Abstract
The oral cavity is colonized by microorganisms growing in biofilms in which interspecies interactions take place. Streptococcus mutans grows in biofilms on enamel surfaces and is considered one of the main etiological agents of human dental caries. Candida albicans is also commonly found in the human oral cavity, where it interacts with S. mutans. C. albicans is a polymorphic fungus, and the yeast-to-hypha transition is involved in virulence and biofilm formation. The aim of this study was to investigate interkingdom communication between C. albicans and S. mutans based on the production of secreted molecules. S. mutans UA159 inhibited C. albicans germ tube (GT) formation in cocultures even when physically separated from C. albicans. Only S. mutans spent medium collected in the early exponential phase (4-h-old cultures) inhibited the GT formation of C. albicans. During this phase, S. mutans UA159 produces a quorum-sensing molecule, competence-stimulating peptide (CSP). The role of CSP in inhibiting GT formation was confirmed by using synthetic CSP and a comC deletion strain of S. mutans UA159, which lacks the ability to produce CSP. Other S. mutans strains and other Streptococcus spp. also inhibited GT formation but to different extents, possibly reflecting differences in CSP amino acid sequences among Streptococcus spp. or differences in CSP accumulation in the media. In conclusion, CSP, an S. mutans quorum-sensing molecule secreted during the early stages of growth, inhibits the C. albicans morphological switch.
The oral cavity is colonized by many different microbial species, where most reside in biofilms. Because of its multispecies nature, the oral microbial community is one of the best biofilm models for studying interspecies interactions (17). The gram-positive bacterium Streptococcus mutans shows a high prevalence in dental biofilms, and it is considered to be the major etiological agent involved in human dental caries (21). The fungal species Candida albicans constitutes a minor part of the total microbial flora (19) and can be isolated as a commensal from the oral cavity of 50% to 60% of healthy adults (33). However, in immunocompromised individuals (for example, due to human immunodeficiency virus infection or as a result of chemotherapy) and elderly patients, this fungus often leads to candidiasis (24). C. albicans is a polymorphic fungus that can exist in three morphotypes: budding yeast, pseudohypha, and true hypha (5). The morphological switch from yeast to hyphal cells is important in many processes, such as virulence (22) and biofilm formation (10, 18), and is therefore the subject of many studies.
Bacteria and yeasts are often found together in vivo, and there is growing evidence that interspecies, and even interkingdom, interactions occur within these populations (7). These interactions can be mediated through signaling molecules (40), as recently described for the interaction between C. albicans and Pseudomonas aeruginosa, an opportunistic bacterial pathogen (15). N-3-oxo-C12 homoserine lactone (HSL), a signaling molecule involved in bacterial quorum sensing, completely represses C. albicans hypha formation without altering the growth rate. Although many gram-negative bacteria produce HSLs with shorter acyl chains (e.g., C4-HSL), the inhibition of C. albicans hypha formation is caused specifically by long-chained HSL molecules. In addition, related, non-HSL molecules with long acyl chains, such as dodecanol and farnesol, also inhibit the hypha formation of C. albicans (8).
A recent report described the coculturing of C. albicans and S. mutans in model oral biofilms on hydroxyapatite (26). It was shown that S. mutans increased the growth of C. albicans by stimulating coadhesion while simultaneously suppressing the formation of hyphae. S. mutans is a gram-positive bacterium and does not produce HSL-type molecules, and the nature of the interaction with C. albicans is presently unknown. In this study, the interaction between S. mutans and C. albicans was investigated by studying the effect of secreted molecules of S. mutans on C. albicans hypha formation.
MATERIALS AND METHODS
Strains and growth conditions.
