Abstract
16S rRNA gene libraries from the lithoautotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture described by Straub et al. (K. L. Straub, M. Benz, B. Schink, and F. Widdel, Appl. Environ. Microbiol. 62:1458-1460, 1996) were dominated by a phylotype related (95% 16S rRNA gene homology) to the autotrophic Fe(II) oxidizer Sideroxydans lithotrophicus. The libraries also contained phylotypes related to known heterotrophic nitrate reducers Comamonas badia, Parvibaculum lavamentivorans, and Rhodanobacter thiooxidans. The three heterotrophs were isolated and found to be capable of only partial (12 to 24%) Fe(II) oxidation, suggesting that the Sideroxydans species has primary responsibility for Fe(II) oxidation in the enrichment culture.
A variety of microorganisms oxidize Fe(II) with nitrate under anaerobic, circumneutral pH conditions (29) and may contribute to an active microbially driven anoxic Fe redox cycle (1, 27-29, 31, 32). Straub et al. (28) obtained the first Fe(II)-oxidizing, nitrate-reducing (enrichment) culture capable of fully autotrophic growth by a reaction such as 5Fe2+ + NO3− + 12H2O → 5Fe(OH)3 + 0.5N2 + 9H+. This process has since been demonstrated in detail with the hyperthermophilic archaeon Ferroglobus placidus (9) and with the mesophilic Proteobacteria Chromobacterium violacens strain 2002 (34) and Paracoccus ferrooxidans strain BDN-1 (16). Nitrate-dependent Fe(II) oxidation in the presence of fixed carbon has been documented for Dechlorosoma suillum strain PS (4), Geobacter metallireducens (7), Desulfitobacterium frappieri (23), and Acidovorax strain BoFeN1 (15). In addition to oxidizing insoluble Fe(II)-bearing minerals (33), the enrichment culture described by Straub et al. (28) is the only autotrophic Fe(II)-oxidizing, nitrate-reducing culture capable of near-complete oxidation of uncomplexed Fe(II) with reduction of nitrate to N2. During Fe(II) oxidation, F. placidus reduces nitrate to nitrite, which may play a significant role in overall Fe(II) oxidation. Although both C. violacens and Paracoccus ferrooxidans reduce nitrate to N2, C. violacens oxidizes only 20 to 30% of the initial Fe(II), and P. ferrooxidans uses FeEDTA2− but not free (uncomplexed) Fe(II) in medium analogous to that used for cultivation of the enrichment culture described by Straub et al. (28). The enrichment culture described by Straub et al. (28) is thus the most robust culture capable of autotrophic growth coupled to nitrate-dependent Fe(II) oxidation available at present. The composition and activity of this culture was investigated with molecular and cultivation techniques. The culture examined is one provided by K. L. Straub to E. E. Roden in 1998 for use in studies of nitrate-dependent oxidation of solid-phase Fe(II) compounds (33) and has been maintained in our laboratory since that time.
Fe(II) oxidation experiments.
The enrichment culture was grown with 10 mM FeCl2·H2O and 4 mM KNO3 (28) in defined, bicarbonate-buffered (pH 6.8) medium (35) at 30°C. Fe(II) oxidation experiments were conducted with either nitrate or nitrite. Nitrite was added periodically to achieve final concentrations of less than 1 mM to prevent chemical oxidation of Fe(II). Total Fe(II) concentrations were determined by 0.5 M HCl extraction and Ferrozine (26) analysis. Nitrate, nitrite, and acetate levels were determined by ion chromatography. Aqueous-phase samples were exposed to air to promote rapid chemical Fe(II) oxidation, centrifuged at 10,000 × g for 5 min, and filtered through a 0.2-μm nylon filter. Cell numbers were determined with acridine orange (13) after Fe(III) oxides were dissolved with ammonium oxalate (13, 18). The ability of the enrichment culture to grow under microaerophilic conditions was evaluated using Fe(II)-O2 opposing-gradient cultures (25).
