Abstract
Ascorbate peroxidase from Leishmania major (LmAPX) is one of the key enzymes for scavenging of reactive oxygen species generated from the mitochondrial respiratory chain. We have investigated whether mitochondrial LmAPX has any role in oxidative stress-induced apoptosis. The measurement of reduced glutathione (GSH) and protein carbonyl contents in cellular homogenates indicates that overexpression of LmAPX protects Leishmania cells against depletion of GSH and oxidative damage of proteins by H2O2 or camptothecin (CPT) treatment. Confocal microscopy and fluorescence spectroscopy data have revealed that the intracellular elevation of Ca2+ attained by the LmAPX-overexpressing cells was always below that attained in control cells. Flow cytometry assay data and confocal microscopy observation strongly suggest that LmAPX overexpression protects cells from H2O2-induced mitochondrial membrane depolarization as well as ATP decrease. Western blot data suggest that overexpression of LmAPX shields against H2O2- or CPT-induced cytochrome c and endonuclease G release from mitochondria and subsequently their accumulation in the cytoplasm. Caspase activity assay by flow cytometry shows a lower level of caspase-like protease activity in LmAPX-overexpressing cells under apoptotic stimuli. The data on phosphatidylserine exposed on the cell surface and DNA fragmentation results show that overexpression of LmAPX renders the Leishmania cells more resistant to apoptosis provoked by H2O2 or CPT treatment. Taken together, these results indicate that constitutive overexpression of LmAPX in the mitochondria of L. major prevents cells from the deleterious effects of oxidative stress, that is, mitochondrial dysfunction and cellular death.
In multicellular organisms, mitochondria are the major physiological source of reactive oxygen species (ROS) within cells and also are important checkpoints for the control of programmed cell death (27). There are increasing numbers of reports that describe apoptosis- or programmed cell death-like processes in unicellular organisms also, such as trypanosomatids (4, 60), bacteria (20, 25), yeasts (34), and Plasmodium (3). Among the kinetoplastid parasites, Trypanosoma and Leishmania are the most carefully studied genera where apoptotic features are well established (49). Several reports have shown that mitochondrial dysfunction or an imbalance of antioxidant homeostasis causes an increase in mitochondrion-generated ROS, which include H2O2, superoxide radical anions, singlet oxygen, and hydroxyl radicals. These species have all been implicated in apoptosis (16, 26, 28, 41). Increasing evidence has been presented to support that ROS homeostasis regulates two major types of important physiological processes and exerts diverse functions within cells. One type of function includes damage or oxidation of cellular macromolecules (DNA, proteins, and lipids), which can lead to necrotic cell death or protein modification (7). The second type of function includes the activation of cellular signaling cascades that regulate proliferation, detoxification, DNA repair, or apoptosis (11). The detoxification of toxic mitochondrial ROS in cells occurs through a variety of cellular antioxidant enzymes, such as superoxide dismutase, which detoxifies cells from superoxide released into the mitochondrial matrix, and several other antioxidant proteins, such as catalase, glutathione (GSH) peroxidase, and peroxiredoxins, which are known to catalyze further degradation of H2O2 (44). During its life cycle, the Leishmania sp. encounters a pool of ROS that is generated either by its own physiological processes or as a result of host immune reaction and drug metabolism. However, unlike most eukaryotes, Leishmania lacks catalase- and selenium-containing GSH peroxidases, enzymes that play a front-line role in detoxifying ROS. Hence, the mechanism by which it resists the toxic effects of H2O2 remains poorly understood.
Recently, we cloned, expressed and characterized the unusual heme-containing ascorbate peroxidase from Leishmania major (LmAPX) and observed that the expression of LmAPX is increased when Leishmania cells are treated with exogenous H2O2 (1, 18). This enzyme is a functional hybrid between cytochrome c peroxidase and APX, owing to its ability to use both ascorbate and cytochrome c as reducing electron donors (58). Colocalization studies by confocal microscopy, submitochondrial fractionation analysis of the isolated mitochondria, and subsequent Western blot analysis with anti-LmAPX antibody have confirmed that the mature enzyme is present in intermembrane space side of the inner membrane. It has also been shown that overexpression of LmAPX causes a decrease in the mitochondrial ROS burden, an increase in tolerance to H2O2, and protection against cardiolipin oxidation under oxidative stress (18). Although previous studies have shown that Leishmania species use superoxide dismutase (23), peroxiredoxins (8), intracellular thiols (14), lipophosphoglycan (13), trypanothione (5), HSP 70 (a heat shock protein) (36), tryparedoxin peroxidase (29), and APX (18) for detoxification of ROS, it is still unclear how the antioxidants protect against oxidative stress-induced apoptotic events in the unicellular organism Leishmania.
Since the LmAPX protein is localized in the mitochondria, we hypothesized that it would be a key protein for the maintenance of mitochondrial functions due to its antioxidant properties via its ROS-scavenging function (18). To test this hypothesis, we overexpressed LmAPX in Leishmania major cells and investigated whether overexpression of LmAPX can confer resistance to oxidant-mediated mitochondrial damage as well as oxidative stress-induced cell death. In this study, we provide evidence that the overexpression of LmAPX in Leishmania cells can indeed protect against camptothecin (CPT) or H2O2-mediated mitochondrial damage as measured by various parameters, including disruption of mitochondrial membrane potential (Δψm), decrease of ATP production, and cytochrome c and endonuclease G release from mitochondria. Cells overexpressing LmAPX were also protected against oxidative stress-induced protein carbonylation, DNA fragmentation, and apoptosis. To the best of our knowledge, this is the first report of a mitochondrial hemeperoxidase that controls the ROS-induced mitochondrial death pathway.
MATERIALS AND METHODS
Materials.
The Mitoprobe JC-1 assay kit for flow cytometry, Vibrant apoptosis assay kit no. 3 for annexin V and propidium iodide (PI), caspase 3 and 7 assay kit, Fluo 4-AM, Pluronic F127, calcium ionophore A23187, ionomycin, and fetal bovine serum were purchased from Molecular Probes (Eugene, OR). The GSH detection kit, caspase inhibitor VAD-fmk (Val-Ala-Asp-fluoromethyl ketone), and terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) kit were procured from Clontech. The Oxyblot protein oxidation detection kit was from Millipore. CPT and all other chemicals were obtained from Sigma or from sources mentioned previously (1, 18).
Parasite culture and treatments.
The promastigote form of Leishmania major (5ASKH) was cultured in M199 medium supplemented with 10% heat-inactivated fetal bovine serum as described previously (1, 18). pXG B2863 vector alone (control) or pXG B2863 vector with LmAPX gene-transfected L. major cells was maintained in the presence of 200 μg/ml G418. Ectopic expression of LmAPX was monitored regularly by Western blotting and activity assay. Western blot experiments and flow cytometry assays (using the fluorogenic peroxidase substrate dihydrorhodamine 123) showed at least 4-fold- and 2.5-fold-higher LmAPX expression and peroxidase activity in the LmAPX-overexpressing parasites compared to control cells (data not shown). For experimental purposes, 3-day-old exponentially growing cultures that contained almost 100% motile promastigotes were used. Control and LmAPX-overexpressing cells were treated with either 1 mM H2O2 or 5 μM CPT to induce apoptosis.
Viability assay in the presence of various concentration of H2O2.