All bacterial and fungal strains used in this study are shown in Table 1. C. albicans was plated onto yeast nitrogen base (YNB) (pH 7.0; BD Becton, Dickinson, and Company, Sparks, MD) supplemented with 0.5% glucose and 1.5% (wt/vol) agar from a 15% (vol/vol) glycerol stock maintained at −80°C and incubated 48 h at 30°C. Streptococcus spp. were cultured on blood agar, except for the S. mutans UA159 ΔcomC mutant, which was cultured on brain heart infusion (BHI; Oxoid, Basingstoke, United Kingdom) agar plates supplemented with erythromycin (10 μg/ml). YNB broth was used to grow C. albicans cells overnight at 30°C while shaking at 120 rpm. Cultures of Streptococcus spp. were grown statically in BHI broth overnight in 5% CO2 at 37°C and supplemented with erythromycin (10 μg/ml) when appropriate. To obtain spent-medium samples, Streptococcus spp. were grown in 200 ml BHI broth; 10 ml of medium was taken after 4, 6, 8, and 24 h and centrifuged at 10,000 × g for 5 min; the pH was checked and, if lower than 7, was set to 7; and cultures were filter sterilized and stored at −20°C. Cocultures of C. albicans and S. mutans were grown in medium containing 70% YNB and 30% BHI broth (vol/vol). Where indicated, spent medium replaced the 30% fresh-BHI fraction. Competence-stimulating peptide (CSP), with an amino acid sequence of NH2-SGSLSTFFRLFNRSFTQALGK-COOH (2) and with a purity of >95%, was purchased from Ansynth Service BV, Roosendaal, The Netherlands. A 12 mM stock of synthetic CSP was prepared by dissolving the peptide in demineralized water and stored at −20°C.
TABLE 1.
Strains used in this study
| Strain | Description | Reference or sourcea |
|---|---|---|
| C. albicans SC5314 | 12 | |
| C. albicans HB12 | 31 | |
| S. mutans ATCC 25175 | ATCC | |
| S. mitis ATCC 33399 | ATCC | |
| S. mutans NS | Clinical isolate | UMCG |
| S. salivarius HB | UMCG | |
| S. sanguis ATCC 10556 | ATCC | |
| S. sobrinus ATCC 33478 | ATCC | |
| S. oralis J22 | P. E. Kolenbrander, NIH, Bethesda, MD | |
| S. gordonii 10558 | Clinical isolate | ACTA |
| S. mutans UA159 | Wild type | 1 |
| S. mutans UA159 ΔcomC | Mutant lacking CSP production | This study |
ACTA, Academic Centre for Dentistry Amsterdam, Amsterdam, The Netherlands; UMCG, University Medical Center Groningen, Groningen, The Netherlands.
Construction of the S. mutans UA159 ΔcomC strain.
To delete the comC gene, we used a previously described PCR ligation mutagenesis method (20): the flanking regions of the comC gene were amplified from S. mutans UA159 genomic DNA with PCR primer pairs comCuf/comCur and comCdf/comCdr (see Table 2 for details). The erythromycin resistance gene (Ermr gene) was amplified from an ermAM cassette using primer pair ermF/ermR. These three PCR fragments were digested with the appropriate restriction enzymes and ligated. The ligation mix was used as a template for the amplification of the knockout construct using the primers comCuf/comCdr. The resulting PCR product was purified and transformed into S. mutans UA159 cells. The comC knockout strain was obtained via double crossover, resulting in the replacement of a major part of the chromosomal comC gene by the Ermr gene. The insertion of the Ermr gene was verified by PCR. The lack of CSP production by the comC knockout strain was confirmed by its incapability of bacteriocin production in competition assays (36).
TABLE 2.
Primer sequences used for S. mutans UA159 comC constructiona
| Primer (restriction site) | Sequence (5′→3′) |
|---|---|
| comCuf | GCTATCAGCTGCGCTGTT |
| comCur (NotI) | GCGGCCGCATTTTATATCTCCTTTTTTTGATTA |
| comCdf (AscI) | GGCGCGCCAAATAAGATAGGCTAACATT |
| comCdr | CATCAATTGCAGGATACC |
| ErmF (AscI) | GGCGCGCCTCGTGCTGACTTGCACCATATC |
| ErmR (NotI) | GCGGCCGCTTACAAAAGCGACTCATAGAAT |
Sequences of primers are indicated from the 5′-to-3′ direction, with the restriction sites, when applicable, indicated in boldface type.
Saliva preparation and coating.
Freeze-dried saliva was prepared as described previously (35). Briefly, stimulated saliva (by chewing parafilm) was collected from healthy volunteers, put on ice, and centrifuged twice at 10,000 × g for 5 min at 10°C. Phenylmethylsulfonyl fluoride was added to a final concentration of 1 mM to prevent protein degradation. Afterwards, the solution was again centrifuged at 10,000 × g for 5 min, dialyzed against demineralized water overnight at 4°C, and freeze-dried before storage at −20°C. For experiments, lyophilized saliva was dissolved in adhesion buffer (1 mM CaCl2, 2 mM potassium phosphate, 50 mM KCl) at a concentration of 1.5 mg/ml. This solution was centrifuged at 10,000 × g for 5 min at 10°C, and the supernatant was checked for the absence of bacteria by microscopy and subsequently used to coat 12-well tissue culture polystyrene plates (Costar; Corning, Inc.) overnight. Before the start of experiments, saliva was removed, and the wells were washed once with adhesion buffer. All volunteers gave their informed consent to saliva donation in accordance with the rules set out by the ethics committee at the University Medical Center Groningen.
