone 1B8-F11; VRMD, Inc., Pullman, WA) antibody for 20 min at 4°C in the dark and then washed twice with PBS and further incubated with the secondary goat anti-mouse immunoglobulin G (IgG; whole molecule, fluorescein isothiocyanate [FITC] conjugated; Sigma, Saint Louis, MO). After 20 min cells were washed and were resuspended in 500 μl PBS. Five microliters of 7-aminoglycoside adenylyltransferase solution were added to assess cell death. Cells were acquired with a FACSCalibur flow cytometer (Becton Dickinson) equipped with a 15-mW argon laser using a 488-nm band pass filter, calibrated with Calibrite 3 beads (Becton Dickinson) and FACSComp Software (Becton Dickinson). Analyses were performed using Cell Quest software.
Cell infection and recovery assay.
Cell cultures in six-well plates were infected with virus at an MOI of 1 TCID50/cell for 1 h. After infection, the inactivation of extracellular virus was carried out by low-pH treatment (20). Briefly, the medium was removed and the plates were washed once with PBS and then incubated for 2 min with either PBS (control plates) or a buffer (pH 3) containing 40 mM citric acid, 10 mM KCl, and 135 mM NaCl. This procedure completely inactivated the absorbed nonpenetrated infectious particles. Cultures were washed with medium and cultured until CPE completely destroyed the cell monolayer, after which 1 ml of the medium was removed from each well and centrifuged for 5 min at 3,000 rpm in a bench top centrifuge to remove any cellular debris. TCID50 were determined on MDBK cells by limiting dilution.
Viral growth curves.
MDBK cells were infected at an MOI of 0.5 PFU/cell and incubated at 37°C for 4 h. Infected cells were washed with serum-free Eagle's minimal essential medium and than overlaid with Eagle's minimal essential medium containing 10% FBS, 2 mM of l-glutamine, 100 IU/ml of penicillin (Sigma), 100 μg/ml of streptomycin (Sigma) and 2.5 μg/ml of amphotericin B. The supernatants of infected cultures were harvested every 24 h, and the amount of infectious virus was determined by plaque assays with BEK or MDBK cells by analyzing PFU.
Serological tests.
Serum neutralization tests were performed as follows. Twenty-five microliters of each serum sample was added to the first line of wells of 96-well plates. Twenty-five microliters of DMEM was added to each well, and for each serum tested, serial twofold dilutions were made. Positive and negative serum controls were included. Twenty-five microliters of virus suspension containing 100 TCID50 of BVDV, BoHV-1, or BoHV-4-A was added to each well. After 1 h of incubation at 37°C, 50 μl of an MDBK cell suspension was added to each well and the plates were incubated for 3 days at 37°C in a humidified atmosphere of 95% air-5% CO2. Expression of viral infectivity and serum-neutralizing activity through CPE were detected by microscopy and/or by crystal violet staining of the cell monolayer. The neutralization antibody titers were expressed as the reciprocal (log2) of the final dilution of serum that completely inhibited viral infectivity. An enzyme-linked immunosorbent assay (ELISA) was carried out with a commercial kit according to the instructions of the manufacturer (ELISA IBR-IPV [Institute Pourquier] or ELISA BVDV [Svanova Biotech AB, Sweden]); the only modification was the use, as a secondary antibody, of a goat anti-rabbit IgG (whole-molecule) peroxidase conjugate (Sigma), diluted 1:20,000.
An indirect fluorescent antibody test (IFAT) for BoHV-4 antibodies was performed as follows. BoHV-4-infected and noninfected BEK cells were seeded onto 18-well glass slides, fixed in cold acetone, and stored at −20°C until used. Sera were serially diluted in PBS, and 30 μl/well was added to the infected and uninfected cells. For each slide, negative- and positive-control sera were used, as well as control wells containing BoHV-4-infected or uninfected cells treated only with the secondary antibody. After 1 h of incubation at 37°C, the slides were washed three times in PBS and incubated for 1 h at 37°C with FITC-conjugated goat anti-rabbit IgG (EBD; Sigma). At the same time, cells were counterstained with Evans blue dye (EBD; Sigma). Fluorescence was detected using a Zeiss Axiovert epifluorescent microscope, and images were acquired with the Axiocam Zeiss system. Sera were considered positive for BoHV-4 antibody when green fluorescence was detected at a dilution of ≥1:40, with no fluorescence for noninfected cells at 1:20.
Animal handling and care.
Rabbits were cared for and used in accordance with Italian laws for animal experimentation. Rabbits were maintained at 24°C with a controlled light cycle (12 h of light, starting at 6:00 a.m.) and with food and water ad libitum. Blood samples were obtained and viral injections were performed via the auricular vein at scheduled intervals.
RESULTS
Construction and expression of a cell membrane-linked gE2/gD-TM chimeric peptide.
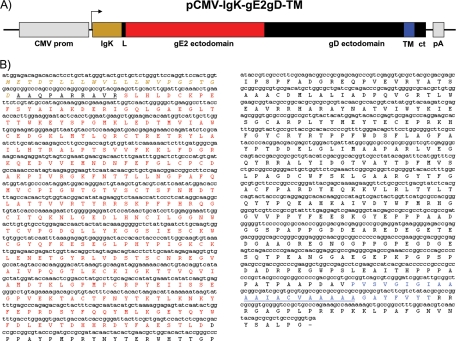
Starting from the idea that the cellular location of viral antigens could be crucial in terms of antigen exposure and processing for the immune system, an expression cassette expressing a chimeric peptide was engineered to target the cellular surface membrane. Using a previously optimized construct, pCMV-IgK-gE2gD-14 (8), used for protein secretion, a new version, pCMV-IgK-gE2gD-TM, with the following characteristics was constructed: (ì) the CMV promoter, chosen because an enhancement of the CMV promoter activity was observed in cells infected with BoHV-4; (ìì) an IgK light chain leader sequence addressing the rough endoplasmic reticulum of heterologous proteins; and (ììì) the BVDV gE2 open reading frame (ORF) ectodomain fused with the BoHV-1 full-length gD deprived of the signal peptide but containing the transmembrane domain and the intracellular domain, to specify cell membrane linking (Fig. 1A and B).