Leishmania major promastigote viability during oxidative stress was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma) assay as described by Gantt et al. with minor modifications (22). Exponentially growing promastigotes (2 × 106) in M199 medium were exposed to different concentrations of H2O2 for 2.0 to 8.0 h. After treatment, cells were washed with ice-cold 1× phosphate-buffered saline (PBS) and incubated in fresh M199 medium with 10% heat-inactivated fetal bovine serum with 0.5 mg/ml MTT for 3 h. After 3 h, cells were pelleted by centrifugation (1,200 × g for 5.0 min) and washed twice with 1× PBS, and 100 μl of 0.04 N HCl in isopropanol was added. The dark blue formazan generated from MTT by living mitochondria is soluble in acid-isopropanol. The absorbance of these solutions was measured on a microplate reader at 570 nm. The percentage of viability was calculated from optical density readings in wells with H2O2 compared with those in wells without. All experiments were performed in triplicate. The viability assay showed that 90% cellular death occurred in Leishmania major promastigotes as a result of treatment for 8.0 h with 1.0 mM H2O2 (see Fig. S1 in the supplemental material).
Measurement of GSH.
The cellular GSH content was measured with a GSH detection kit (Clontech). After treatment with H2O2 or CPT, 107 cells were pelleted and lysed with 1× lysis buffer. Cell lysates were then incubated with monochlorobimane dye (2 mM) for 1 hour at 37°C, and the GSH level was detected with a fluorescence plate reader at 395-nm excitation and 480-nm emission wavelengths.
Measurement of free cytosolic calcium.
Cytosolic Ca2+ in cells was monitored using Fluo 4AM (Molecular Probes, Eugene, OR). A total of 107 cells were loaded with 5 μM Fluo-4/AM for 60 min at room temperature in the presence of 10 μM pluronic acid F127. After incubation, cells were washed with fresh serum-free medium and analyzed immediately with a fluorescence spectrophotometer with excitation at 490 nm and emission at 518 nm. The concentration of free Ca2+ was calculated using the formula Kd (F − FMIN)/(FMAX − F), where Kd is 345 nM, F is the fluorescence intensity of the cells, FMIN is the minimum fluorescence of the cells obtained by treating cells with 10 μM calcium ionophore in the presence of 3 mM EGTA, and FMAX is maximum fluorescence of cells achieved in the presence of calcium ionophore. Different aliquots of same sample were visualized with a Leica TCS-SP confocal microscope with excitation at 488 nm and emission at 530 nm.
Subcellular fractionation and Western blot analysis for quantitation of cytochrome c and endonuclease G release.
Subcellular fractionation was performed by hypotonic lysis followed by use of the Percoll density gradient method at 4°C as described previously (18). The mitochondrial fraction was judged by cytochrome c oxidation assay and kynurenine hydroxylase (an outer membrane marker) assay. Western blot analysis was performed as described previously (18). The primary antibodies used were as follows: rabbit anti-Trypanosoma brucei cytochrome c antibody (1:500), rabbit anti-Leishmania donovani endonuclease G (1:500), goat anti-HSP 60 antibody (1:5,000), rabbit anti-Leishmania donovani adenosine kinase (1:50), and mouse anti-α-tubulin antibody (1:5,000). The horseradish peroxidase-conjugated secondary antibodies used were anti-rabbit (1:10,000), anti-mouse (1:6,000), and anti-goat (1:6,000). In each experiment 50 μg of total protein was loaded as described for each case. Precise quantitation was done by densitometric analysis to correct the expression of the protein of interest with that of α-tubulin, HSP 60, or adenosine kinase, which were immunodetected in the same sample. Densitometric analysis was performed by importing images to a personal computer using Total Lab TL 100 software (Nonlinear Dynamics Ltd.).
Δψm measurement.
Δψm was assayed by flow cytometry with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole carbocyanide iodide (JC-1) as a probe. JC-1 is a cationic and lipophilic vital dye that concentrates in mitochondria in a potential-dependent manner. Measurements were performed according to manufacturer's instruction. Briefly, after treatment, cells were washed twice and resuspended in 1 ml PBS at 106 cells/ml. JC-1 probe was added to a 6 μM final concentration and incubated for 20 min at 26°C. For a positive control, 50 μM of the mitochondrial uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was added to nontreated control cells 15 min prior to addition of JC-1. Analysis was performed on FacsCanto flow cytometer (Becton Dickinson) equipped with 488-nm excitation and 530/610-nm emission filters for green (monomeric form) and red (J-aggregate formation) fluorescence, respectively, after appropriate fluorescence compensation. Data were analyzed with FACSDiva software.
For microscopy, JC-1-labeled equivalent cells were allowed to adhere to poly-l-lysine-coated slides and visualized with a Leica TCS-SP confocal microscope. Mitochondria with higher transmembrane potential accumulate more dye, and emission shifts from 530 nm (green fluorescence) at lower concentrations to 590 nm (red fluorescence) at higher concentrations due to J-aggregate formation.
Measurement of protein carbonyl.
The protein carbonyl content was detected with an Oxyblot protein oxidation detection kit (Chemicon International Ltd., Hampshire, United Kingdom) according to the manufacturer's protocol. Total proteins were extracted from 1 × 107 cells with Laemmli buffer containing 6% sodium dodecyl sulfate. Fifty micrograms of total protein was incubated with 1× 2,4-dinitrophenylhydrazine for 20 min at room temperature to form the carbonyl derivative dinitrophenylhydrazone. Carbonylated proteins were detected with anti-2,4-dinitrophenol rabbit antibody (diln-200) followed by horseradish peroxidase-conjugated anti-rabbit antibody (diln-300). Blots were developed with an Amersham ECL kit.
Detection of caspase-like protease activity.
The detection of caspase activity by FLICA (a caspase 3- and 7-specific fluorogenic inhibitor, FAM-DEVD-FMK) is based on affinity labeling of the reactive-center cysteine residue of activated caspases by the FMK moiety of FLICA via the caspase-specific recognition sequence aspartic acid-glutamic acid-valine-aspartic acid (DEVD). Apart from the FMK moiety, FLICA also contains a fluorogenic carboxyfluorescein group as a reporter molecule. Due to the cell membrane permeability, unbound FLICA molecules diffuse out of the cell, and the green fluorescence indicates the amount of FLICA-bound active caspases within the cell. Activation of caspase-like protein was detected by flow cytometry as per the manufacturer's instructions. Briefly after treatment with H2O2 or CPT, 106 cells were resuspended in 300 μl of fresh medium and incubated for 1 h in the presence of 10 μl caspase inhibitor. Cells were further washed with 1× wash buffer and finally suspended in 500 μl 1× wash buffer and analyzed immediately by flow cytometry (BD FacsCanto) with 488-nm excitation and 530-nm emission wavelengths.
TUNEL staining.
Cells undergoing apoptosis produce DNA fragments in the nuclei. TUNEL staining was performed with an Apoalert DNA fragmentation assay kit (Clontech, Mountain View, CA) to detect in situ DNA fragmentation according to manufacturer's manual. Briefly H2O2- or CPT-treated cells were harvested at different time intervals, washed with PBS, and fixed with 4% formaldehyde in PBS. Cells were then applied to poly-l-lysine-coated slides and permeabilized with 0.2% Triton X-100 in PBS. Equilibration was performed by incubating cells in equilibration buffer for 10 min at room temperature, followed by incubation with equilibration buffer containing nucleotide mix and terminal deoxynucleotidyl transferase enzyme for 1 h in 37°C humidified incubator. Samples were counterstained with 10 μg/ml PI with 1 μg/ml RNase and visualized under a Leica TCS-SP confocal microscope.
Measurement of ATP.
The cellular ATP concentration was measured by the bioluminescence method using an ATP determination kit (Molecular Probes). Briefly, differently treated cells (1 × 107) were mixed with reaction buffer containing 1 mM dithiothreitol, 0.5 mM luciferin, and 12.5 μg/ml luciferase. The luminescence intensity was measured in a luminometer (Promega). ATP concentrations were calculated from an ATP standard curve, and cellular ATP levels were expressed as nmol/106 cells.
Apoptosis assessment by annexin V staining.
