Abstract
Some protozoans are able to encyst as a protective response to a harmful environment. The cyst wall usually contains chitin as its main structural constituent. Acanthamoeba is an exception since its cyst wall contains cellulose. Specific cytochemical differentiation between cellulose and chitin by microscopy has not been possible due to the similarity of the constituent β-1,4-linked hexose backbones of these molecules. Thus, various fluorescent brightening agents and lectins bind to both cellulose and chitin. The identification of Acanthamoeba spp., which is based primarily on morphological and biochemical features, is labor-intensive and requires cloning and axenization. We describe a novel immunocytochemical method for identification of Acanthamoeba spp. based on selective binding of Trichoderma reesei cellulase to protozoan cyst wall cellulose. A recombinant cellulose-binding protein consisting of two cellulose-binding domains (CBDs) from T. reesei cellulases was coupled to the fluorescent dyes Alexa Fluor 350 and Alexa Fluor 568 or was labeled with biotin using EZ-Link sulfo-NHS-biotin. No staining reaction was observed with chitin-containing preparations of fungi. Thus, the recombinant CBDs can be used as a marker to distinguish between cellulose and chitin. This allows rapid identification of Acanthamoeba cyst wall cellulose in paraffin or frozen sections of infected tissues.
Laboratory diagnosis of infections with Acanthamoeba spp. is based on identification of the parasite in infected tissue. Although various techniques, including immunocytological and molecular methods, have been described, recovery of viable parasites by cultivation on agar is still the basic procedure used (16). This method is usually associated with histopathological examination of the specimen to prove tissue invasion by the parasite.
Recognition of parasites in tissue sections is often difficult and depends on the expertise of the pathologist. In addition to traditional histological staining methods, immunohistology using parasite-specific antibodies, lectin conjugates, and calcofluor white have been used for visualization of parasites in tissue sections (3).
Some protozoan parasites have the ability to protect themselves by forming a cyst wall, which is resistant to environmental stresses such as desiccation, lack of nutrients, and variations in temperature and pH. In most pathogenic protozoans studied, chitin is the carbohydrate polymer providing the required structural toughness to the cyst wall. Acanthamoeba spp. are exceptions, as their cysts are made up of cellulose. Recently, cellulose has also been identified as a cyst wall component in a closely related amoeba, Balamuthia mandrillaris (15). Cellulose consists of β-d-glucosyl units linked by β-1,4-glucosidic bonds. Chitin is very similar but contains N-acetylglucosamine as the monomer. Both polymers form very similar crystalline macroscopic structures. Specific cytochemical differentiation between cellulose and chitin by microscopy has not been possible due to the similarity of the constituent β-1,4-linked hexose backbones. This is especially true for various fluorescent brightening agents, such as calcofluor white, used as cytochemical markers in microscopic diagnosis of protozoan and fungal infections. A two-domain structural organization is often observed in cellulose-degrading enzymes. Most Trichoderma reesei cellulases consist of a catalytic domain and a cellulose-binding domain (CBD) joined by a linker. The catalytic domain contains the active site with the amino acid residues responsible for the hydrolytic mechanism. The role of the CBD is to bind to the solid cellulose. The ability of CBDs to attach to cellulose can be utilized in various applications. Individual types of CBDs can vary significantly in their properties, such as affinity, preference for crystalline or amorphous cellulose, and cross-reactivity with other similar carbohydrates (7, 8, 9, 10).
We have previously described a novel immunocytochemical method for identification of Acanthamoeba spp. based on selective binding of T. reesei cellulase to protozoan cyst wall cellulose (12). In that study we used a recombinant dimeric CBD (D-CBD) fusion protein in an indirect immunofluorescence analysis to specifically stain the cellulose and visualize its localization in the cyst wall. In preliminary studies, this method was also shown to be useful detection of parasites in tissue sections (11).
The aim of the present study was to simplify the detection method by preparing D-CBDs as fluorescent and biotinylated conjugates that could be used for direct and rapid detection of cellulose in Acanthamoeba by both fluorescence and ordinary light microscopy.
MATERIALS AND METHODS
Cellulase.
D-CBD, obtained as a recombinant fusion protein (8), was coupled to fluorescent dyes using an Alexa Fluor 350 (blue) and Alexa Fluor 568 (red) protein labeling kit (Molecular Probes, Eugene, OR) or was labeled with biotin using EZ-Link sulfo-NHS-biotin (Pierce, Rockford, IL). All coupling procedures were performed according to the methods provided by the manufacturers.