FIG. 1.
(A) pCMV-IgK-gE2gD-TM vector diagram (not to scale) containing the cytomegalovirus enhancer promoter (CMV prom), the IgK signal peptide (IgK) in frame with the gE2 ectodomain (gE2), the full-length gD (gD) provided with its transmembrane domain (TM) together with the cytoplasmic tail (ct), and the growth hormone polyadenylation signal (pA). (B) Chimeric peptide sequence (IgK-gE2/gD-TM) and predicted amino acid product. Italic ocher letters indicate the signal peptide, and the underlined black letters indicate the linker sequence connecting the signal peptide and the gE2 ectodomain (in red). gE2 is followed by the gD ectodomain in black and its transmembrane domain in blue, containing the highly hydrophobic peptide (underlined) and the cytoplasmic tail (in black).
gE2/gD-TM is expressed on the cell surface of transfected cells.
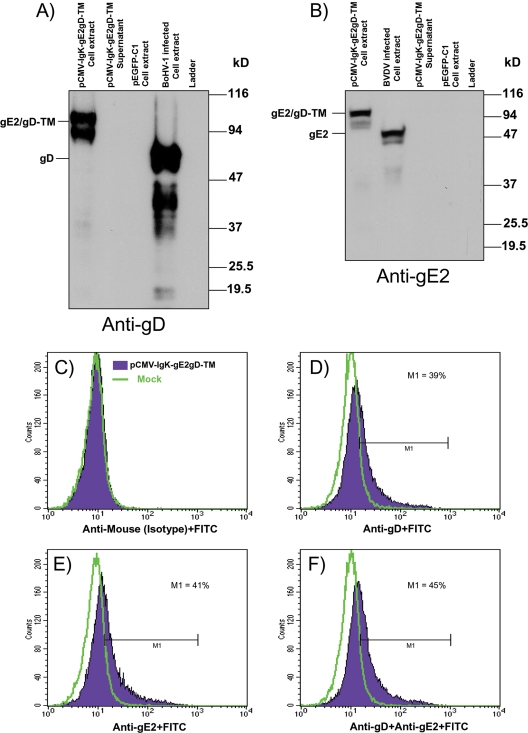
pCMV-IgK-gE2gD-TM was transiently transfected in HEK 293T cells, and cell membrane-associated protein expression was detected by both anti-gD and gE2 monoclonal antibodies by Western immunoblotting (Fig. 2A and B), as well as in cells infected with BoHV-1 or BVDV used as a positive control but not in cells transfected with pEGFP-C1. In contrast no secreted antigens were detected when cell-transfected supernatants were analyzed for gE2/gD-TM secretion. Although gE2/gD-TM was detectably associated with the membrane fraction of pCMV-IgKgE2/gD-TM-transfected cells, to guarantee its presence on the cell surface, nonpermeabilized pCMV-IgKgE2/gD-TM-transfected cells were analyzed by flow cytometry and the gE2/gD-TM chimeric peptide was displayed on the cell surface of transfected cells when assayed with both anti-gE2 and anti-gD monoclonal antibodies (Fig. 2C to F).
FIG. 2.
(A and B) Western immunoblotting of cell extract and supernatant from HEK 293T cells transfected with pCMV-IgK-gE2gD-TM or with pEGFP-C1 (plasmid expressing EGFP) and probed with both anti-gD (A) and anti-gE2 (B) monoclonal antibodies. (C to F) Fluorescence-activated cell sorting analysis of gE2/gD-TM chimeric peptide expression on the cell surface of pCMV-IgK-gE2gD-TM-transfected cells. The green line corresponds to mock-transfected cells, and the violet line corresponds to pCMV-IgK-gE2gD-TM-transfected cells. In panel C, pCMV-IgK-gE2gD-TM-transfected and mock-transfected cells were both assayed with the anti-isotype antibody (FITC), and no nonspecific signals for the two cell populations were observed, as shown by the complete overlapping of the curves. When mock- and pCMV-IgK-gE2gD-TM-transfected cells were assayed with anti-gD (D) or anti-gE2 (E) plus the secondary antibody, a significant shift was observed for the pCMV-IgK-gE2gD-TM-transfected cells, and such a shift was further increased when anti-gD and anti-gE2 were assayed together (F). The experiment was repeated three times, giving identical results.
Generation of a recombinant BoHV-4 expressing gE2/gD-TM chimeric peptide.