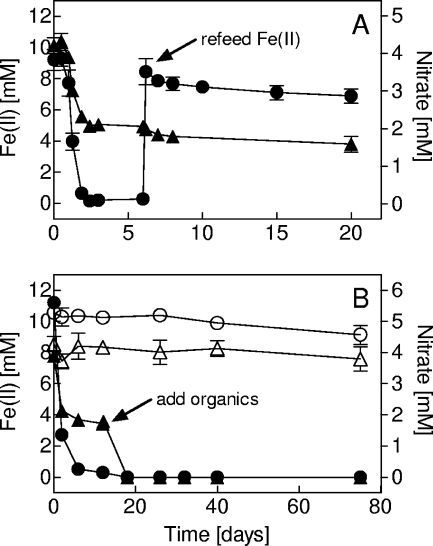
The culture consistently oxidized more than 90% of the added Fe(II) with nitrate (Fig. 1A). Approximately 3 × 107 cells were produced per μmol Fe(II) oxidized, similar to values reported for other Fe(II)-oxidizing bacteria (FeOB) (21, 24). No further reduction of nitrate was observed once Fe(II) oxidation stopped. The molar ratio of Fe(II) oxidized to nitrate reduced was ca. 5:1, in agreement with results of previous studies (28, 33). Fe(II) oxidation with nitrite was slower (Fig. 1B), with a molar ratio of ca. 2.5, close to that expected based on the following reaction: 3Fe2+ + 1NO2− + 7H+ → 3Fe(OH)3 + 0.5N2 + 5H2O. No significant Fe(II) oxidation or reduction of nitrate or nitrite was observed in the absence of cells. Although nitrate was added in excess, Fe(II) was never oxidized completely, even during prolonged incubation (several months). Oxidation of additional Fe(II) added to freshly grown, Fe(III) oxide-rich cultures was slow and incomplete (Fig. 2A). No Fe(III) reduction was observed upon the addition of organic carbon (0.5% yeast extract, 0.5% tryptic soy broth, and 0.5 mM acetate) after Fe(II) oxidation stopped, although residual nitrate was rapidly consumed (Fig. 2B). There was no evidence for growth (i.e., a distinct growth band; see reference 24) of the enrichment culture in Fe(II)-O2 opposing-gradient cultures.
FIG. 1.
(A) Cell growth and Fe(II) oxidation by the enrichment culture with nitrate as the electron acceptor. (B) Fe(II) oxidation with nitrite as the electron acceptor. Data are presented as means ± standard deviations (SD) for triplicate cultures. Filled and open symbols represent results from inoculated and uninoculated cultures, respectively. • and ○, Fe(II); ▴ and ▵, nitrate; ▪ and □, nitrite; ×, cell numbers.
FIG. 2.
Response of a freshly grown (ca. 1-week-old) enrichment culture to the addition of 8 mM Fe(II) (refeed) (A) or a combination of 0.5% yeast extract, 0.5% tryptic soy broth, and 0.5 mM acetate (add organics) (B). Data are presented as means ± SD for triplicate cultures. Symbols are as in Fig. 1.
Clone libraries.
DNA was extracted from ca. 1-week-old, stationary-phase enrichment cultures using a SoilKit from MoBio. 16S rRNA genes were amplified (20) and cloned using the pGEM-T vector (Promega). Three separate clone libraries obtained between 2005 and 2007 (total of 119 clones) revealed a consistent composition of the enrichment culture, which was transferred more than 60 times during this period. Four different operational taxonomic units (OTUs; 97% 16S rRNA gene similarity cutoff [8]) were distinguished in each library. All libraries were dominated by phylotypes OTU1 and OTU2, with frequencies of 62 to 72% and 14 to 21%, respectively. OTU1 was 95% and 94% similar to the lithoautotrophic, microaerophilic Fe(II)-oxidizing Betaproteobacteria Sideroxydans lithotrophicus (6) and Gallionella ferruginea (10), respectively. OUT2 was most closely related (94%) to Comamonas badia (Betaproteobacteria) (30). OTU3 and OTU4 were 96 and 99% similar to Parvibaculum lavamentivorans (Alphaproteobacteria) (22) and Rhodanobacter thiooxidans (Gammaproteobacteria) (17), respectively.