GT assay.
As a measure of filamentation, a germ tube (GT) assay was performed using 12-well tissue culture polystyrene plates as described previously (15), with minor modifications. Saliva-coated culture plates filled with 1 ml of mixed medium were inoculated with C. albicans SC5314 cells to a final optical density at 600 nm of 0.1 (corresponding to 1 × 106 cells/ml). When appropriate, bacteria were added to 1 × 107 cells/ml. The plates were incubated for 3.5 h at 37°C while shaking at 80 rpm. The morphological state of C. albicans was analyzed by microscopy using a 20× objective lens on an inverted microscope (Olympus, Tokyo, Japan). For selected experiments, ThinCerts tissue culture polystyrene inserts (Greiner Bio-One) with a 0.4-μm pore size were used to physically separate yeasts from bacteria.
Fluorescent microscopy of GFP expression.
C. albicans HB12 cultures carrying green fluorescent protein (GFP) under the control of the HWP1 promoter (31) were grown with the addition of 1 μM CSP in a GT assay. HB12 expresses GFP only in true hyphae and not in pseudohyphae or yeast cells. Fluorescence microscopy was performed with a Leica DM4000B fluorescence microscope. Cells were observed with a 20× objective. For visualization of all cells, calcofluor white (Fluorescence Brightener 28; Sigma-Aldrich) staining was applied in a final concentration of 3.6 mM. Separate images were taken using filter sets for GFP and UV. Overlay images were created using Leica Application Suite, version 2.8.0.
Statistical analysis.
Differences in percentages of GT formation were tested using Student's t test with the threshold of a P value of <0.05 for statistical significance.
RESULTS
Effect of saliva and S. mutans UA159 on C. albicans SC5314 GT formation.
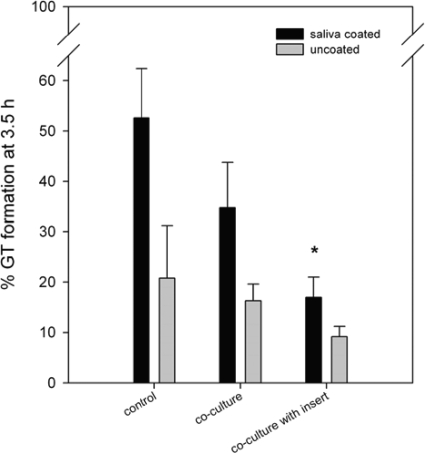
Upon the induction of GT formation at 37°C, C. albicans showed a higher fraction of germinating cells in saliva-coated wells (52%) than in uncoated wells (20%) (Fig. 1). Coculture with S. mutans UA159 resulted in only 35% of all cells being germinated. This inhibition of the fraction of germinating cells from 52% to 35% was not significant (P = 0.06) for saliva-coated and uncoated wells (20% to 16%; P = 0.25). When C. albicans and S. mutans UA159 cells were physically separated from each other by polystyrene inserts, GT formation was significantly inhibited in saliva-coated wells (from 52% to 17%; P = 0.007) but not in uncoated wells (from 20% to 9%; P = 0.06), indicating that physical contact is not involved in this inhibition of GT formation. The effect of S. mutans UA159 on C. albicans is best visualized after saliva coating, and hence, these conditions were applied for all following experiments.
FIG. 1.
Effect of different coculturing techniques on GT formation of C. albicans. C. albicans was grown without (control) or in coculture with S. mutans UA159 either without or with an insert physically separating both species. The morphology of C. albicans was determined after 3.5 h of growth. Wells were uncoated (gray bars) or coated with saliva (black bars). The results are averages of data from four independent experiments, with at least 300 cells counted per sample for each experiment. An asterisk indicates a significant (P < 0.05) difference compared to the control.
Effect of spent medium of S. mutans on C. albicans GT formation.