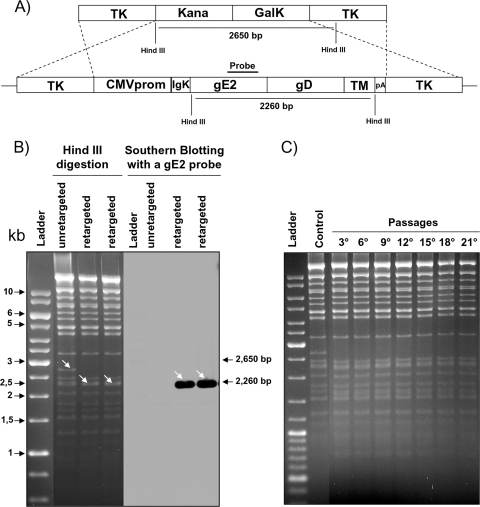
For making the CMV-IgK-gE2gD-TM expression cassette a vector and generating recombinant virus expressing the gE2/gD-TM chimeric peptide, an apathogenic strain of BoHV-4 cloned as a BAC as previously described (8) was utilized. The BAC recombineering system approach modified by the introduction of a kanamycin selection step (9) was used for this purpose, where the BoHV-4 TK gene was chosen as a target site for the insertion of the CMV-IgK-gE2gD-TM expression cassette. The BoHV-4 TK gene has been disrupted in BoHV-4 by the insertion of foreign sequences without interfering with viral replication in vitro (9). Furthermore, the BoHV-4 TK genomic region is highly conserved among BoHV-4 isolates (19), ensuring the stability of the genomic locus for the insertion of foreign expression cassettes. The generated targeting vector (TK-CMV-IgK-gE2gD-TM-TK) obtained by subcloning the CMV-IgK-gE2gD-TM cassette (Fig. 3A) into pINT2 (4) was excised from the plasmid backbone and used for heat-inducible homologous recombination in Escherichia coli SW102 containing the BAC-BoHV-4-A TK-KanaGalK-TK genome targeted into the TK locus with the KanaGalK selector cassette (8). Hence, retargeting was performed to the same site to replace the KanaGalK cassette with the CMV-IgK-gE2gD-TM cassette, with a negative selection on deoxygalactose minimal plates and a negative selection with medium containing kanamycin and promoting clone survival in medium containing chloramphenicol but clone death in medium containing kanamycin. That procedure allowed the isolation of positive clones for the right targeting, pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM (Fig. 3B). Because pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM was produced and propagated in E. coli SW102 carrying heat-inducible recombinases and repeated passages could lead to a bacterial phenotype with a leaking transcription of the recombinases even at temperatures that could not induce growth, SW102 carrying pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM was serially cultured over 20 passages, pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM was isolated and analyzed by HindIII restriction enzyme digestion, and no differences among restriction profiles at various periods of culture were observed (Fig. 3C).
FIG. 3.
(A) Diagram showing (not to scale) the retargeting by heat-inducible homologous recombination in SW102 containing pBAC-BoHV-4-A-TK-KanaGalK-TK, where the Kana/GalK cassette was removed and replaced with the CMV-IgK-gE2gD-TM expression cassette. (B) Representative colonies tested by HindIII restriction enzyme analysis, agar gel electrophoresis, and Southern blotting showing the right retargeting, where the 2,650-bp band (nonretargeted; indicated by an arrow) disappeared and a 2,260-kb band (retargeted; indicated by arrows), detected by Southern blotting with a gE2 probe, appeared. (C) Stability of the pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM plasmid in E. coli SW102 cells. SW102 cells containing the pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM were passaged for 21 consecutive days, and BAC DNA from the culture was prepared on the indicated days, analyzed by HindIII digestion and agarose gel electrophoresis, and compared to the parental unretargeted pBAC-BoHV-4-A-TK-KanaGalK-TK (control) growth in DH10B cells lacking recombinase.
Reconstitution of viable BoHV-4-A-CMV-IgK-gE2gD-TM in eukaryotic cells.
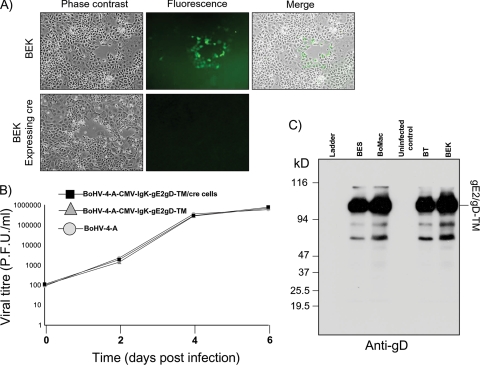
To reconstitute the infectious virus, BoHV-4-A-CMV-IgK-gE2gD-TM, pBAC-BoHV-4-A-CMV-IgK-gE2gD-TM was electroporated into BEK or BEKcre cells to excise the BAC cassette. Viable virus was obtained in both cases (Fig. 4A), and the production of the virus without the BAC cassette was monitored by the loss of the green fluorescence into the viral plaques obtained due to the loss of GFP contained in the floxed BAC backbone (8). When viruses with BAC excised or not excised were assessed for growth characteristics, no differences between them were observed (Fig. 4B), confirming that the replication competence of BoHV-4 cloned as a BAC can be maintained after the insertion of large fragments of foreign DNA. When cell lines were infected with BoHV-4-A-CMV-IgK-gE2gD-TM, they robustly expressed membrane-associated gE2/gD-TM (Fig. 4C).
FIG. 4.
(A) Representative fluorescent microscopic images of plaques formed by viable reconstituted recombinant BoHV-4-A-CMV-IgK-gE2gD-TM after DNA electroporation into BEK cells or in BEK cells expressing cre recombinase. Magnification, ×10. (B) Replication kinetics of BoHV-4-A-CMV-IgK-gE2gD-TM growth on cre-expressing cells, compared with those of the parental BoHV-4-A-CMV-IgK-gE2gD-TM still containing the BAC cassette and the BoHV-4-A isolate. The data presented are the means ± standard errors of triplicate measurements (P > 0.05 for all time points as measured by Student's t test). (C) Western immunoblotting of BoHV-4-A-CMV-IgK-gE2gD-TM-infected cell extracts. Uninfected BEK cell extract was used as a negative control.