Samples of Acanthamoeba spp.
Corneal samples from humans with Acanthamoeba keratitis and tissues from mice infected by intranasal inoculation with trophozoites of Acanthamoeba spp. (13) were fixed in formalin, embedded in paraffin or in freezing medium (Tissue-tek; Miles, Naperville, IL), and sectioned (5 μm). Prior to staining, paraffin sections were deparaffinized and rehydrated using double changes of xylene and ethanol (99.9%, 95.5%, and 70%; 5 min each), while frozen sections were fixed in cold acetone for 20 min. A reference preparation of Acanthamoeba cysts was obtained from an axenic culture grown in PYG medium (2). Cysts and empty cyst walls were collected from cultures after prolonged incubation, washed three times with sterile Page's saline, and immunostained in suspension or after air drying and fixation in cold acetone for 20 min on microscope slides.
Samples of fungi.
Samples of the following species were used for investigations: for division Zygomycota and class Zygomycetes, Absidia corymbifera (6); for phylum Ascomycota and class Pezizomycetes, Aspergillus fumigatus, Aspergillus niger, and Penicillium notatum; for phylum Ascomycota and class Euascomycetes, Scedosporium apiospermum (5); for phylum Ascomycota and class Saccharomycetes, Candida albicans, Candida dubliniensis, Candida famata, Candida inconspicua, Candida kefyr, Candida rugosa, Candida sake, Candida utilis, Candida zeylanoides, Geotrichum candidum, Geotrichum capitatum, Kluyveromyces fragilis, Pichia membranaefaciens, and Pichia spp.; for phylum Basidiomycota and class Agaricomycotina, Cryptococcus neoformans; and for phylum Basidiomycota and class Urediniomycetes, Rhodotorula spp. and Sporobolomyces roseus (1, 4). Fungi were cultivated on Sabouraud agar (14). Then hyphae and spores of fungi were collected from cultures, washed three times with sterile Page's saline, and immunostained in suspension after air drying and fixation in cold acetone for 20 min on microscope slides or as frozen sections prepared in freezing medium (Tissue-tek; Miles, Naperville, IL).
D-CBD-Alexa Fluor conjugates.
For staining with D-CBD coupled to the fluorescent dyes, slides were immersed in phosphate-buffered saline (PBS), incubated with D-CBD for 30 min at room temperature, washed three times with PBS, and mounted with Vectashield nonfading medium (Vector Laboratories Inc., Burlingame, CA). For microscopy a Leica DMRB fluorescence microscope (Leica Mikroskopie und Systeme GmbH) equipped with dicroic mirror filter combinations for UV excitation light (A) and green tetramethyl rhodamine isocyanate excitation light (K3) was used. For photography, a Nikon-Kodak (Eastman Kodak Inc.) digital camera was used.
D-CBD-biotin conjugates.
In order to reduce the nonspecific reaction due to the presence of endogenous biotin in both tissues and Acanthamoeba cysts, slides with tissue sections and control cyst preparations were incubated with StreptABComplex/HRP (Dako, Denmark) for 1 h at 37°C and washed three times in PBS. Endogenous peroxidase was blocked by incubation with 5% H2O2 in PBS for 30 min at room temperature. After blocking, slides were rinsed with PBS and incubated with biotinylated D-CBD for 30 min at room temperature. Subsequent washes in three changes of PBS were followed by incubation with StreptABComplex/HRP (Dako, Denmark) for 30 min at room temperature. After a wash with PBS, 3,3-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO) was added for 15 min. Slides were rinsed with water, dried, mounted permanently with coverslips, and examined with the light microscope.