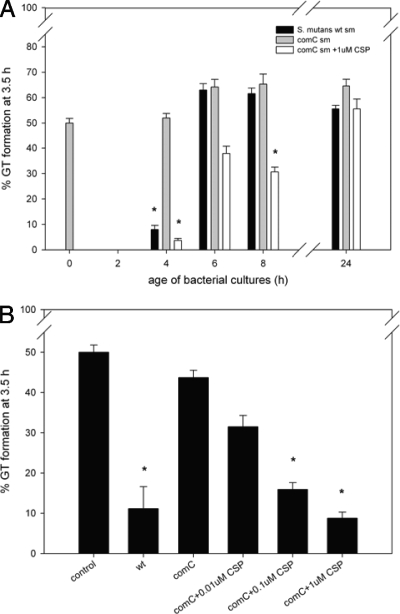
Spent medium obtained from S. mutans UA159 and S. mutans ΔcomC cultures at different phases during growth (4-, 6-, 8-, and 24-h-old cultures) was tested for its ability to affect the germination of C. albicans. Spent medium from 4-h-old culture of S. mutans UA159 inhibited C. albicans GT formation (from 50% to 8%), whereas spent medium of 6-, 8-, and 24-h-old cultures did not show any inhibition (Fig. 2A). CSP, a quorum-sensing molecule involved in the stimulation of competence (27), encoded by comC (14), is produced by S. mutans during the beginning of the exponential growth phase (25). Spent medium from the S. mutans ΔcomC strain did not show any inhibition, whereas the addition of 1 μM CSP to 4-, 6-, and 8-h-old cultures inhibited GT formation to 4%, 38%, and 31% respectively (Fig. 2A). It should be noted that the pH of the spent medium in the GT assay was always set to 7.
FIG. 2.
(A) Effect of spent medium from S. mutans UA159 and S. mutans UA159 ΔcomC strain cultures from different growth phases on GT formation by C. albicans. Spent medium (sm) of S. mutans UA159 (black bars) and the S. mutans UA159 ΔcomC strain (gray bars), harvested at early exponential (4 h old), mid-exponential (6 h old), early stationary (8 h old), and late stationary phases, show very different effects on GT formation. In addition, spent medium of the ΔcomC strain (white bars) from all time points was supplemented with 1 μM CSP. Time point zero depicts fresh medium (no bacterial growth), while 2-h-old medium was not tested. (B) Effect of 4-h-old spent medium from the S. mutans ΔcomC strain supplemented with various concentrations of synthetic CSP. A total of 1 μM CSP inhibited GT to a level similar to that of wild-type (wt) spent medium. Decreased synthetic CSP concentrations resulted in a dose-dependent behavior. The pH of all samples was set to 7 prior to the GT assay. The results are averages of data from two experiments, with at least 300 cells counted per experiment. An asterisk indicates a significant (P < 0.05) difference compared to the control.
To chemically complement the comC mutation, synthetic CSP was added to 4-h-old spent medium of the ΔcomC strain at various concentrations. When 1 μM CSP was added, the effect was very similar to that of 4-h-old wild-type spent medium, showing a rate of GT formation of 8% (Fig. 2B). Together with the reduction in the concentration of CSP to 0.1 or 0.01 μM, the inhibitory effect was reduced (16% and 32% GT formation, respectively).
Effect of synthetic CSP on C. albicans GT formation.
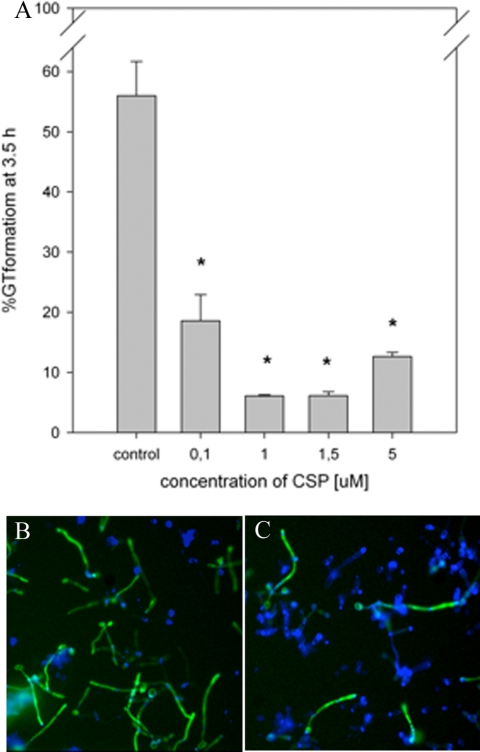
The effect of increasing concentrations of synthetic CSP, ranging from 0.1 to 5 μM, is shown in Fig. 3A. Synthetic CSP at 0.1 μM significantly inhibited GT formation by 60% compared to the control. Using 1.0 μM CSP, an inhibition of GT formation by 90% compared to the control was obtained, indicating that the inhibition of GT formation is dependent on the concentration of synthetic CSP added to the culture; however, concentrations up to 5.0 μM did not enhance the effect. Synthetic CSP did not inhibit the growth of C. albicans at the concentrations tested (data not shown). The effect of synthetic CSP on C. albicans GT formation was confirmed using C. albicans HB12, expressing GFP under the control of the hypha-specific HWP1 promoter. HWP1 is expressed only in germinating cells and not in pseudohyphae or yeast cells. A decrease in the number of green fluorescent (germinating) cells and an increase in the number of pseudohyphae and yeast cells compared to the control were observed in the presence of 1 μM CSP (Fig. 3B and C).