Isolation of heterotrophic nitrate reducers.
Heterotrophic nitrate-reducing organisms were isolated by the roll tube method (14) with 4 mM KNO3 and 5 mM acetate. Fe(II) oxidizers were isolated in the same way with 4 mM KNO3 and 10 mM FeCl2·H2O with or without 0.25 mM acetate. Roll tubes with 4 mM KNO3 and 5% H2 in the gas phase were used to evaluate potential autotrophic H2 oxidation activity. Colonies were picked and transferred to 5 ml of fresh medium inside an anaerobic chamber by using sterile Pasteur pipettes. On two occasions, 10 to 15 colonies were transferred into filter-sterilized culture medium, amended with 10 mM Fe(II) and 4 mM nitrate, from a pregrown enrichment culture. Three heterotrophic nitrate-reducing bacteria were isolated from the 10−4 dilution. None of the isolates corresponded to the heterotrophic organisms BrG1, BrG2, and BrG3 from the original enrichment culture (28, 29); instead, pure cultures with 16S rRNA gene sequences identical to OTU2, -3, and -4 from the clone libraries were obtained (Table 1). Because our culture was propagated from the original described culture, the heterotrophic community has apparently changed during 10 years of independent cultivation. As documented for strains BrG1 to -3 (29) and the related Acidovorax strain BoFeN1 (15), none of the heterotrophic isolates grew autotrophically with Fe(II). With 0.2 mM acetate, the isolates oxidized between 12 and 18% of the initial 10 mM Fe(II) (Table 1). Different combinations of isolates oxidized slightly larger amounts of Fe(II). Less than 10% oxidation occurred in the absence of acetate. In contrast to results with the enrichment culture, none of the pure or mixed cultures could be sustained for more than four transfers with or without 0.2 mM acetate. None of the pure or mixed cultures produced reddish-brown Fe(III) oxides like the enrichment culture; rather, greenish-blue phases similar to those described by Weber et al. (34) were observed.
TABLE 1.
Extent of Fe(II) oxidation and ratio of Fe(II) oxidized to nitrate reduced by the enrichment culture and heterotrophic nitrate-reducing isolates
| Culture (class and genus) or culture combination | % Fe(II) oxidized | Fe(II) oxidized/nitrate reduceda |
|---|---|---|
| Enrichment culture | 96 | 5.23 ± 0.14 |
| OTU2 (Betaproteobacteria, Comamonas sp.) | 16 | 5.10 ± 0.21 |
| OTU3 (Alphaproteobacteria, Parvibaculum sp.) | 18 | 4.85 ± 0.11 |
| OTU4 (Gammaproteobacteria, Rhodanobacter sp.) | 12 | 5.31 ± 0.15 |
| OTU2 + OTU3 | 21 | 5.03 ± 0.16 |
| OTU3 + OTU4 | 19 | 4.93 ± 0.18 |
| OTU2 + OTU4 | 23 | 4.81 ± 0.27 |
| OTU2 + OTU3 + OTU4 | 24 | 5.07 ± 0.20 |
Data are the means ± SD for triplicate cultures. The only culture that could be continuously transferred was the enrichment culture. The carbon source was 0.2 mM acetate for all cultures or combinations except the enrichment culture, in which dissolved inorganic carbon was the carbon source. All cultures contained 8 to 10 mM FeCl2·4H2O and 4 mM KNO3.
Isolation of nitrate-dependent Fe(II) oxidizers.