RESULTS AND DISCUSSION
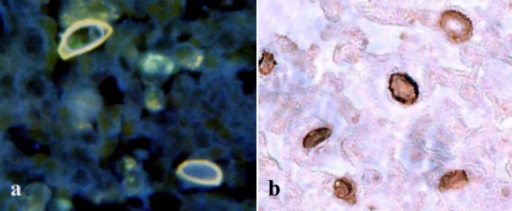
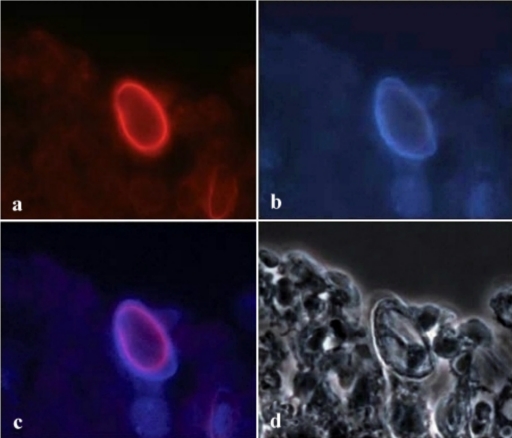
One of the problems that we encountered in the previous study was exposing the cellulose located in the inner wall of the Acanthamoeba cyst. Using paraffin or frozen sections, however, allows CBD to access the target molecules more easily. Figures 1 and 2 show that conjugated D-CBD reagents can be used for demonstration of Acanthamoeba cysts in the infected cornea of an eye (Fig. 1a and b) and in infected mouse tissues (Fig. 2a and c). D-CBD prepared as a biotinylated protein can be easily used for light microscopy (Fig. 1b). Due to the high affinity of the binding, avidin-biotin technology is widely used in histochemical procedures. However, the presence of endogenous biotin in various cells and tissues and its potential interference with the test can cause false-positive results (17). With the blocking step included in the test described here we could significantly reduce the background staining.
FIG. 1.
Localization of cellulose in the Acanthamoeba cyst wall after D-CBD staining with two different conjugates of frozen sections of corneas from a patient with keratitis. (a) Note the distinct binding to the inner cyst wall and the orange autofluorescence of the outer cyst wall after staining with the D-CBD-Alexa Fluor 350 blue conjugate. There is some blue autofluorescence of the connective tissue background. (b) Identification of Acanthamoeba cyst in corneal tissue stained with biotinylated D-CBD.
FIG. 2.
Detection of an Acanthamoeba cyst in a paraffin section of experimentally infected mouse tissue with the fluorescent D-CBD conjugate. (a) Red staining of the cyst wall with D-CBD coupled to Alexa Fluor 568. (b) Autofluorescence of Acanthamoeba cyst wall viewed under UV excitation light. (c) Alexa Fluor 568 staining superimposed on the blue autofluorescence, showing cellulase binding at the inner part of the autofluorescent cyst wall. (d) Same area as the area in panel c examined with phase-contrast illumination.
Although the additional incubations increased the time needed to complete the test, blocking resulted in satisfactory resolution of the staining. Using the fluorescent conjugates, detection of Acanthamoeba cysts is a single-step procedure, which allows rapid and specific demonstration of parasites. Alexa Fluor 568 conjugate appears to be superior to Alexa Fluor 350 in this application, especially when it is used with tissue sections. Bright autofluorescence of Acanthamoeba ectocysts with the UV excitation light interferes with the blue staining of cellulose when Alexa Fluor 350 is used (Fig. 1a and Fig. 2a and c).
The red fluorochrome in the Alexa Fluor 568 conjugate, however, offers the possibility of distinguishing between the autofluorescence of the outer wall and the specific reaction in the inner wall and thus precisely localizing cellulose in the cysts of Acanthamoeba spp. (Fig. 2b and c).
A positive staining reaction was seen only with Acanthamoeba cysts. No reactivity of D-CBD was seen with sections of fungi. The negative results for the reaction of D-CBD with fungi, which contain chitin in the cell wall, confirmed the specificity of the D-CBD of T. reesei for cellulose. The D-CBD reacts with cellulose, but it does not react with chitin. Earlier investigations showed that D-CBD does not react with chitin in walls of Entamoeba dispar, Giardia isteninalis, and Pneumocystis carinii cysts (11, 12).
The D-CBD conjugates for direct staining of Acanthamoeba cysts described here are alternative, potentially useful diagnostic tools. Also, these conjugates may be useful markers for the identification and classification of environmental amoebae.
Acknowledgments
M.D. received financial support from the Swedish Institute, and M.B.L. acknowledges support from the Academy of Finland.
Corneal samples were obtained from Elisabet Holst, Department of Infectious Diseases and Medical Microbiology, Lund University, Lund, Sweden, and tissues from infected mice were donated by Edward Hadas, Department of Biology and Medical Parasitology, Poznan University of Medical Sciences, Poznan, Poland. Fungal samples were a kind gift from Victor Fernandez, Swedish Institute for Infectious Disease Control, Stockholm, Sweden.
Footnotes
Published ahead of print on 4 September 2009.