FIG. 3.
(A) Inhibition of GT formation by synthetic CSP is concentration dependent. The percentage of GT formation decreases with increasing synthetic CSP concentrations from 0.1 to 1.0 μM; concentrations up to 5.0 μM did not enhance the effect. The results are averages of data from two experiments, with at least 300 cells counted per experiment. An asterisk indicates a significant (P < 0.05) difference compared to the control. (B and C) Fluorescence microscopy of C. albicans HB12 carrying the HWP1 promoter-GFP fusion grown without (B) or with (C) 1 μM CSP. Germinating cells express HWP1 (green), while calcofluor white stains the cell wall of all cells (blue), showing a simultaneous decrease in numbers of germinating cells and an increase in numbers of yeast cells and pseudohyphae in the culture with 1 μM CSP compared to the control.
Effect of synthetic CSP on preformed GT.
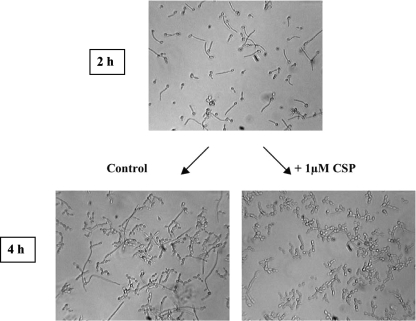
To investigate whether synthetic CSP can revert preexisting hyphae back to yeast cells, C. albicans was grown under hypha-inducing conditions for 2 h, after which 1 μM of CSP was added, followed by an additional culturing under hypha-inducing conditions for 2 h (Fig. 4). After 2 h of growth, 62% of all cells had germinated. The addition of 1 μM of synthetic CSP resulted in 5% GT, compared to 40% in the untreated control. Our results show that synthetic CSP not only inhibits germination but may also stimulate the hypha-to-yeast transition.
FIG. 4.
Effect of synthetic CSP on germinating cells. (Top) GT formation after 2 h of incubation. (Bottom) Lack of germination 2 h after the addition of 1 μM synthetic CSP (right) compared to a control without synthetic CSP (left).
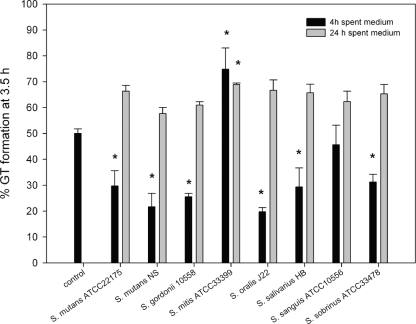
Effect of spent medium from other Streptococcus strains and species on C. albicans GT formation.
The influence of other S. mutans strains and other Streptococcus spp. on C. albicans GT formation is shown in Fig. 5. All 4-h-old spent media from the S. mutans strains tested, as well as Streptococcus gordonii 10558, S. oralis J22, S. salivarius HB, and S. sobrinus ATCC 33478, showed a rate of GT formation of approximately 20 to 30%. Four-hour-old spent medium obtained from Streptococcus mitis ATCC 33399 and S. sanguis ATCC 10556 showed rates of GT formation of 74% and 45%, respectively. None of the 24-h-old spent media, corrected for pH, showed inhibition.
FIG. 5.
Effect of spent media of several Streptococcus spp. on GT formation of C. albicans. Shown are data for spent media of various Streptococcus spp. grown for 4 h (black bars) or 24 h (gray bars). The pH of all samples was set to 7 prior to the GT assay. The results are averages of data from two experiments, with at least 300 cells counted per experiment. An asterisk indicates a significant (P < 0.05) difference compared to the control.