Single brownish colonies of putative FeOB appeared in acetate-free Fe(II)/nitrate roll tubes after 2 to 3 weeks of incubation. No growth was observed for H2-containing roll tubes. A total of 26 colonies were picked from the 10−4 and 10−6 dilutions, and acetate (0.25 mM) was added to 13 of these transfers. Eight acetate-amended cultures from the 10−4 dilution showed significant Fe(II) oxidation. No Fe(II) oxidation was observed without acetate or from 10−6 dilutions. Fe(II)-oxidizing cultures could be successfully transferred only when acetate was provided. The extent of Fe(II) oxidation declined from 40 to 70% in the first transfer to 10 to 15% in the fourth transfer, after which no Fe(II) oxidation occurred. Similar results were obtained with colonies inoculated into filter-sterilized spent media. Clone libraries (18 to 25 clones) from four Fe(II)-oxidizing cultures revealed that brownish colonies represent mixed rather than pure colonies. All recovered sequences revealed 100% similarity to either OTU1, -2, -3, or -4. OTU1 was present in all cultures, OTU2 was found in three cultures, and OTU3 and OTU4 were found in two cultures only.
Fe redox cycling coculture experiments.
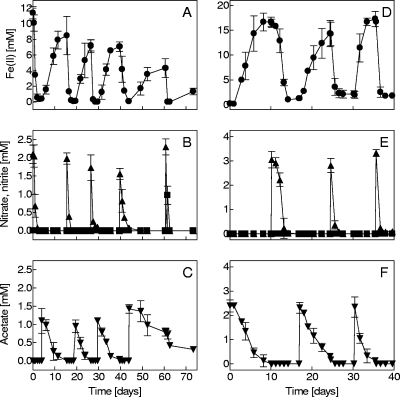
Geobacter sulfurreducens (3) was grown on acetate with autoclaved natural amorphous Fe(III) oxide from a groundwater Fe seep (2). The enrichment culture was combined with G. sulfurreducens to determine whether alternating the addition of organic carbon (acetate) and nitrate could lead to sustained cycles of Fe oxidation and reduction. G. sulfurreducens does not reduce nitrate (3); therefore, the organisms in the enrichment culture were responsible for Fe(II) oxidation in the cocultures. The first experiment was initiated by amending a freshly grown enrichment culture with a 10% inoculum of G. sulfurreducens plus ca. 1 mM acetate. A second experiment was initiated by amending a grown G. sulfurreducens culture with a 10% inoculum of the enrichment culture plus ca. 3 mM nitrate.
Alternating the addition of nitrate and acetate produced multiple cycles of Fe oxidation and reduction. The first experiment began with Fe(II) oxidation, and subsequent additions of acetate and nitrate pushed the coculture through five consecutive Fe redox cycles (Fig. 3A to C). The second experiment, beginning with Fe(III) reduction, produced three full cycles of Fe reduction and oxidation (Fig. 3D to F). Only small amounts of nitrite were detected in both experiments, a result which suggests a direct coupling between Fe(II) oxidation and nitrate reduction.
FIG. 3.
Fe redox cycling by the enrichment culture and G. sulfurreducens driven by alternating addition of acetate and nitrate. Cocultures were initiated with either Fe(II) oxidation (A to C) or Fe(III) reduction (D to F). Data are presented as means ± SD for triplicate cultures. Symbols are as in Fig. 1; acetate, ▾.
Activity of the enrichment culture.
The clone library data suggest a robust, stable microbial community in the enrichment culture. Based on the predominance of OTU1 and the physiological properties of the heterotrophic nitrate-reducing isolates (Table 1), we infer that OTU1 is the autotroph with primary responsibility for Fe(II) oxidation and for supplying the heterotrophic organisms with fixed carbon. This argument is consistent with the finding that none of the isolated heterotrophs could be transferred for more than a few generations. The role of the three heterotrophs during the autotrophic growth of the enrichment culture is not clear. Unsuccessful attempts to propagate picked colonies from roll tubes in filter-sterilized spent medium from a previously grown enrichment culture argue against production of essential secondary metabolites by the heterotrophs. One explanation that can be ruled out is that the heterotrophs carry out dissimilatory Fe(III) oxide reduction that delays or prevents encrustation of the autotroph by Fe(III) oxides: no formation of Fe(II) was observed for a freshly grown enrichment culture with additional carbon sources (Fig. 2B).