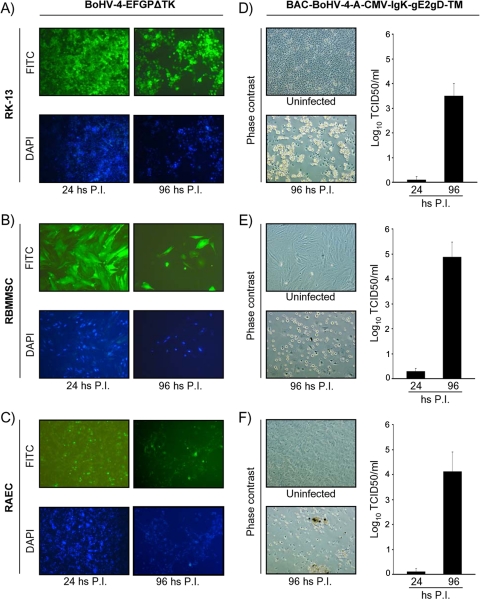
BoHV-4 replication in rabbit cells.
To test the replication competence of BoHV-4 in rabbits, a rabbit cell line (RK-13) and two rabbit primary cultures (RBMSC and RAEC) were infected either with BoHV-4-EFGPΔTK or BoHV-4-A-CMV-IgK-gE2gD-TM. When cells were infected with BoHV-4-EFGPΔTK, a recombinant BoHV-4 expressing GFP (4), infection was monitored by enhanced GFP (EGFP) expression and CPE spread through the cell monolayer (Fig. 5A to C), whereas when cells were infected with BoHV-4-A-CMV-IgK-gE2gD-TM, after infection the inactivation of extracellular virus was carried out by low-pH treatment and viral replication was monitored by CPE spread through the cell monolayer and by determining the titers of the newly produced progeny virus (Fig. 5D to F). Indeed, BoHV-4 infected and replicated in rabbit cells.
FIG. 5.
(A to C) Representative pictures of BoHV-4-EFGPΔTK-infected RK-13 cells (A), RBMSC (B), and RAEC (C) at 24 and 96 h postinfection, visualized by fluorescence microscopy with a FITC filter for EGFP expression or with a DAPI (4′,6-diamidino-2-phenylindole) filter for nuclear counterstaining. (D to F) Representative phase-contrast pictures of BoHV-4-A-CMV-IgK-gE2gD-TM-infected RK-13 cells (D), RBMSC (E), and RAEC (F) at 24 and 96 h postinfection with respective titers (expressed as log10 TCID50 per ml) of viral particles released at 24 and 96 h postinfection. Values are the means ± standard errors of three independent experiments. Magnification, ×10 (all panels).
Animals inoculated with BoHV-4-CMV-IgK-gE2gD-TM produced serum-neutralizing antibodies against both BVDV and BoHV-1.
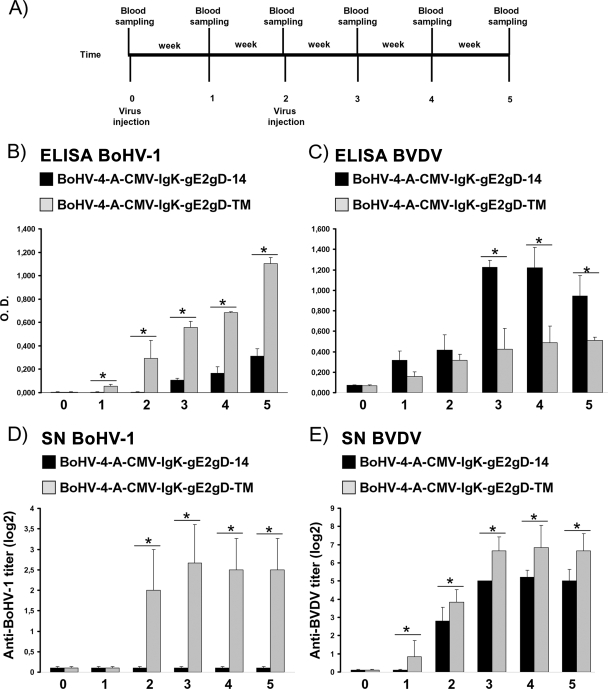
In accordance with Italian laws on animal experimentation, which suggest minimizing the number of animals used, a group of five BVDV- and BoHV-1-serum-negative rabbits were used for intravenous inoculation of BoHV-4-CMV-IgK-gE2gD-TM. After collection of preimmune serum, rabbits were intravenously inoculated with 1 ml of 105 TCID50/ml of BoHV-4-CMV-IgK-gE2gD-TM. An identical inoculation was performed 2 weeks later. Blood samples were collected weekly (Fig. 6A) from all animals for the assessment of anti-BoHV-1 and anti-BVDV antibodies and compared with sera coming from five rabbits of the identical strain, sex, age, and weight, identically inoculated with a secreted version of the recombinant virus (BoHV-4-CMV-IgK-gE2gD-14) (8), and stored at −20°C. Furthermore, body temperature and clinical sign development were monitored daily. None of the animals developed fever or other clinical signs during the time of observation (5 weeks) as previously observed for BoHV-4-CMV-IgK-gE2gD-14-inoculated animals (8). All animals developed an antibody response against BoHV-1, detectable in the second week after the first viral inoculation. Because the antibody titer was observable 2 weeks following the first viral inoculation and the blood samples were collected before the second viral inoculation, it was possible to note that a single inoculation of BoHV-4-CMV-IgK-gE2gD-TM was able to elicit a humoral immune response against BoHV-1 and BVDV, as demonstrated by ELISA (Fig. 6B and C). However, such antibody production was significantly higher in animals inoculated with BoHV-4-CMV-IgK-gE2gD-TM than in those inoculated with BoHV-4-CMV-IgK-gE2gD-14 (P < 0.005) for BoHV-1 but lower in animals inoculated with BoHV-4-CMV-IgK-gE2gD-TM than in those inoculated with BoHV-4-CMV-IgK-gE2gD-14 for BVDV (P < 0.005).