DISCUSSION
S. mutans can affect GT formation of C. albicans in cocultures even if the bacteria and the fungi are physically separated. In addition, filter-sterilized spent medium of S. mutans inhibited GT formation. These results indicate that S. mutans secretes one or more diffusible molecules that affect C. albicans hypha formation. The inhibition of GT formation occurs through several mechanisms, one of which involves the secreted quorum-sensing molecule CSP. This is supported by several lines of evidence: (i) there is a strong inhibitory effect of 4-h-old spent medium on GT formation (CSP was previously described to be produced specifically in the early exponential phase [25]), (ii) synthetic CSP inhibits GT formation in a concentration-dependent manner, and (iii) a mutant of S. mutans that is unable to produce CSP showed a reduced inhibition of GT formation compared to the wild-type strain but could be restored to wild-type levels of GT inhibition by the addition of synthetic CSP.
Saliva was present in all experiments to better resemble the conditions in the oral cavity, where saliva affects the behavior of microorganisms. In the present study, it was shown that saliva induces the GT formation of C. albicans. CSP did not affect GT formation induced by serum (data not shown), which may indicate that saliva is a weaker inducer of hypha formation than serum. This is in line with data from a previous report showing that contact-stimulated hypha formation was more pronounced in the presence of serum than in the presence of saliva (23). Biofilms of S. mutans and C. albicans grown under comparable conditions on hydroxyapatite, polymethylmetacrylate, and soft denture liner discs showed that the presence of S. mutans leads to the suppression of hypha formation in C. albicans without affecting the extent of biofilm formation (26). The extent of C. albicans biofilm formation, when grown in 96-well tissue culture polystyrene plates with saliva or serum coating, was not significantly affected by synthetic CSP (data not shown). A more detailed study of the effect of CSP and other secreted molecules of S. mutans is needed to obtain a deeper understanding of the mechanism governing this interaction in mixed-species biofilms.
Another quorum-sensing molecule secreted by Streptococcus spp., autoinducer 2 (AI-2) (39), has been implicated in interactions within mixed C. albicans biofilms. The presence of S. gordonii cells appeared to induce more extensive hypha formation but not in a luxS mutant (3). There was no significant effect of exogenous dihydroxypentanedione (precursor to AI-2) on hypha formation. However, because many different bacterial species produce slightly different AI-2 derivatives (9), the role of AI-2 in S. mutans-C. albicans interactions should be carefully investigated in the future.
A maximal inhibition of GT formation (Fig. 3 A) was realized by the addition of 1 μM synthetic CSP. This CSP concentration is commonly used to induce competence, and it was speculated to be a physiologically relevant concentration (29, 30). Several attempts to determine CSP concentrations in the spent media used in the current study were unsuccessful. We also note that there are no reports of this in the literature, although many groups have studied CSP-related phenomena. In a paper where a reporter gene was used, there was also no CSP concentration mentioned (32). Unfortunately, there is only speculation regarding the physiological concentrations of CSP produced by S. mutans (28). Based on our chemical complementation of the ΔcomC strain and a comparison between the response of C. albicans to spent medium and the response to synthetic CSP, we predict that the natural concentration of CSP can reach an order of magnitude of 1 μM. Synthetic CSP is not toxic to C. albicans, as it does not inhibit growth, nor does it induce the expression of genes within the general stress response (11), such as CTA1 and HSP12 (data not shown).