Phosphatidylserine exposure was assessed with the Vybrant apoptosis assay kit no. 3 (Molecular Probes). After incubation with H2O2 or CPT, cells were harvested by centrifugation for 5 min at 1,200 × g and washed twice with cold 1× PBS. Cells (1 × 106/ml) were then resuspended in 100 μl 1× annexin binding buffer with 5 μl fluorescein isothiocyanate (FITC)-conjugated annexin V and 1 μg/ml PI and incubated for 20 min. After staining, 400 μl of 1× buffer was added to the cells, and samples were stored on ice until data acquisition by flow cytometry. To eliminate the emission spectral overlap of fluophores, fluorescence compensation was performed with unstained and 8-h-treated single-stained (with either PI or with FITC) samples. Measurements were completed within 1 h. For confocal imaging, only annexin V-bound cells were used.
Statistical analysis.
All results were expressed as the mean ± standard error (SE) from at least three independent experiments. Statistical analysis for parametric data was done by Student's t test or analysis of variance wherever applicable using Origin 6.0 software (Microcal Software, Inc. Northampton, MA). The analysis of variance was followed by post hoc analysis (multiple-comparison t test) for the evaluation of the difference between individual groups. A P value of less than 0.05 was considered statistically significant.
RESULTS
LmAPX prevents both cellular GSH depletion and accumulation of oxidized proteins.
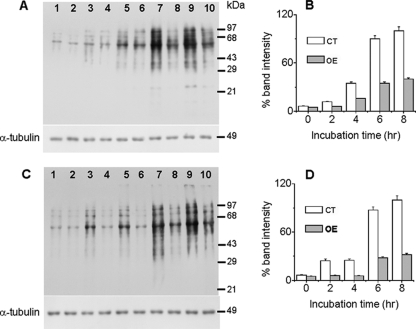
Like H2O2, CPT is an in vivo ROS-producing agent that acts by inhibiting class I DNA topoisomerase and induces apoptotic death in cultured mammalian cells (50). In Leishmania cells, CPT has been shown to induce apoptosis by generation of ROS, an imbalance in cytosolic cations, mitochondrial dysfunction, and subsequent activation of caspase-like molecules (48). Depletion of cellular GSH or protein oxidation is often used as a measure of the level of ROS within the cell. We measured the cellular GSH content in control and LmAPX-overexpressing cells treated for up to 4 h with H2O2 or CPT to induce oxidative stress. Cells overexpressing LmAPX show more than a 2.5-fold-higher level of GSH after 1 h of treatment and more than 1.5-fold protection throughout the 4 h of treatment, indicating a less-oxidizing environment in overexpressing cells (Table 1). Protein carbonyl content is the most general and well-used biomarker of protein oxidation within cells (15). Since a decrease in ROS production could probably cause less oxidative damage to proteins, we monitored the protein carbonyl content of cellular homogenates by derivatization of the carbonyl group with 2,4-dinitrophenylhydrazine and subsequent immunodetection of the resulting hydrazone with the Oxyblot protein oxidation detection kit (Fig. 1). Treatment of H2O2 and CPT in both control and LmAPX-overexpressing cells resulted in a time-dependent increase in band intensity (Fig. 1A and C). Densitometry quantification of the bands revealed 16-fold and 10-fold increases in the carbonyl content of proteins in control cells subjected to 8 h of H2O2 and CPT treatment, respectively, whereas LmAPX-overexpressing cells under identical treatment conditions showed increases of only 6-fold and 4-fold in protein carbonyl content in H2O2- and CPT-treated overexpressing cells, respectively (Fig. 1 B and D). The band intensity in LmAPX-overexpressing cells was at least 2.5-fold lower than that in the control cells throughout the 8 h of treatment. These results indicate that overexpression of LmAPX protected Leishmania cells against oxidative damage of proteins by H2O2 or CPT treatment.
TABLE 1.
Effect of LmAPX overexpression on cellular GSH levela
| Time (h) | Fluorescence units (mean ± SEM)b after treatment with: |
|||
|---|---|---|---|---|
| H2O2 |
CPT |
|||
| Control cells | LmAPX-overexpressing cells | Control cells | LmAPX-overexpressing cells | |
| 0 | 318 ± 12 | 322 ± 10 | 309 ± 15 | 312 ± 20 |
| 1 | 185 ± 10* | 272 ± 13* | 209 ± 10* | 296 ± 22* |
| 2 | 134 ± 8* | 231 ± 11* | 172 ± 12* | 260 ± 15* |
| 3 | 75 ± 5* | 139 ± 8* | 148 ± 10* | 216 ± 14* |
| 4 | 62 ± 4* | 112 ± 7* | 102 ± 4* | 172 ± 8* |
The intracellular GSH level was measured using monochlorobimane after treatment with either CPT (5μM) or H2O2 (1 mM). An increase in fluorescence units reflects a larger amount of GSH present in the cells. All experiments were performed in triplicate.
*, P < 0.01.
FIG. 1.
Effect of LmAPX overexpression on cellular protein oxidation. Total cell lysates were derivatized with 2,4-dinitrophenylhydrazine as described in Materials and Methods, and 20 μg of each was subjected to immunoblot analysis either with anti-2,4-dinitrophenol antibody or α-tubulin antibody (as a loading control). (A) Immunoblot of H2O2-treated control cells (lanes 3, 5, 7, and 9) or cells overexpressing LmAPX (lanes 4, 6, 8, and 10). (B) The bar graph represents the percentage of carbonylated proteins in H2O2-treated cells expressed as percentage of band intensity. CT, control cells; OE, cells overexpressing LmAPX. (C) Immunoblot of CPT-treated control cells (lanes 3, 5, 7, and 9) or cells overexpressing LmAPX (lanes 4, 6, 8, and 10). Lanes 1 and 2 of both panels A and C represented untreated control and LmAPX-overexpressing cells, respectively. (D) The bar graph represents the percentage of carbonylated proteins in CPT-treated cells expressed as percentage of band intensity. Band intensity was quantified by Total Lab TL100 software, and error bars represent the SE from three independent experiments. The positions of molecular mass standards are indicated.
LmAPX overexpression prevents oxidative stress-induced elevation of cytosolic calcium.
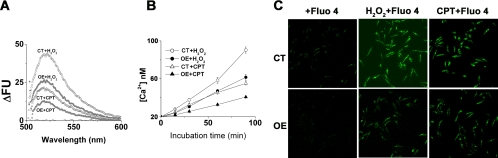
It is now recognized that ROS in mitochondria play a key role in intracellular Ca2+ homeostasis (38, 51). Perturbation of homeostasis and elevation of cytosolic Ca2+ can lead to apoptotic cell death. Hence, we asked whether overexpression of LmAPX defended against oxidative stress-mediated elevation of cytosolic Ca2+. We measured Ca2+ levels in both control cells and LmAPX-overexpressing cells after treatment with H2O2 and CPT at different time points by confocal microscopy and fluorescence spectroscopy using the Fluo 4 dye as a calcium sensor (Fig. 2). Elevation of Ca2+ was usually monitored by an increase in the green fluorescence intensity at 518 nm resulting from fluorescence emission of Ca2+-bound Fluo 4 (excitation, 490 nm). The fluorescence emission peak at 518 nm was raised when either cell type was treated with H2O2 or CPT for 90 min. However, the intensity of the peak at 518 nm for the LmAPX-overexpressing cells was at least twofold lower than that for the control cells, indicating that overexpression of LmAPX protected cells against oxidative stress-mediated elevation of cytosolic Ca2+ (Fig. 2A). Also, at other time points after H2O2 and CPT treatment, the elevation of cytosolic Ca2+ was much lower in LmAPX-overexpressing cells than in control cells (Fig. 2B).
FIG. 2.