REFERENCES
- 1.Adl, S. M., A. G. Simpson, M. A. Farmer, R. A. Andersen, O. R. Anderson, J. R. Barta, S. S. Bowser, G. Brugerolle, R. A. Fensome, S. Fredericq, T. Y. James, S. Karpov, P. Kugrens, J. Krug, C. E. Lane, L. A. Lewis, J. Lodge, D. H. Lynn, D. G. Mann, R. M. McCourt, L. Mendoza, O. Moestrup, S. E. Mozley-Standridge, T. A. Nerad, C. A. Shearer, A. V. Smirnov, F. W. Spiegel, and M. F. Taylor. 2005. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 52:99-451. [DOI] [PubMed] [Google Scholar]
- 2.American Type Culture Collection. 1993. Catalogue of protists, p. 68. American Type Culture Collection, Manassas, VA.
- 3.Bottone, E. J. 1993. Free-living amebas of genera Acanthamoeba and Naegleria: an overview and basic microbiologic correlates. Mt. Sinai J. Med. 60:260-270. [PubMed] [Google Scholar]
- 4.Eriksson, O. E., and K. Winka. 1997. Supraordinal taxa of Ascomycota. Myconet 1:1-16. http://www.fieldmuseum.org/myconet/. [Google Scholar]
- 5.Gilgado, F., J. Cano, J. Gené, D. A. Sutton, and J. Guarro. 2008. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J. Clin. Microbiol. 46:766-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann, K., S. Discher, and K. Voigt. 2007. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol. Res. 111:1169-1183. [DOI] [PubMed] [Google Scholar]
- 7.Linder, M., I. Salovuori, L. Ruohonen, and T. T. Teeri. 1996. Characterization of double cellulose-binding domain. J. Biol. Chem. 35:21268-21272. [DOI] [PubMed] [Google Scholar]
- 8.Linder, M., K. Selber, T. Nakari-Setälä, M. Qiao, M. R. Kula, and M. Penttilä. 2001. The hydrophobins HFBI and HFBII from Trichoderma reesei showing efficient interactions with nonionic surfactants in aqueous two-phase systems. Biomacromolecules 2:f511-517. [DOI] [PubMed] [Google Scholar]
- 9.Linder, M., and T. T. Teeri. 1996. The cellulose-binding domain of the major cellobiohydrolase of Trichoderma reesei exhibits true reversibility and a high exchange rate on crystalline cellulose. Proc. Natl. Acad. Sci. USA 93:12251-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder, M., and T. T. Teeri. 1997. The roles and function of cellulose-binding domains. J. Biotechnol. 57:15-28. [DOI] [PubMed] [Google Scholar]
- 11.Linder, M., J. Winiecka-Krusnell, and E. Linder. 2001. Identification of Acanthamoebae in clinical and environmental samples using cellulose-binding domains of cellulase as immunocytochemical marker for cyst wall cellulose, p. 198-201. In Proceedings of the IX International Meeting on the Biology and Pathogenicity of Free-Living Amoebae. Editions John Libbey Eurotext, Montrouge, France.
- 12.Linder, M., J. Winiecka-Krusnell, and E. Linder. 2002. Use of recombinant cellulose-binding domains of Trichoderma reesei cellulase as a selective immunocytochemical marker for cellulose in protozoa. Appl. Environ. Microbiol. 68:2503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazur, T., E. Hadas, L. Gustowska, J. Winiecka-Krusnell, and E. Linder. 1999. Secondary amoebic eye infections in mice due to Acanthamoeba spp. Parasitol. Res. 85:776-778. [DOI] [PubMed] [Google Scholar]
- 14.Sandven, P., and J. Lassen. 1999. Importance of selective media for recovery of yeasts from clinical specimens. J. Clin. Microbiol. 37:3731-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui, R., N. A. Khan, and E. L. Jarroll. 2009. The cyst wall carbohydrate composition of Balamuthia mandrillaris. Parasitol. Res. 104:1439-1443. [DOI] [PubMed] [Google Scholar]
- 16.Visvesvara, S., H. Moura, and F. Schuster. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthis mandrillaris, Naegleria fowleri and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50:1-26. [DOI] [PubMed] [Google Scholar]
- 17.Wang, H., and J. Pevsner. 1999. Detection of endogenous biotin in various tissues: novel functions in the hippocampus and implications for its use in avidin-biotin technology. Cell Tissue Res. 296:511-516. [DOI] [PubMed] [Google Scholar]