The suppressing effect on C. albicans GT formation was obtained only with 4-h-old S. mutans UA159 spent medium, coinciding with maximal CSP production (25). The pH of the medium is one of the factors that is known to affect GT formation in C. albicans (4), and S. mutans is known to produce significant amounts of acid during growth (13). GT formation was significantly inhibited with spent medium from 8- and 24-h-old cultures of S. mutans UA159 and the ΔcomC strain, which was caused by acidification (pH 4.8) (data not shown), whereas this inhibition disappeared when the spent medium was set to pH 7. However, after 4 h, the pH of the spent medium had not yet changed and was still 7 (data not shown); therefore, pH is not causing the inhibitory effect on GT formation. The richness of BHI medium affects GT formation; the addition of 10% or more BHI medium in YNB significantly inhibited GT formation (data not shown). An exhaustion of BHI medium in spent-medium experiments would therefore stimulate GT formation. Both 6-h-old and 8-h-old spent media of S. mutans UA159 and the ΔcomC strain induced GT formation slightly, by 15%. This effect could be explained by the exhaustion of BHI medium. Alternatively, the effect could be explained by the production of an inducer of GT formation in both strains, possibly AI-2, as reported previously for S. gordonii (3). In addition, the induction of GT formation using 6- and 8-h-old spent media illustrates the reduction of the level of CSP production in S. mutans UA159, as described previously (34). Spent media of 4-h-old cultures from S. mutans ATCC 22175, S. mutans NS, and several other streptococcal strains also inhibited GT formation albeit to different extents. This may indicate some level of redundancy toward CSP sequences recognized by C. albicans, as different streptococcal strains and species produce CSP with different amino acid sequences (38). Interestingly, an induction of GT was found for S. mitis ATCC 33399, which may indicate the production of GT-inducing factors. Twenty-four-hour-old cultures of all species did not show an inhibition of GT formation.
A number of C12 acyl chain-containing molecules that affect the morphological transition of C. albicans have been described. Farnesol is produced and sensed by C. albicans (16) to levels as high as 58 μM (37). N-3-Oxo-C12 HSL, produced by P. aeruginosa (15), and dodecanol (8), which mimics farnesol, were the first bacterial signaling molecules described to inhibit the yeast-to-hypha transition in C. albicans. Recently, cis-2-dodecenoic acid, produced by Burkholderia cenocepacia (6), was shown to inhibit the morphological transition at much lower concentrations than farnesol and N-3-oxo-C12 HSL. Because of the structural similarities, it is possible that the regulation of morphological transitions occurs through a shared pathway. CSP of S. mutans is a small, 22-amino-acid-containing peptide that is structurally unrelated to the bacterial signaling molecules known to affect the C. albicans yeast-to-hypha transition. The discovery of S. mutans CSP as a signaling molecule involved in interactions with C. albicans adds a structurally unrelated molecule to the list of known bacterial signaling molecules affecting C. albicans.
Acknowledgments
This work was supported by a young investigator grant of the Human Frontier Science Program (grant RGY0072/2007) to B.P.K.
Footnotes
Published ahead of print on 28 August 2009.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, E., H. A. Hussain, K. R. Crawford, S. Miah, Z. K. Ascott, M. H. Khwaja, and A. H. Hosie. 2007. Genetic variation in comC, the gene encoding competence-stimulating peptide (CSP) in Streptococcus mutans. FEMS Microbiol. Lett. 268:47-51. [DOI] [PubMed] [Google Scholar]
- 3.Bamford, C. V., A. d'Mello, A. H. Nobbs, L. C. Dutton, M. M. Vickerman, and H. F. Jenkinson. 2009. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 77:3696-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensen, E. S., S. J. Martin, M. Li, J. Berman, and D. A. Davis. 2004. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol. 54:1335-1351. [DOI] [PubMed] [Google Scholar]
- 5.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 6.Boon, C., Y. Deng, L. H. Wang, Y. He, J. L. Xu, Y. Fan, S. Q. Pan, and L. H. Zhang. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2:27-36. [DOI] [PubMed] [Google Scholar]
- 7.Buijssen, K. J., H. J. Harmsen, H. C. van der Mei, H. J. Busscher, and B. F. van der Laan. 2007. Lactobacilli: important in biofilm formation on voice prostheses. Otolaryngol. Head Neck Surg. 137:505-507. [DOI] [PubMed] [Google Scholar]
- 8.Davis-Hanna, A., A. E. Piispanen, L. I. Stateva, and D. A. Hogan. 2008. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol. Microbiol. 67:47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Keersmaecker, S. C., K. Sonck, and J. Vanderleyden. 2006. Let LuxS speak up in AI-2 signaling. Trends Microbiol. 14:114-119. [DOI] [PubMed] [Google Scholar]