LmAPX overexpression suppresses cytosolic Ca2+ release. The intracellular Ca2+ level was measured using Fluo 4 AM as an indicator after treatment with either CPT (5 μM) or H2O2. CT, control cells; OE, cells overexpressing LmAPX. (A) Changes in intracellular release of Ca2+ were compared by fluorescence spectrophotometry. (B) Analysis of time-dependent elevation of intracellular Ca2+ level. The data shown are means ± SEs. (C) The increase in Ca2+ level was visualized by confocal microscopy. Data are representative of at least three independent experiments.
To validate the above results by an alternative method, the level of cytosolic Ca2+ was analyzed by Fluo 4AM fluorescence imaging by microscopy. The data presented in Fig. 2C clearly confirmed the data obtained with the fluorescence spectrophotometer. Although the nontreated control had very low green signals, nontreated overexpressing cells had no green signal. The treatment with H2O2 or CPT caused elevation of Ca2+ at 0 to 1.5 h in both control and LmAPX-overexpressing cells, as shown by an increase in fluorescence at 518 nm for control cells and LmAPX-overexpressing cells. The level of elevation of Ca2+ attained by the LmAPX-overexpressing cells was clearly lower than that observed in control cells from 0 to 1.5 h intervals.
LmAPX overexpression prevents oxidative stress-induced loss of Δψm and a decline in cellular ATP generation.
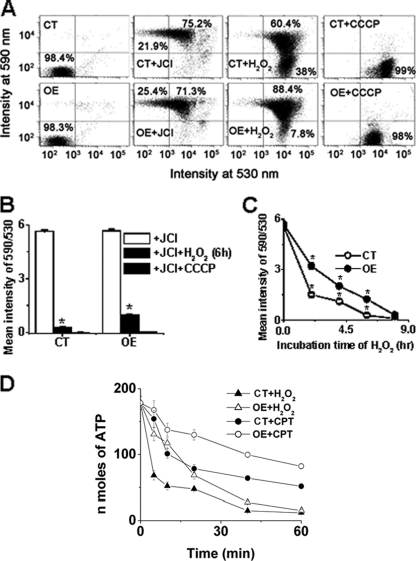
Intracellular ROS buildup and elevation of cytosolic Ca2+ can induce mitochondrial dysfunction, which was highly coupled with a collapse in Δψm (7). To examine whether overexpression of LmAPX shielded against oxidative stress-mediated loss of Δψm, we measured the Δψm by flow cytometry assay and confocal microscopy using the potentiometric fluorescent dye JC-1 (Fig. 3; see Fig. S2 in the supplemental material). A shift in the fluorescence emission from green (535 nm) to red (595 nm) indicates accumulation of JC-1 in the mitochondria, which is dependent solely on the membrane potential of the mitochondria. Consequently, mitochondrial membrane depolarization is usually accompanied by a decrease in the fluorescence intensity ratio (red/green). We observed that incubation with the mitochondrial uncoupler CCCP reduced the JC-1 fluorescence intensity ratio, indicating that the JC-1 response in Leishmania cells is sensitive to changes in membrane potential (Fig. 3A and B). Treatment with H2O2 in both control and LmAPX-overexpressing cells resulted in a time-dependent shift in the fluorescence intensity from red (595 nm) to green (535 nm). However, careful analysis of the data revealed that the red/green ratio for the LmAPX-overexpressing cells was at least twofold higher than that for the control cells (Fig. 3C). This observation strongly suggested that LmAPX overexpression protects cells from H2O2-induced mitochondrial membrane depolarization.
FIG. 3.
Effect of LmAPX overexpression on preservation of Δψm in L. major. L. major cells (107/ml) were incubated with the potential-sensitive probe JC-1 (6 μM) for 15 min at 25°C to asses Δψm after treatment with H2O2 (1 mM) or CPT (5 μM) for the indicated times and analyzed by flow cytometry and confocal microscopy with excitation at 488 nm. Emission was detected at 530 nm (monomer) and 590 nm (aggregate). A drop in Δψm is identified as a change in JC-1 properties from forming J-aggregates (emission at 590 nm, red color) at high Δψm to forming J-monomers (emission at 530 nm, green color) at low Δψm. The nearly complete monomer was induced by treating cells with 50 μM CCCP, an uncoupler of mitochondrial respiration, 15 min prior to addition of JC-1. (A) Dot plots of blank cells (cells without JC-1), cells with JC-1, H2O2-treated JC-1-stained cells, and CCCP-treated JC-1-stained cells. CT and OE, control cells and LmAPX-overexpressing cells, respectively. (B) Effect of H2O2 (6 h) and CCCP treatment on Δψm in CT and OE cells. Bar graphs represent the ratio of mean fluorescence intensity (590/530) of total cells analyzed by flow cytometry. Error bars indicate the SE from three independent experiments. The asterisks indicate the level of statistical significance (<0.05). (C) Time-dependent analysis of 590/530 values of H2O2-treated CT and OE cells. (D) Time-dependent measurement of intracellular ATP levels in CT and OE cells in the presence of either CPT or H2O2. ATP concentration is expressed as nanomoles of ATP/106 cells.
To further substantiate the lower Δψm of control cells compared with LmAPX-overexpressing cells, fluorescence imaging of samples analyzed by fluorescence-activated cell sorting (FACS) was performed in parallel by confocal microscopy. JC-1 fluorescence imaging (see Fig. S2 in the supplemental material) clearly confirmed the data obtained by FACS. Nontreated control and LmAPX-overexpressing cells showed marked peripheral red and green signals in a punctuated manner. Treatment with H2O2 caused depolarization of mitochondria at 0 to 8 h in both control and LmAPX-overexpressing cells, as shown by an increase in green fluorescence at 535 nm for control cells and LmAPX-overexpressing cells. The observation of lower green fluorescence in overexpressing cells indicated that the level of depolarization attained by the LmAPX-overexpressing cells was always below that for control cells.
Δψm could affect mitochondrial generation of ATP, which is directly proportional to Δψm (24). The ATP level is important, as progression to necrosis or apoptosis depends on the availability of ATP (32). Thus, to examine whether overexpression of LmAPX protects against H2O2- and CPT-induced loss of ATP generation, we measured the ATP content by bioluminescence assay using the Molecular Probes ATP determination kit (A22066) (Fig. 3D). Although there was a gradual fall in the ATP levels in both control cells and LmAPX-overexpressing cells, the ATP content for the H2O2- or CPT-treated control cells was at least twofold lower than that for the LmAPX-overexpressing cells (Fig. 3D). At various time points, LmAPX-overexpressing cells also showed protection against ATP decrease compared to control cell.
LmAPX overexpression prevents oxidative stress-induced release of cytochrome c and endonuclease G from mitochondria to the cytosol.
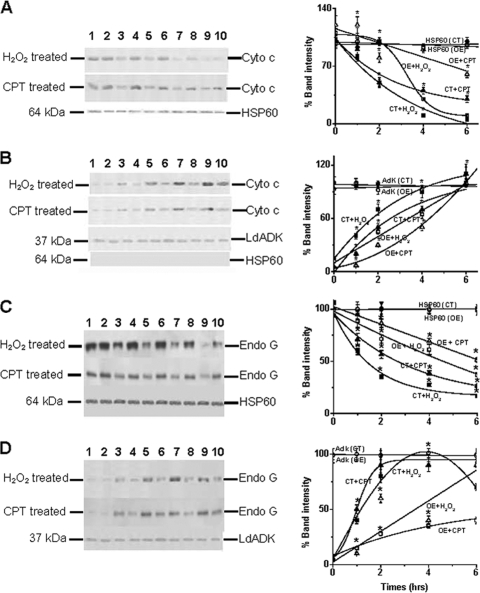
Cytochrome c was identified as a factor that is released from mitochondria to the cytoplasm in apoptotic cells (31). To investigate whether overexpression of LmAPX protected against oxidative stress-mediated cytochrome c release, we compared H2O2- or CPT-induced cytochrome c release from mitochondria to the cytoplasm in control and LmAPX-overexpressing cells. Subcellular fractionation followed by Western blotting indicated that CPT and H2O2 treatment causes time-dependent cytochrome c release from mitochondria (Fig. 4A) and subsequent accumulation in the cytoplasm (Fig. 4B). Our experimental results showed that the band intensities of mitochondrial cytochrome c for the control cells were ∼2-fold lower than those for the LmAPX-overexpressing cells. Simultaneously, the band intensities of cytoplasm cytochrome c for the control cells were ∼2-fold higher than those for the LmAPX-overexpressing cells. As control markers for mitochondria and cytoplasm, we used the mitochondrial HSP 60 and cytosolic adenosine kinase, respectively.