FIG. 6.
Kinetics of the humoral immune responses of rabbits immunized with BoHV-4-A-CMV-IgK-gE2gD-TM (transmembrane) and compared with those of rabbits immunized with BoHV-4-A-CMV-IgK-gE2gD-14 (secreted). (A) Diagram showing the rabbit immunization scheme and blood sample collection. (B and C) Sera collected from rabbits before immunization and after immunization were evaluated for anti-gD (B) and anti-gE2 (C) antibodies by ELISA. Antibodies detected were expressed as the optical density at 504 nm; each value represents the mean response of five rabbits ± the standard error of the mean (*, P < 0.005 as measured by Student's t test or one-way analysis of variance). (D and E) Anti-BoHV-1 (D) and anti-BVDV (E) serum-neutralizing antibodies (SN) as measured by serum neutralization test. SN antibodies were expressed as the reciprocal of the highest dilution of the serum that inhibited the development of virus-induced CPE in MDBK cells. Virus neutralization titers of >2 (log2) were considered positive. Each value represents the mean response of five rabbits ± the standard error of the mean (*, P < 0.005 as measured by Student's t test or one-way analysis of variance).
Because serum-neutralizing antibodies are predictive in terms of vaccine efficacy (1, 11, 12, 14, 26), whether the antibodies produced had neutralizing activity against both BoHV-1 and BVDV was investigated. Serum neutralization tests were performed with the same sera, and serum-neutralizing antibodies against both BoHV-1 and BVDV appeared in sera from animals inoculated with BoHV-4-CMV-IgK-gE2gD-TM expressing the membrane-linked form of the chimeric peptide. In contrast, for animals inoculated with BoHV-4-CMV-IgK-gE2gD-14, expressing the secreted form of the chimeric peptide, serum-neutralizing antibodies appeared for only BVDV, as previously observed (8), and with a titer lower than that for BoHV-4-CMV-IgK-gE2gD-TM-inoculated animals (Fig. 6D and E). These data point out the requirement of a specific engineered antigen cellular location for an effective immune response.
Recombinant BAC-BoHV-4-A-CMV-IgK-gE2gD-TM incorporates gE2/gD-TM into its envelope membrane and does not acquire serum neutralizable properties.
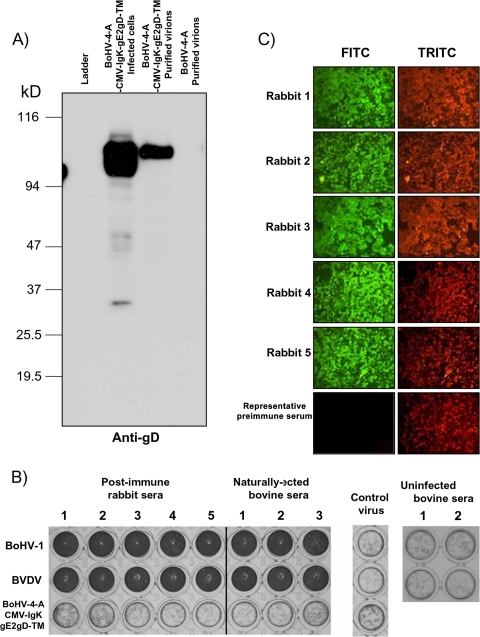
The capability of BoHV-1 gD to be incorporated in the BoHV-4 envelope was previously demonstrated (9). Now, because BoHV-1 gD is a typical type 1 integral membrane glycoprotein and the full-length gD deprived of the signal peptide but containing the transmembrane domain and the intracellular tail is positioned at the carboxyl terminus of the chimeric peptide gE2gD-TM, its capability to drive the incorporation of gE2/gD-TM on the BoHV-4 envelope was investigated. A virus preparation from BoHV-4-A-CMV-IgK-gE2gD-TM was purified by CsCl2 gradient centrifugation and analyzed by Western immunoblotting with monoclonal antibody against gD (Fig. 7A) or gE2 (data not shown). As expected, a strong signal was detected in BoHV-4-A-CMV-IgK-gE2gD-TM virions but not in wild-type BoHV-4 virions. Thus, these results indicate the incorporation of gE2/gD-TM into BoHV-4 virus particles.
FIG. 7.