The issue of cell encrustation is potentially significant in terms of the activity of the enrichment culture. Encrustation of FeOB cell surfaces with Fe(III) oxide precipitates may limit substrate diffusion and uptake (19), and this phenomenon is commonly assumed to limit growth of neutrophilic FeOB (11, 12). Production of stalks or sheaths that serve as loci for Fe(III) oxide deposition is a recognized mechanism for avoiding/delaying cell encrustation of neutrophilic FeOB (5). Because these types of extracellular structures are not present in the enrichment culture, it is possible that binding of Fe(III) oxides by the heterotrophic organisms is responsible for retarding encrustation of the autotrophic Fe(II) oxidizer. The Fe(II) readdition experiment (Fig. 2A) indicated that grown cultures were unable to efficiently oxidize Fe(II) in the presence of abundant Fe(III) oxide precipitates. In contrast, the coculture experiments with G. sulfurreducens showed that periodic conversion of Fe(III) oxides back to Fe(II) allowed for sustained Fe(II) oxidation activity through multiple Fe redox cycles. Thus, it seems likely that Fe(III) oxide accumulation plays a role in the regulation of Fe(II) oxidation by the enrichment culture.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences from the clone libraries and pure culture isolates have been deposited into GenBank under accession numbers FN430653 to FN430670.
Acknowledgments
We thank Kristina Straub and Evgenya Shelobolina for review of early versions of the manuscript.
This research was supported by the NASA Astrobiology Institute (University of California—Berkeley node).
Footnotes
Published ahead of print on 11 September 2009.
REFERENCES
- 1.Benz, M., A. Brune, and B. Schink. 1998. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch. Microbiol. 169:159-165. [DOI] [PubMed] [Google Scholar]
- 2.Blöthe, M., and E. E. Roden. 2009. Microbial iron redox cycling in a circumneutral-pH groundwater seep. Appl. Environ. Microbiol. 75:468-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caccavo, F., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri, S. K., J. G. Lack, and J. D. Coates. 2001. Biogenic magnetite formation through anaerobic biooxidation of Fe(II). Appl. Environ. Microbiol. 67:2844-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson, D. 2000. Microbial oxidation of Fe(II) and Mn(II) at circumneutral pH, p. 31-52. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, DC.
- 6.Emerson, D., J. A. Rentz, and T. Plaia. Sideroxydans lithotrophicus, gen. nov., sp. nov. and Gallionella capsiferriformans sp. nov., oxygen-dependent ferrous iron-oxidizing bacteria that grow at circumneutral pH. Int. J. Syst. Evol. Microbiol, in press.
- 7.Finneran, K. T., M. E. Housewright, and D. R. Lovley. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510-516. [DOI] [PubMed] [Google Scholar]
- 8.Gillis, M., P. Vandamme, P. DeVos, J. Swings, and K. Kersters. 2001. Polyphasic taxonomy, p. 43-48. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer, New York, NY. [Google Scholar]
- 9.Hafenbradl, D., M. Keller, R. Dirmeier, R. Rachel, P. Rossnagel, S. Burggraf, H. Huber, and K. O. Stetter. 1996. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 166:308-314. [DOI] [PubMed] [Google Scholar]
- 10.Hallbeck, L., F. Stahl, and K. Pedersen. 1993. Phylogeny and phenotypic characterization of the stalk-forming and iron-oxidizing bacterium Gallionella ferruginea. J. Gen. Microbiol. 139:1531-1535. [DOI] [PubMed] [Google Scholar]
- 11.Hallberg, R., and F. G. Ferris. 2004. Biomineralization by Gallionella. Geomicrobiol. J. 21:325-330. [Google Scholar]
- 12.Hanert, H. H. 1992. The genus Gallionella, p. 4082-4088. In H. G. T. A. Balows, M. Dworkin, W. Harder, and K.-H. Schliefer (ed.), The prokaryotes. Springer-Verlag, New York, NY.