FIG. 4.
Western blot analysis for cytosolic cytochrome c and endonuclease G release from mitochondria isolated from control cells (CT) and LmAPX-overexpressing cells (OE). The concentrations of H2O2 and CPT used were 1 mM and 5 μM, respectively. Lanes 1 and 2, untreated control and LmAPX-overexpressing cells, respectively; lanes 3, 5, 7, and 9 control cells with 1 h, 2 h, 4 h, and 6 h of treatment, respectively; lanes 4, 6, 8, and 10, LmAPX-overexpressing cells with 1 h, 2 h, 4 h, and 6 h of treatment, respectively. Panels A, B, C, and D represent immunoblot and densitometric analysis of cytochrome c in the mitochondrial fraction, cytochrome c in the cytosolic fraction, endonuclease G in the mitochondrial fraction, and endonuclease G in the cytosolic fraction, respectively. Band intensity is presented as the percentage of cytochrome c or endonuclease G released from untreated cells. Fifty micrograms of total protein was analyzed by 13% sodium dodecyl sulfate-polyacrylamide gel electrophoresis with antibodies specific for T. brucei cytochrome c, L. donovani endonuclease G, HSP 60 (mitochondrial matrix protein), and L. donovani adenosine kinase (cytosolic protein). No HSP 60 was detected in the cytosolic fraction. All the data are representative of at least three independent experiments. *, statistically significant P value of less than 0.05 (P value is less than 0.027).
We next tested whether overexpression of LmAPX protected L. major cells against oxidative stress-mediated release of another cell death protein, Leishmania endonuclease G (9, 21, 47), by comparing endonuclease G released from the mitochondria to the cytoplasm in control and LmAPX-overexpressing cells upon treatment with cell death stimuli. Immunoblotting with anti-endonuclease G antibody for mitochondrial and cytosolic extracts prepared from parasites showed gradual translocation of endonuclease G to the cytosol with time upon H2O2 or CPT treatment (Fig. 4C and D). Our immunoblotting results showed that the band intensity of mitochondrial endonuclease G for the control cells was ∼2-fold lower than that for the LmAPX-overexpressing cells (Fig. 4C). Concurrently, the band intensity of cytoplasm endonuclease G for the control cells was ∼2-fold higher than that for the LmAPX-overexpressing cells (Fig. 4D). Although mitochondrial endonuclease G gradually decreased with time of treatment in both types of cells, cytosolic endonuclease G was not increased after 4 h. These results suggested that the cytosolic endonuclease G might be translocated into the nucleus after 4 h of treatment (9, 21, 47). The equal band intensities corresponding to HSP 60 for the mitochondrial extracts and L. donovani adenosine kinase for the cytoplasm extracts served as loading controls.
LmAPX-overexpressing cells show low caspase-like activity under oxidative stress.
Caspases are a class of cysteine proteases and are the main actors in the metazoan apoptotic pathway. Caspase-like activities have been reported in both Leishmania donovani and Leishmania major (6, 16). To check whether control and LmAPX-overexpressing cells show any difference in caspase-like activity in the presence of apoptotic stimuli, we used the caspase 3- and caspase 7-specific fluorogenic inhibitor FAM-DEVD-FMK. Flow cytometric analysis showed a 56% increase in caspase-like activity from a basal level in H2O2-treated control cells, compared to only 9% in LmAPX-overexpressing cells (Table 2). CPT-treated cells showed 42% and 9% increases in basal activity in control and overexpressing cells, respectively. In an assessment of caspase-like activity in the presence of a nonfluorogenic caspase inhibitor (VAD-fmk), pretreated control cells showed almost 86 to 90% inhibition of caspase-like activity. LmAPX-overexpressing cells showed a basal level of caspase activity in the presence of the inhibitor (Table 2).
TABLE 2.
Depletion of drug-induced caspase-like protease activity by LmAPX
| Cells | FITC green fluorescence (mean ± SEM)a with: |
|||||
|---|---|---|---|---|---|---|
| No FLICA | FLICA | FLICA + H2O2 | FLICA + CPT | H2O2 + VAD-fmk + FLICA | CPT + VAD-fmk + FLICA | |
| Control | 126 ± 5 | 278 ± 10 | 434 ± 18* | 397 ± 30* | 328 ± 20* | 307 ± 22 |
| LmAPX overexpressing | 142 ± 8 | 258 ± 9 | 281 ± 7* | 283 ± 12* | 271 ± 10* | 277 ± 15 |
Increased FLICA green fluorescence indicates increased caspase-like protease activity. Unlabeled control cells were used to set up a flow cytometer by adjusting the forward and side scatter of the cell population. Values without FLICA indicate the background fluorescence level of unlabeled cells. The increase in fluorescence intensity was calculated with respect to background fluorescence intensity. The fluorescence data are representative of three independent experiments. Quantitation of the relative change in fluorescence was analyzed with FACSDiVa software. *, P < 0.05.
LmAPX overexpression prevents apoptosis.
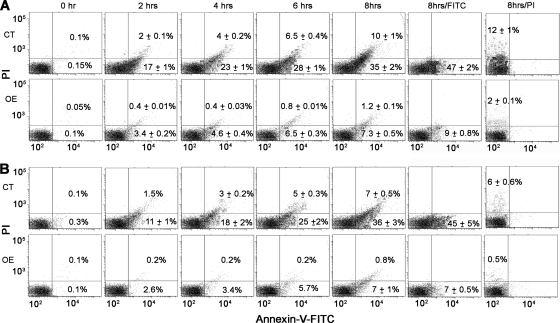
Elevation of cytosolic Ca2+ and mitochondrial dysfunction are known to be important events promoting apoptosis (46). To investigate whether overexpression of LmAPX shielded against oxidative stress-mediated apoptosis, we measured phosphatidylserine externalization (Fig. 5) and nuclear DNA fragmentation (Fig. 6) as markers of apoptosis. Representative FACS dot plots of FITC-conjugated annexin V- and PI-labeled samples are shown in Fig. 5A and B. Both H2O2 treatment and CPT treatment led to a gradual conversion of viable cells (lower left quadrant) into early apoptotic (lower right quadrant) and late apoptotic (upper right quadrant) cells in both control and LmAPX-overexpressing cells. However, overexpression of LmAPX clearly protected against H2O2- or CPT-induced cell death by maintaining a higher percentage of viable cells in time-dependent manner. LmAPX overexpression also protects against late apoptosis, with a minimum difference of ninefold for the longest time interval. This result suggested that treated control cells reached the late apoptotic stage faster than LmAPX-overexpressing cells. For further validation, fluorescence imaging of FACS-analyzed samples was performed in parallel by confocal microscopy. Annexin V-FITC fluorescence imaging (see Fig. S3 in the supplemental material) clearly confirmed the data obtained by FACS.
FIG. 5.