(A) Incorporation of gE2/gD into recombinant BoHV-4 particles. Extracts of virus-infected cells and virus purified through CsCl2 gradient centrifugation were analyzed by Western immunoblotting with an anti-gD monoclonal antibody. Positive and negative controls were performed with BoHV-4-A-CMV-IgK-gE2gD-TM-infected cell extract and BoHV-4-A purified virus, respectively. (B) Rabbit (1 to 5) or vaccinated bovine (1 to 3) sera containing neutralizing antibodies against both BVDV and BoHV-1 were tested against BVDV, BoHV-1, or BoHV-4-A-CMV-IgK-gE2gD-TM at a single dilution of 1:2 to increase the chances of detecting serum-neutralizing antibody against BoHV-4-A-CMV-IgK-gE2gD-TM. Control virus was established in the absence of sera or with sera from cows not infected with BVDV or BoHV-1. Crystal violet staining allows macroscopic evaluation of the integrity (dark wells) or the destruction (clear transparent wells) of the cell monolayer. The test was repeated three times, and the same results were obtained. (C) Representative microscopic IFAT images of sera from BoHV-4-A-CMV-IgK-gE2gD-TM-immunized rabbits. The presence of anti-BoHV-4 antibodies in the rabbit serum samples (5 weeks postimmunization) is detectable by green cells when observed with an FITC filter. Negative controls were established with preimmune sera as well as control wells containing BoHV-4-infected or uninfected cells treated only with the secondary antibody. Counterstaining with Evans blue dye observed with a tetramethyl rhodamine isothiocyanate (TRITC; red pictures) filter was used to monitor the integrity of the cell substrate. Magnification, ×10.
Because BoHV-4-CMV-IgKgE2gD-TM elicits the production of neutralizing antibodies against BoHV-1 and BVDV and gE2/gD is incorporated into the recombinant virions, we performed serum neutralization tests with sera from BoHV-1- and BVDV-positive cows and sera from BoHV-4-CMV-IgKgE2gD-TM-immunized rabbits to determine whether BoHV-4-CMV-IgKgE2gD-TM acquired serum neutralizable properties. None of the sera were able to neutralize BoHV-4-CMV-IgKgE2gD-TM (Fig. 7B). However, antibodies against BoHV-4, despite the fact that they were not serum neutralizing, were produced in all immunized rabbits, as shown by IFAT (Fig. 7C). Therefore, the immune response induced by BoHV-4-CMV-IgKgE2gD-TM did not have neutralizing activity toward the vector, a strong drawback for many viral vectors (28).
DISCUSSION
The E2 protein of the BVDV NADL strain consists of about 370 amino acids and has a calculated molecular mass of 41 kDa. The N terminus of BVDV gE2 is formed by Arg-690, and the C terminus is located around amino acid 1063. The C terminus of E2 includes approximately 30 amino acids, which could function as a transmembrane anchor for E2, and has a translocation signal for the downstream protein. Full-length E2 remains cell associated in virus-infected cells and is glycosylated, and the mature form has a molecular mass of ∼53 kDa (21). The gD protein of the BoHV-1 New York strain consist of 417 amino acid residues and is a typical type 1 integral membrane protein containing a signal peptide of 18 amino acids at the amino terminus and a hydrophobic transmembrane domain between residues 361 and 389, which is followed by a 28-amino-acid cytoplasmic tail (24). The mature form of gD has a molecular mass of ∼71 kDa and contains both N-linked and O-linked oligosaccharides (27). The N-linked glycan addition sites have been located at residues 41 and 102 (25). BVDV gE2 and BoHV-1 gD are both involved in the virus entry process, which is conventionally divided into two steps, initial attachment via interaction of viral attachment proteins with receptors on the cell surface and subsequent penetration involving membrane fusion. Because of this important function, antibodies raised against these two proteins can lead to a serum-neutralizing effect on the virus, and the presence of serum-neutralizing antibodies in the serum of vaccinated animals is considered a predictive indication for vaccine efficacy. BVDV gE2 and BoHV-1 gD have been extensively used for vaccine development (18, 22), and their ORFs have been genetically manipulated and expressed by prokaryotic and eukaryotic systems with variable results.
The aim of coexpressing gE2 and gD ORFs, paving the way for polyvalent specific vaccination, was hampered by the inability of vector technology to guarantee reliable coexpression. Nevertheless, internal ribosome entry sites (IRESs) were the first strategy employed, in which reinitiation ribosomes first translate an upstream cistron, although highly inefficiently, and then resume translation of the second, downstream cistron. However, two main problems blocked the successful use of gE2-IRES-gD complex polycistronic vectors: the large size and imbalance of most IRESs, which made it very difficult to predict the level of expression of the downstream cistron. A second strategy taken into account was the possibility of introducing the target site for a cellular proteinase between two cistrons cloned in frame forming a single ORF. In this way the polyprotein is synthesized as a fusion protein that posttranslationally is proteolytically cleaved to yield the discrete proteins of interest. Unfortunately, this strategy has several practical difficulties: (i) the polyprotein to be cleaved must reside, or at least pass through, the same compartment as the proteinase; (ii) the cleavage is not always independent of the context; (iii) the cleavage may be incomplete and unpredictable; (iv) efficient cleavage will be produced only in cells actively expressing the proteinase; and (v) the posttranslational cleavage is not compatible with all possible subcellular targeting (13).
Although antigen delivery strategies in mammalian cells have employed viruses or simple plasmids, as is the case for DNA vaccination, as vectors, the coexpression strategies previously described have not taken advantage of the dominant ways in which viruses achieve coexpression in cells. It is the polyprotein strategy that many viruses use to coexpress most of their proteins, or even all of them (as in picornaviruses or pestiviruses). Not surprisingly, this strategy is indeed used by cells, although not very often, in particular for the coordinated secretion of different proteins and peptides. Starting from this basic information, gE2 and gD ORFs were cloned in frame, forming a single ORF (gE2/gD), and successfully expressed as a secreted form previously (8) or as a membrane-linked form in the present work. To shuttle the optimized expression cassette into the cells in vitro and in vivo, a strain of BoHV-4 isolated from the milk cellular fraction of a BoHV-4 serologically positive clinically healthy lactating dairy cow and cloned as a BAC was employed. Although BoHV-4 is considered a virus without a clear disease association and only a few investigators have successfully produced an experimental disease (10), the idea of BoHV-4 involvement in bovine postpartum metritis, despite only as a secondary agent along with other agents like bacteria, is going to become consistent (6, 7). The existence of a BoHV-4 biotype potentially able to be correlated with a particular lesion cannot be absolutely excluded when a virus is going to be exploited as a gene delivery vector. A recombinant BoHV-4, BoHV-4-A-CMV-IgK-gE2gD-TM, delivering a CMV-IgK-gE2/gD-TM expression cassette into the TK locus, was successfully obtained by recombination-mediated genetic engineering. The reconstituted virus in eukaryotic cells abundantly expressed gE2/gD-TM chimeric peptide as a cell membrane-associated protein.