- 10.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 11.Enjalbert, B., D. A. Smith, M. J. Cornell, I. Alam, S. Nicholls, A. J. Brown, and J. Quinn. 2006. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell 17:1018-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hata, S., and H. Mayanagi. 2003. Acid diffusion through extracellular polysaccharides produced by various mutants of Streptococcus mutans. Arch. Oral Biol. 48:431-438. [DOI] [PubMed] [Google Scholar]
- 14.Havarstein, L. S., R. Hakenbeck, and P. Gaustad. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 16.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolenbrander, P. E. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54:413-437. [DOI] [PubMed] [Google Scholar]
- 18.Krueger, K. E., A. K. Ghosh, B. P. Krom, and R. L. Cihlar. 2004. Deletion of the NOT4 gene impairs hyphal development and pathogenicity in Candida albicans. Microbiology 150:229-240. [DOI] [PubMed] [Google Scholar]
- 19.Kulak, Y., A. Arikan, and E. Kazazoglu. 1997. Existence of Candida albicans and microorganisms in denture stomatitis patients. J. Oral Rehabil. 24:788-790. [DOI] [PubMed] [Google Scholar]
- 20.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 21.Marsh, P. D. 1999. Microbiologic aspects of dental plaque and dental caries. Dent. Clin. N. Am. 43:599-614. [PubMed] [Google Scholar]
- 22.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 23.Nikawa, H., H. Nishimura, T. Hamada, S. Makihira, and L. P. Samaranayake. 1998. Relationship between thigmotropism and Candida biofilm formation in vitro. Mycopathologia 144:125-129. [DOI] [PubMed] [Google Scholar]
- 24.Odds, F. C. 1997. Mycology in oral pathology. Acta Stomatol. Belg. 94:75-80. [PubMed] [Google Scholar]
- 25.Pakula, R., and W. Walczak. 1963. On the nature of competence of transformable streptococci. J. Gen. Microbiol. 31:125-133. [DOI] [PubMed] [Google Scholar]
- 26.Pereira-Cenci, T., D. M. Deng, E. A. Kraneveld, E. M. Manders, A. A. Del Bel Cury, J. M. Ten Cate, and W. Crielaard. 2008. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral Biol. 53:755-764. [DOI] [PubMed] [Google Scholar]
- 27.Pestova, E. V., L. S. Havarstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853-862. [DOI] [PubMed] [Google Scholar]
- 28.Petersen, F. C., G. Fimland, and A. A. Scheie. 2006. Purification and functional studies of a potent modified quorum-sensing peptide and a two-peptide bacteriocin in Streptococcus mutans. Mol. Microbiol. 61:1322-1334. [DOI] [PubMed] [Google Scholar]
- 29.Petersen, F. C., L. Tao, and A. A. Scheie. 2005. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 187:4392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi, F., J. Kreth, C. M. Levesque, O. Kay, R. W. Mair, W. Shi, D. G. Cvitkovitch, and S. D. Goodman. 2005. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol. Lett. 251:321-326. [DOI] [PubMed] [Google Scholar]
- 31.Staab, J. F., Y. S. Bahn, and P. Sundstrom. 2003. Integrative, multifunctional plasmids for hypha-specific or constitutive expression of green fluorescent protein in Candida albicans. Microbiology 149:2977-2986. [DOI] [PubMed] [Google Scholar]
- 32.Syvitski, R. T., X. L. Tian, K. Sampara, A. Salman, S. F. Lee, D. L. Jakeman, and Y. H. Li. 2007. Structure-activity analysis of quorum-sensing signaling peptides from Streptococcus mutans. J. Bacteriol. 189:1441-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thein, Z. M., Y. H. Samaranayake, and L. P. Samaranayake. 2006. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch. Oral Biol. 51:672-680. [DOI] [PubMed] [Google Scholar]
- 34.Tomasz, A., and R. D. Hotchkiss. 1964. Regulation of the transformability of pneumococcal cultures by macromolecular cell products. Proc. Natl. Acad. Sci. USA 51:480-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Mei, H. C., M. Rustema-Abbing, J. de Vries, and H. J. Busscher. 2008. Bond strengthening in oral bacterial adhesion to salivary conditioning films. Appl. Environ. Microbiol. 74:5511-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Ploeg, J. R. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber, K., R. Sohr, B. Schulz, M. Fleischhacker, and M. Ruhnke. 2008. Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob. Agents Chemother. 52:1859-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whatmore, A. M., V. A. Barcus, and C. G. Dowson. 1999. Genetic diversity of the streptococcal competence (com) gene locus. J. Bacteriol. 181:3144-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida, A., T. Ansai, T. Takehara, and H. K. Kuramitsu. 2005. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 71:2372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, L. H., and Y. H. Dong. 2004. Quorum sensing and signal interference: diverse implications. Mol. Microbiol. 53:1563-1571. [DOI] [PubMed] [Google Scholar]