Changes in plasma membrane phosphatidylserine distribution during apoptosis induced either by H2O2 (1 mM) or CPT (5 μM). Control (CT) and LmAPX-overexpressing (OE) cells were double stained with annexin V and PI and analyzed by flow cytometric analysis at the indicated times. Representative dot plots are divided in four quadrants. Viable cells that did not bind annexin V and also did not incorporate PI are represented by the lower left quadrant of each dot plot, early apoptotic cells that bind only annexin V are represented by the lower right quadrant, late apoptotic cells that bind annexin V and incorporate PI are represented by the upper right quadrant. Percentages of cells are indicated in the corresponding quadrants. (A) Time-dependent distribution of viable, early apoptotic, and late apoptotic cells among H2O2-challenged control cells (upper panel) and LmAPX-overexpressing cells (lower panel). (B) Time-dependent distribution of viable, early apoptotic, and late apoptotic cells among CPT-treated control cells (upper panel) and LmAPX-overexpressing cells (lower panel). A total of 30,000 cells were analyzed in each condition. All results are representative of three independent experiments.
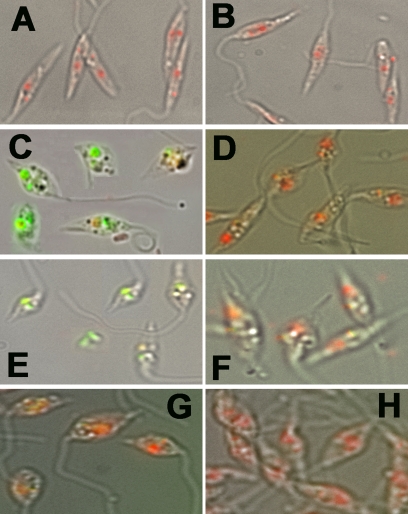
FIG. 6.
TUNEL staining to determine the level of in situ oxidative stress-induced DNA fragmentation. TUNEL-staining cells were counterstained with PI in the presence of RNase. (A) Merged image of control cells under bright-field microscopy. (B) Merged image of LmAPX-overexpressing cells under bright-field microscopy. (C) Control cells with H2O2 treatment for 6 h. (D) Overexpressing cells with H2O2 treatment for 6 h. (E) Control cells with CPT treatment for 6 h. (F) Overexpressing cells with CPT treatment for 6 h. (G) Control cells with H2O2 treatment for 6 h in the presence of a caspase inhibitor (VAD-fmk). (H) Overexpressing cells with H2O2 treatment for 6 h in the presence of a caspase inhibitor (VAD-fmk). All results are representative of three independent experiments.
DNA fragmentation was assessed by TUNEL staining (Fig. 6). As shown in Fig. 6A and B, the formation of red color in both nucleus and kinetoplast DNAs with PI staining indicated that there was no FITC-conjugated dUTP-labeled DNA in either control or LmAPX-overexpressing cells. On the other hand, H2O2- or CPT-treated control cells at 6 h showed significantly more dUTP-labeled green nuclei (Fig. 6C and E) than H2O2- or CPT-treated LmAPX-overexpressing cells (Fig. 6D and F), indicating that a larger amount of DNA fragmentation occurred in H2O2- or CPT-treated control cells. On the basis of dUTP-labeled cell counting, LmAPX overexpression reduced by around 85% and 80% TUNEL-positive cells compared to control cells after H2O2 and CPT treatment, respectively (data not shown). The results for overexpressing cells with H2O2 or CPT treatment indicated that there was 4 to 5 times more dUTP-labeled DNA in control cells than in LmAPX-overexpressing cells. Furthermore, in the presence of VAD-fmk, H2O2- or CPT-induced fragmentation of nuclear DNA was inhibited and there was less labeling with FITC-conjugated dUTP in both control and overexpressing cells (Fig. 6G and H). These results suggested that greater DNA fragmentation occurred in H2O2- or CPT-treated control cells than in LmAPX-overexpressing cells.
DISCUSSION
LmAPX is an important enzyme for ROS detoxification and protection against cardiolipin oxidation under oxidative stress (18). Though plant APX has been extensively characterized and has been shown to be responsive to several environmental stresses (37, 43, 52, 59), the exact physiological function of the mitochondrial LmAPX enzyme has not been yet clarified. The work presented here indicates, for the first time, that LmAPX functions to protect the Leishmania parasite from oxidative stress-induced cell death. The protective function of LmAPX is mediated by detoxification of excess ROS burden, which is an early and critical event in preventing release of Ca2+, loss of mitochondrial potential, and cytochrome c and endonuclease G release from mitochondria to the cytosol.
Considerable evidence has accumulated to suggest that ROS (e.g., hydrogen peroxide and NO) may act as mediators of apoptosis in a variety of cell types (2, 30, 42). Although mitochondrial respiration, enzymatic reactions (aconitase and α-ketoglutarate dehydrogenase) of the tricarboxylic acid cycle, the microsomal cytochrome P450 system, and plasma membrane NADPH oxidase generate intracellular ROS, the ROS from mitochondria are responsible for a close association between the activities of mitochondria and cell death (35). Indeed, H2O2 or CPT treatment of L. donovani control cells results in mitochondrial dysfunction and is accompanied by cellular death (16, 38, 48). It has been previously shown that LmAPX is localized exclusively in the mitochondria (18) and utilizes H2O2 and reduced cytochrome c in the reaction cycle; hence, it may act directly on mitochondria and preserve these organelles, which represent one of the main targets for ROS-induced damage.
It has long been known that catalase and classical selenium-containing GSH peroxidase, two major hydroperoxide-eliminating enzymes generally present in eukaryotes, are missing in trypanosomatid genome sequences. Instead, mainly three distinct families of peroxidase (2-Cys peroxiredoxins, nonselenium GSH peroxidase-like enzymes, and APXs) have been shown to be crucial in elimination of hydroperoxide and peroxynitrite in trypanosomatids (12). By knockdown analysis of trypanothione synthetase, it was suggested that this enzyme is essential for T. brucei survival (5). The trypanothione-reducing enzyme trypanothione reductase has been shown to be critical for survival and/or infectivity of L. donovani and T. brucei (39). Another group of highly abundant redox proteins, tryparedoxins, have diverse cell functions, such as peroxide metabolism, synthesis of deoxynucleotides, and regulation of mitochondrial DNA replication (19, 40). The results from studies with peroxiredoxin-overexpressing Leishmania and Trypanosoma cruzi cells and double-stranded RNA interference studies with T. brucei show that peroxiredoxins have an important role in eliminating hydroperoxides and peroxynitrite (8, 33, 55). In eukaryotes, peroxiredoxins are considered to act as a regulator of H2O2-mediated intracellular signaling processes and not as general antioxidant devices. Considerable evidence has suggested that nonselenium GSH peroxidase-like enzymes in trypanosomatids metabolize fatty acid and phospholipid hydroperoxides (56), although the activity is very low compared to that of selenium-containing GSH peroxidase. Leishmania also possess trypanothione S transferase activity associated with the eukaryotic translation elongation factor 1B, which reacts preferentially with linoleic acid hydroperoxide but not with H2O2 (54). Recent reports also demonstrate that overexpression of APX within Leishmania and T. cruzi is more resistant to exogenously added H2O2 (18, 57). This heme protein is involved in the elimination of H2O2 with a very high catalytic efficiency (k1 = 6.7 × 107 M−1 s−1) (58), and it acts as a pseudocatalase in the presence of low concentration of ascorbate (17). Because LmAPX can oxidize ferrocytochrome c in the presence of endogenous H2O2, it is an excellent candidate for playing dual roles in mitochondria by elimination of both H2O2 and superoxide.