Rabbits were immunized with BoHV-4-A-CMV-IgK-gE2gD-TM, and sera were compared with sera obtained from BoHV-4-A-CMV-IgK-gE2gD-14-immunized rabbits. In general a better level of immunization was observed with the membrane-associated form than with the secreted one. A complete rescue of the serum-neutralizing antibody fraction against BoHV-1 was obtained for the membrane-associated form. The only discrepancy observed was for the ELISA antibody titer against BVDV, where a higher response was obtained in BoHV-4-A-CMV-IgK-gE2gD-14-immunized rabbits than in BoHV-4-A-CMV-IgK-gE2gD-TM-immunized ones. The reason for such a discrepancy in terms of immune response definitely resides in the presence of the BoHV-1 gD transmembrane domain, raising several hypotheses. First, the absence of a gD transmembrane domain, which was deleted to make gE2/gD in a secreted soluble form, could be important in terms of specific epitopes determinant for the formation of serum-neutralizing antibodies. However, BoHV-1 gD expressed as a secreted form in a DNA vaccine vector was able to confer serum-neutralizing properties (17). Second, relevant epitopes for a serum-neutralizing response, which could be encrypted by the protein folding variation due to the fusion of the two glycoproteins when expressed as a secreted form, could be restored when the hydrophobic transmembrane domain is reintroduced. Third, the presence of the transmembrane domain in the carboxy terminus of the gE2/gD-TM targeting the protein to the cellular surface could allow a better recognition and processing of the antigen from the immune system. Antigen presentation by antigen-presenting cells as macrophages, where BoHV-4 tends to infect and to establish persistent infection (21), is a crucial component of the process which needs to be taken into account when antigens are delivered by a BoHV-4-based vector or other viral vectors with the same characteristics, beside the expression of the antigen individually, in combination with other antigens, or as a chimeric protein as described in the present work.
Acknowledgments
We thank the Italian Ministry of University and Scientific Research (Italian National Grant MIUR, PRIN 2005, 2005078885) and the Fondazione Cariparma (Cassa di Risparmio di Parma, Italy) for funding contributions to the project.
This paper is dedicated to Cesidio Filippo Flammini, who will retire this November after having worked for 42 years in the Veterinary School of Parma University (Italy).
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Babiuk, L. A., S. van Drunen Littel-van den Hurk, and S. K. Tikoo. 1996. Immunology of bovine herpesvirus 1 infection. Vet. Microbiol. 53:31-42. [DOI] [PubMed] [Google Scholar]
- 2.Collings, D. F., E. P. Gibbs, and L. P. Stafford. 1972. Concurrent respiratory and genital disease associated with infectious bovine rhinotracheitis-infectious pustular vulvo-vaginitis (IBR-IPV) virus in a dairy herd in the United Kingdom. Vet. Rec. 91:214-219. [DOI] [PubMed] [Google Scholar]
- 3.Donofrio, G., A. Cavaggioni, M. Bondi, S. Cavirani, C. F. Flammini, and C. Mucignat-Caretta. 2006. Outcome of bovine herpesvirus 4 infection following direct viral injection in the lateral ventricle of the mouse brain. Microbes Infect. 8:898-904. [DOI] [PubMed] [Google Scholar]
- 4.Donofrio, G., S. Cavirani, T. Simone, and V. L. van Santen. 2002. Potential of bovine herpesvirus 4 as a gene delivery vector. J. Virol. Methods 101:49-61. [DOI] [PubMed] [Google Scholar]
- 5.Donofrio, G., S. Cavirani, A. Vanderplasschen, L. Gillet, and C. F. Flammini. 2006. Recombinant bovine herpesvirus 4 (BoHV-4) expressing glycoprotein D of BoHV-1 is immunogenic and elicits serum-neutralizing antibodies against BoHV-1 in a rabbit model. Clin. Vaccine Immunol. 13:1246-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donofrio, G., S. Herath, C. Sartori, S. Cavirani, C. F. Flammini, and I. M. Sheldon. 2007. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction 134:183-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donofrio, G., L. Ravanetti, S. Cavirani, S. Herath, A. Capocefalo, and I. M. Sheldon. 2008. Bacterial infection of endometrial stromal cells influences bovine herpesvirus 4 immediate early gene activation: a new insight into bacterial and viral interaction for uterine disease. Reproduction 136:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donofrio, G., C. Sartori, V. Franceschi, A. Capocefalo, S. Cavirani, S. Taddei, and C. F. Flammini. 2008. Double immunization strategy with a BoHV-4-vectorialized secreted chimeric peptide BVDV-E2/BoHV-1-gD. Vaccine 26:6031-6042. [DOI] [PubMed] [Google Scholar]