- 13.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3B:117-132. [Google Scholar]
- 15.Kappler, A., B. Schink, and D. K. Newman. 2005. Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology 3:235-245. [Google Scholar]
- 16.Kumaraswamy, R., K. Sjollema, G. Kuenen, M. vanLoosdrecht, and G. Muyzer. 2006. Nitrate-dependent [Fe(II)EDTA]2− oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor. Syst. Appl. Microbiol. 29:276-286. [DOI] [PubMed] [Google Scholar]
- 17.Lee, C. S., K. K. Kim, Z. Aslam, and S.-T. Lee. 2007. Rhodanobacter thiooxydans sp. nov., isolated from a biofilm on sulfur particles used in an autotrophic denitrification process. Int. J. Syst. Evol. Microbiol. 57:1775-1779. [DOI] [PubMed] [Google Scholar]
- 18.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mac Rae, I. C., and J. S. Celo. 1975. Influence of colloidal iron on respiration of a species of the genus Acinetobacter. Appl. Microbiol. 29:837-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muyzer, G., A. Teske, C. O. Wirsen, and H. W. Jannasch. 1995. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164:165-172. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer, S. C., D. Emerson, and J. P. Megonigal. 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl. Environ. Microbiol. 68:3988-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schleheck, D., B. J. Tindall, R. Rossello-Mora, and A. M. Cook. 2004. Parvibaculum lavamentivorans gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear alkylbenzenesulfonate. Int. J. Syst. Evol. Microbiol. 54:1489-1497. [DOI] [PubMed] [Google Scholar]
- 23.Shelobolina, E. S., C. Gaw-VanPraagh, and D. R. Lovley. 2003. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 20:143-156. [Google Scholar]
- 24.Sobolev, D., and E. Roden. 2004. Characterization of a neutrophilic, chemolithoautotrophic Fe(II)-oxidizing β-proteobacterium from freshwater wetland sediments. Geomicrobiol. J. 21:1.-10. [Google Scholar]
- 25.Sobolev, D., and E. E. Roden. 2001. Suboxic deposition of ferric iron by bacteria in opposing gradients of Fe(II) and oxygen at circumneutral pH. Appl. Environ. Microbiol. 67:1328-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 27.Straub, K. L., M. Benz, and B. Schink. 2001. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 34:181-186. [DOI] [PubMed] [Google Scholar]
- 28.Straub, K. L., M. Benz, B. Schink, and F. Widdel. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straub, K. L., W. A. Schonhuber, B. E. E. Buchholz-Cleven, and B. Schink. 2004. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 21:371-378. [Google Scholar]
- 30.Tago, Y., and K. Aida. 1977. Exocellular mucopolysaccharide closely related to bacterial floc formation. Appl. Environ. Microbiol. 34:308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber, K. A., L. A. Achenbach, and J. D. Coates. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4:752-764. [DOI] [PubMed] [Google Scholar]
- 32.Weber, K. A., P. F. Churchill, M. M. Urrutia, R. K. Kukkadapu, and E. E. Roden. 2006. Anaerobic redox cycling of iron by wetland sediment microorganisms. Environ. Microbiol. 8:100-113. [DOI] [PubMed] [Google Scholar]
- 33.Weber, K. A., F. W. Picardal, and E. E. Roden. 2001. Microbially-catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 35:1644-1650. [DOI] [PubMed] [Google Scholar]
- 34.Weber, K. A., J. Pollock, K. A. Cole, S. M. O'Connor, L. A. Achenbach, and J. D. Coates. 2006. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microbiol. 72:686-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widdel, F., and F. Bak. 1991. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, NY.