Recently we have proposed that cyclic oxidation/reduction of the cytochrome c by the LmAPX system may function as a ROS-scavenging system (18, 58). In mammalian cells, an increase in cellular ROS production has been claimed to be responsible for cell death (53). From this study, it is established that overexpression of LmAPX in Leishmania cells clearly protects cells against H2O2 (an exogenous oxidant) or CPT (an endogenous oxidant)-mediated mitochondrial apoptotic cell death. It is well known that apoptosis in mammalian cells has been tightly linked to activation of caspases (45), which are missing in the Leishmania genome sequence. It is notable that ROS act as mediators in many cases of caspase-like protein-dependent as well as -independent DNA fragmentation in Leishmania cells (10, 21). Another peculiar aspect emerging from our experiments is the fact that although the TUNEL assay suggests that oxidant-induced DNA cleavage is blocked by overexpression of LmAPX, DNA degradation could not be completely blocked by using a caspase inhibitor. The reason for this discrepancy could be an involvement of oxidant-dependent caspase-like protease activation on one hand (6, 16), but on the other hand it suggests the existence of some alternative oxidant-dependent apoptotic pathway (endonuclease G dependent) leading to nuclear fragmentation in Leishmania (9, 21, 47). Our endonuclease G release data presented in Fig. 4C and D clearly confirmed the data obtained by TUNEL assay.
Mitochondria are both producers and targets of ROS, and an increase of ROS is known to trigger cells to undergo apoptosis through the activation of caspases, especially caspase 3 (10). The prevention of phosphatidylserine exposure and DNA fragmentation in the LmAPX-overexpressing cells after exposure to oxidative stress indicates their ability to resist proapoptotic changes induced by oxidative stress. In the absence of Bcl2 family proteins in Leishmania that regulate apoptosis induced by diverse stimuli in human host cells, LmAPX appears to be an important protein in ROS-induced apoptosis regulation. Thus, understanding the molecular function of single-copy mitochondrial LmAPX provides opportunities for discovering and evaluating molecular targets for drug design, which now forms a rational basis for the development of improved therapy against leishmaniasis.
Supplementary Material
Acknowledgments
We thank S. M. Beverley for providing pXG-B2863 vector, A. K. Datta for L. donovani adenosine kinase antibody, H. K. Majumder for L. donovani endonuclease G antibody, and Andre Schneider (University of Berne, Switzerland) for T. brucei cytochrome c antibody. We thank Arunima Biswas for her help with flow cytometry.
This work was supported by Council of Scientific and Industrial Research (CSIR) project NWP 0038 and CSIR fellowships (to S.D., R.K.Y., and S.P.).
Footnotes
Published ahead of print on 11 September 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Adak, S., and A. K. Datta. 2005. Leishmania major encodes an unusual peroxidase that is a close homologue of plant ascorbate peroxidase: a novel role of the transmembrane domain. Biochem. J. 390:465-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albina, J. E., S. Cui, R. B. Mateo, and J. S. Reichner. 1993. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J. Immunol. 150:5080-5085. [PubMed] [Google Scholar]
- 3.Al-Olayan, E. M., G. T. Williams, and H. Hurd. 2002. Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int. J. Parasitol. 32:1133-1143. [DOI] [PubMed] [Google Scholar]
- 4.Ameisen, J. C., T. Idziorek, O. Billaut-Mulot, M. Loyens, J. P. Tissier, A. Potentier, and A. Ouaissi. 1995. Apoptosis in a unicellular eukaryote (Trypanosoma cruzi): implications for the evolutionary origin and role of programmed cell death in the control of cell proliferation, differentiation and survival. Cell Death Differ. 2:285-300. [PubMed] [Google Scholar]
- 5.Ariyanayagam, M. R., S. L. Oza, M. L. Guther, and A. H. Fairlamb. 2005. Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome. Biochem. J. 391:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnoult, D., K. Akarid, A. Grodet, P. X. Petit, J. Estaquier, and J. C. Ameisen. 2002. On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 9:65-81. [DOI] [PubMed] [Google Scholar]
- 7.Balaban, R. S., S. Nemoto, and T. Finkel. 2005. Mitochondria, oxidants, and aging. Cell 120:483-495. [DOI] [PubMed] [Google Scholar]
- 8.Barr, S. D., and L. Gedamu. 2003. Role of peroxidoxins in Leishmania chagasi survival. Evidence of an enzymatic defense against nitrosative stress. J. Biol. Chem. 278:10816-10823. [DOI] [PubMed] [Google Scholar]
- 9.BoseDasgupta, S., B. B. Das, S. Sengupta, A. Ganguly, A. Roy, S. Dey, G. Tripathi, B. Dinda, and H. K. Majumder. 2008. The caspase-independent algorithm of programmed cell death in Leishmania induced by baicalein: the role of LdEndoG, LdFEN-1 and LdTatD as a DNA ‘degradesome.’ Cell Death Differ. 15:1629-1640. [DOI] [PubMed] [Google Scholar]
- 10.Burkle, A. 2005. Poly(ADP-ribose). The most elaborate metabolite of NAD+. FEBS J. 272:4576-4589. [DOI] [PubMed] [Google Scholar]
- 11.Cadenas, E. 2004. Mitochondrial free radical production and cell signaling. Mol. Aspects Med. 25:17-26. [DOI] [PubMed] [Google Scholar]
- 12.Castro, H., and A. M. Tomas. 2008. Peroxidases of trypanosomatids. Antioxid. Redox Signal. 10:1593-1606. [DOI] [PubMed] [Google Scholar]
- 13.Chan, J., T. Fujiwara, P. Brennan, M. McNeil, S. J. Turco, J. C. Sibille, M. Snapper, P. Aisen, and B. R. Bloom. 1989. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Natl. Acad. Sci. USA 86:2453-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Channon, J. Y., and J. M. Blackwell. 1985. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. II. Possible mechanisms involved in protective H2O2 scavenging. Parasitology 91:207-217. [DOI] [PubMed] [Google Scholar]
- 15.Dalle-Donne, I., D. Giustarini, R. Colombo, R. Rossi, and A. Milzani. 2003. Protein carbonylation in human diseases. Trends Mol. Med. 9:169-176. [DOI] [PubMed] [Google Scholar]
- 16.Das, M., S. B. Mukherjee, and C. Shaha. 2001. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J. Cell Sci. 114:2461-2469. [DOI] [PubMed] [Google Scholar]
- 17.Dolai, S., R. K. Yadav, A. K. Datta, and S. Adak. 2007. Effect of thiocyanate on the peroxidase and pseudocatalase activities of Leishmania major ascorbate peroxidase. Biochim. Biophys. Acta 1770:247-256. [DOI] [PubMed] [Google Scholar]
- 18.Dolai, S., R. K. Yadav, S. Pal, and S. Adak. 2008. Leishmania major ascorbate peroxidase overexpression protects cells against reactive oxygen species-mediated cardiolipin oxidation. Free Radic. Biol. Med. 45:1520-1529. [DOI] [PubMed] [Google Scholar]
- 19.Dormeyer, M., N. Reckenfelderbaumer, H. Ludemann, and R. L. Krauth-Siegel. 2001. Trypanothione-dependent synthesis of deoxyribonucleotides by Trypanosoma brucei ribonucleotide reductase. J. Biol. Chem. 276:10602-10606. [DOI] [PubMed] [Google Scholar]
- 20.Engelberg-Kulka, H., and G. Glaser. 1999. Addiction modules and programmed cell death and antideath in bacterial cultures. Annu. Rev. Microbiol. 53:43-70. [DOI] [PubMed] [Google Scholar]
- 21.Gannavaram, S., C. Vedvyas, and A. Debrabant. 2008. Conservation of the pro-apoptotic nuclease activity of endonuclease G in unicellular trypanosomatid parasites. J. Cell Sci. 121:99-109. [DOI] [PubMed] [Google Scholar]