- 9.Donofrio, G., C. Sartori, L. Ravanetti, S. Cavirani, L. Gillet, A. Vanderplasschen, S. Taddei, and C. F. Flammini. 2007. Establishment of a bovine herpesvirus 4 based vector expressing a secreted form of the bovine viral diarrhoea virus structural glycoprotein E2 for immunization purposes. BMC Biotechnol. 7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egyed, L. 2000. Bovine herpesvirus type 4: a special herpesvirus. Acta Vet. Hung. 48:501-513. [DOI] [PubMed] [Google Scholar]
- 11.Fulton, R. W., and L. J. Burge. 2000. Bovine viral diarrhea virus types 1 and 2 antibody response in calves receiving modified live virus or inactivated vaccines. Vaccine 19:264-274. [DOI] [PubMed] [Google Scholar]
- 12.Fulton, R. W., A. W. Confer, L. J. Burge, L. J. Perino, J. M. d'Offay, M. E. Payton, and R. E. Mock. 1995. Antibody responses by cattle after vaccination with commercial viral vaccines containing bovine herpesvirus-1, bovine viral diarrhea virus, parainfluenza-3 virus, and bovine respiratory syncytial virus immunogens and subsequent revaccination at day 140. Vaccine 13:725-733. [DOI] [PubMed] [Google Scholar]
- 13.Fussenegger, M. 2001. The impact of mammalian gene regulation concepts on functional genomic research, metabolic engineering, and advanced gene therapies. Biotechnol. Prog. 17:1-51. [DOI] [PubMed] [Google Scholar]
- 14.Grossberg, S. E., and Y. Kawade. 1997. The expression of potency of neutralizing antibodies for interferons and other cytokines. Biotherapy 10:93-98. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, Y., B. N. Jahagirdar, R. L. Reinhardt, R. E. Schwartz, C. D. Keene, X. R. Ortiz-Gonzalez, M. Reyes, T. Lenvik, T. Lund, M. Blackstad, J. Du, S. Aldrich, A. Lisberg, W. C. Low, D. A. Largaespada, and C. M. Verfaillie. 2002. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41-49. [DOI] [PubMed] [Google Scholar]
- 16.Keil, G. M., A. Heinze, and B. Mächtig. 1990. Bovine herpesvirus 4 vector for life-virus vaccines? Zentralbl. Bakteriol. 272:375. [Google Scholar]
- 17.Lewis, P. J., S. van Drunen Littel-van den Hurk, and L. A. Babiuk. 1999. Altering the cellular location of an antigen expressed by a DNA-based vaccine modulates the immune response. J. Virol. 73:10214-10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang, R., J. V. van den Hurk, C. Zheng, H. Yu, R. A. Pontarollo, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2005. Immunization with plasmid DNA encoding a truncated, secreted form of the bovine viral diarrhea virus E2 protein elicits strong humoral and cellular immune responses. Vaccine. 23:5252-5262. [DOI] [PubMed] [Google Scholar]
- 19.Lomonte, P., M. Bublot, P. P. Pastoret, and E. Thiry. 1992. Location and characterization of the bovine herpesvirus type 4 thymidine kinase gene; comparison with thymidine kinase genes of other herpesviruses. Arch. Virol. 127:327-337. [DOI] [PubMed] [Google Scholar]
- 20.Mettenleiter, T. C. 1989. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology 171:623-625. [DOI] [PubMed] [Google Scholar]
- 21.Osorio, F. A., D. L. Rock, and D. E. Reed. 1985. Studies on the pathogenesis of a bovine cytomegalo-like virus in an experimental host. J. Gen. Virol. 66:1941-1951. [DOI] [PubMed] [Google Scholar]
- 22.Reddy, P. S., N. Idamakanti, C. Pyne, A. N. Zakhartchouk, D. L. Godson, Z. Papp, M. E. Baca-Estrada, L. A. Babiuk, G. K. Mutwiri, and S. K. Tikoo. 2000. The immunogenicity and efficacy of replication-defective and replication-competent bovine adenovirus-3 expressing bovine herpesvirus-1 glycoprotein gD in cattle. Vet. Immunol. Immunopathol. 76:257-268. [DOI] [PubMed] [Google Scholar]
- 23.Rusko, J., G. Van Slooten, and D. J. Adams. 1995. Caffeine-evoked, calcium-sensitive membrane currents in rabbit aortic endothelial cells. Br. J. Pharmacol. 115:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tikoo, S. K., D. R. Fitzpatrick, L. A. Babiuk, and T. J. Zamb. 1990. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J. Virol. 64:5132-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikoo, S. K., M. D. Parker, J. V. van den Hurk, J. Kowalski, T. J. Zamb, and L. A. Babiuk. 1993. Role of N-linked glycans in antigenicity, processing, and cell surface expression of bovine herpesvirus 1 glycoprotein gIV. J. Virol. 67:726-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Drunen Littel-van den Hurk, S., S. K. Tikoo, J. V. van den Hurk, L. A. Babiuk, and J. Van Donkersgoed. 1997. Protective immunity in cattle following vaccination with conventional and marker bovine herpesvirus-1 (BHV1) vaccines. Vaccine 15:36-44. [DOI] [PubMed] [Google Scholar]
- 27.van Drunen Littel-van den Hurk, S., J. V. van den Hurk, J. E. Gilchrist, V. Misra, and L. A. Babiuk. 1984. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology 135:466-479. [DOI] [PubMed] [Google Scholar]
- 28.Zaiss, A. K., M. J. Cotter, L. R. White, S. A. Clark, N. C. Wong, V. M. Holers, J. S. Bartlett, and D. A. Muruve. 2008. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 82:2727-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]