- 22.Gantt, K. R., T. L. Goldman, M. L. McCormick, M. A. Miller, S. M. Jeronimo, E. T. Nascimento, B. E. Britigan, and M. E. Wilson. 2001. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J. Immunol. 167:893-901. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh, S., S. Goswami, and S. Adhya. 2003. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochem. J. 369:447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb, R. A. 2001. Mitochondria and apoptosis. Biol. Signals Recept. 10:147-161. [DOI] [PubMed] [Google Scholar]
- 25.Grassme, H., V. Jendrossek, and E. Gulbins. 2001. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 6:441-445. [DOI] [PubMed] [Google Scholar]
- 26.Harder, S., M. Bente, K. Isermann, and I. Bruchhaus. 2006. Expression of a mitochondrial peroxiredoxin prevents programmed cell death in Leishmania donovani. Eukaryot. Cell 5:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 28.Hockenbery, D. M., Z. N. Oltvai, X. M. Yin, C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241-251. [DOI] [PubMed] [Google Scholar]
- 29.Iyer, J. P., A. Kaprakkaden, M. L. Choudhary, and C. Shaha. 2008. Crucial role of cytosolic tryparedoxin peroxidase in Leishmania donovani survival, drug response and virulence. Mol. Microbiol. 68:372-391. [DOI] [PubMed] [Google Scholar]
- 30.Kane, D. J., T. A. Sarafian, R. Anton, H. Hahn, E. B. Gralla, J. S. Valentine, T. Ord, and D. E. Bredesen. 1993. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262:1274-1277. [DOI] [PubMed] [Google Scholar]
- 31.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters, J. J., T. Qian, C. A. Bradham, D. A. Brenner, W. E. Cascio, L. C. Trost, Y. Nishimura, A. L. Nieminen, and B. Herman. 1999. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J. Bioenerg. Biomembr. 31:305-319. [DOI] [PubMed] [Google Scholar]
- 33.Lin, Y. C., J. Y. Hsu, S. C. Chiang, and S. T. Lee. 2005. Distinct overexpression of cytosolic and mitochondrial tryparedoxin peroxidases results in preferential detoxification of different oxidants in arsenite-resistant Leishmania amazonensis with and without DNA amplification. Mol. Biochem. Parasitol. 142:66-75. [DOI] [PubMed] [Google Scholar]
- 34.Madeo, F., E. Frohlich, M. Ligr, M. Grey, S. J. Sigrist, D. H. Wolf, and K. U. Frohlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mignotte, B., and J. L. Vayssiere. 1998. Mitochondria and apoptosis. Eur. J. Biochem. 252:1-15. [DOI] [PubMed] [Google Scholar]
- 36.Miller, M. A., S. E. McGowan, K. R. Gantt, M. Champion, S. L. Novick, K. A. Andersen, C. J. Bacchi, N. Yarlett, B. E. Britigan, and M. E. Wilson. 2000. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J. Biol. Chem. 275:33883-33889. [DOI] [PubMed] [Google Scholar]
- 37.Mittler, M., and B. A. Zilinskas. 1994. Regulation of pea cytosolic ascorbate peroxidase and other antioxidants enzymes during the progression of drought stress and following recovery from drought. Plant J. 5:397-405. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee, S. B., M. Das, G. Sudhandiran, and C. Shaha. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717-24727. [DOI] [PubMed] [Google Scholar]
- 39.Muller, S., E. Liebau, R. D. Walter, and R. L. Krauth-Siegel. 2003. Thiol-based redox metabolism of protozoan parasites. Trends Parasitol. 19:320-328. [DOI] [PubMed] [Google Scholar]
- 40.Onn, I., N. Milman-Shtepel, and J. Shlomai. 2004. Redox potential regulates binding of universal minicircle sequence binding protein at the kinetoplast DNA replication origin. Eukaryot. Cell 3:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piacenza, L., F. Irigoin, M. N. Alvarez, G. Peluffo, M. C. Taylor, J. M. Kelly, S. R. Wilkinson, and R. Radi. 2007. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem. J. 403:323-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 43.Prasad, T. K., M. D. Anderson, B. A. Martin, and C. R. Stewart. 1994. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee, S. G., K. S. Yang, S. W. Kang, H. A. Woo, and T. S. Chang. 2005. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 7:619-626. [DOI] [PubMed] [Google Scholar]
- 45.Rich, T., C. J. Watson, and A. Wyllie. 1999. Apoptosis: the germs of death. Nat. Cell Biol. 1:E69-E71. [DOI] [PubMed] [Google Scholar]
- 46.Richter, C., M. Schweizer, A. Cossarizza, and C. Franceschi. 1996. Control of apoptosis by the cellular ATP level. FEBS Lett. 378:107-110. [DOI] [PubMed] [Google Scholar]
- 47.Rico, E., J. F. Alzate, A. A. Arias, D. Moreno, J. Clos, F. Gago, I. Moreno, M. Dominguez, and A. Jimenez-Ruiz. 2009. Leishmania infantum expresses a mitochondrial nuclease homologous to EndoG that migrates to the nucleus in response to an apoptotic stimulus. Mol. Biochem. Parasitol. 163:28-38. [DOI] [PubMed] [Google Scholar]
- 48.Sen, N., B. B. Das, A. Ganguly, T. Mukherjee, G. Tripathi, S. Bandyopadhyay, S. Rakshit, T. Sen, and H. K. Majumder. 2004. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 11:924-936. [DOI] [PubMed] [Google Scholar]
- 49.Shaha, C. 2006. Apoptosis in Leishmania species and its relevance to disease pathogenesis. Indian J. Med. Res. 123:233-244. [PubMed] [Google Scholar]
- 50.Simizu, S., M. Takada, K. Umezawa, and M. Imoto. 1998. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J. Biol. Chem. 273:26900-26907. [DOI] [PubMed] [Google Scholar]
- 51.Tagliarino, C., J. J. Pink, G. R. Dubyak, A. L. Nieminen, and D. A. Boothman. 2001. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J. Biol. Chem. 276:19150-19159. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka, K., Y. Suda, N. Kondo, and K. Sugahara. 1985. O3 tolerance and the ascorbate-dependent H2O2 decomposing system in chloroplasts. Plant Cell Physiol. 26:1425-1431. [Google Scholar]
- 53.Vander Heiden, M. G., N. S. Chandel, E. K. Williamson, P. T. Schumacker, and C. B. Thompson. 1997. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91:627-637. [DOI] [PubMed] [Google Scholar]
- 54.Vickers, T. J., S. Wyllie, and A. H. Fairlamb. 2004. Leishmania major elongation factor 1B complex has trypanothione S-transferase and peroxidase activity. J. Biol. Chem. 279:49003-49009. [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson, S. R., D. Horn, S. R. Prathalingam, and J. M. Kelly. 2003. RNA interference identifies two hydroperoxide metabolizing enzymes that are essential to the bloodstream form of the African trypanosome. J. Biol. Chem. 278:31640-31646. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson, S. R., D. J. Meyer, and J. M. Kelly. 2000. Biochemical characterization of a trypanosome enzyme with glutathione-dependent peroxidase activity. Biochem. J. 352:755-761. [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkinson, S. R., S. O. Obado, I. L. Mauricio, and J. M. Kelly. 2002. Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 99:13453-13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav, R. K., S. Dolai, S. Pal, and S. Adak. 2008. Role of tryptophan-208 residue in cytochrome c oxidation by ascorbate peroxidase from Leishmania major-kinetic studies on Trp208Phe mutant and wild type enzyme. Biochim. Biophys. Acta 1784:863-871. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura, K., Y. Yabuta, T. Ishikawa, and S. Shigeoka. 2000. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 123:223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zangger, H., J. C. Mottram, and N. Fasel. 2002. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ. 9:1126-1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.