Abstract
Resistance of Nosema ceranae to different exposure conditions has been evaluated by using Sytox green and DAPI (4′,6-diamidino-2-phenylindole) to test spore viability. High thermotolerance at 60 and 35°C and resistance to desiccation were observed. However, a significant decrease in viability after freezing and a rapid degeneration of spores maintained at 4°C were also detected.
Two Nosema species have been related to pathology in the honeybee: Nosema apis (18) a parasite of Apis mellifera, the western honeybee, and Nosema ceranae (4), a parasite of Apis cerana, the eastern honeybee. Currently, however, N. ceranae is considered an emergent and important parasite of Apis mellifera (4).
Over the last few years, an increase in infections by this microsporidian has been detected in several European countries, together with an increase in honeybee colony deaths and a consequent decrease in the production of honey (9). However, it is not clear if N. ceranae infection may be the only factor related to this disorder, since this pathogen has also been found in healthy colonies (14). In Spain, Higes et al. (7, 9) have demonstrated the presence of this parasite in honeybee samples from colonies with clear signs of population depletion, relating the colony collapse disorder to N. ceranae. The presence of this microsporidian is not exclusive to Europe, since it has also been described in bee samples collected about a decade ago in the United States (3).
The pathology produced by N. ceranae in A. mellifera bees may be higher than that produced by N. apis, showing a rapid autoinfective capacity of the spores to spread the infection among epithelial cells, producing high mortality (6). On the other hand, reduced longevity of caged N. ceranae-infected worker bees compared to bees infected by N. apis has also been found (15).
To date, continuous cultures of N. ceranae are not available and there is no effective treatment. For this reason, it is important to study the effects of different exposure conditions, such as time, temperature, and desiccation, on the viability of spores kept in the laboratory for use in the search for new treatments and for development of culture protocols. In addition, as different levels of thermotolerance in the environment and different epidemiological patterns have been described for these microsporidia, available data on resistance of N. apis spores cannot be extrapolated to N. ceranae.
Spores.
Spores from N. apis and N. ceranae were provided by the experimental apiary of the Regional Apicultural Center in Marchamalo, Spain. Recently collected spores were purified as described previously (8). The spore concentration was determined by counting with a hematocytometer chamber. Two stocks of N. ceranae spores were used (S1 and S2). All studies were carried out at a concentration of 5 × 105 spores/ml. The Nosema species was determined by multiplex PCR, which amplifies the 16S rRNA locus (12).
Spore treatments.
The effects of time, temperature, and desiccation on viability were measured at different points. All studies were carried out in duplicate.
The effect of temperature on N. ceranae spores (S1) maintained in 1× phosphate-buffered saline (PBS) was measured after treatment for 1, 2, 4, and 6 h at 60°C or 2 h at 35°C or after having been autoclaved. Viability was again tested on the same batch of spores after treatment 1 month later, maintaining the spores at 4°C in 1× PBS. To study the effect of freezing on spores of N. ceranae, they were maintained at −20°C with RPMI medium plus 10% dimethyl sulfoxide (DMSO) or with fetal bovine serum (FBS) plus 10% DMSO or were air dried with no media for 1 week or 3 months. The effect of desiccation was determined with air-dried spores maintained at room temperature (RT). The viability was measured at 12 h, 48 h, and 1 week later. The viability of N. ceranae spores (S2) and N. apis, maintained in 1× PBS at 4°C for 12 months, was also studied. In each group, a negative control consisting of spores kept at 4°C was used to compare the evolution of viability.
Staining procedure.
A modification of the method of Green et al. (5) was used. Aliquots of 50 μl of spores at 5 × 105 spores/ml in H2O were added to 50 μl of 1 μM Sytox green (Molecular Probes, Inc.) and incubated for 20 min at RT. The spores were then washed once in H2O, 100 μl of 2 μg/ml of DAPI (4′,6-diamidino-2-phenylindole) was added, and the mixture was incubated for 30 min at RT. After washing in H2O, spores were diluted in 50 μl of H2O and 15 μl was spotted, in duplicate, onto slides. The slides were allowed to dry and then viewed under oil, using a fluorescent microscope. Dead spores were counted as yellow-green ovals through the 470- to 490-nm excitation wavelength filter used for viewing Sytox green staining (5), and the total number of spores was counted as turquoise ovals through the 395- to 415-nm excitation wavelength filter used for viewing DAPI staining. To differentiate extruded spores, not visible by either Sytox green or DAPI staining, white-light microscopy was employed during the viewing. In each staining, at least 20 fields per well were counted, differentiating live, dead, and extruded spores in each field. The spores of each type were expressed in percentages. A correlation between dual staining and the infectivity of spores was previously established (data not shown).
Statistical analyses.
The chi-square test (SPSS 15.0) was used to compare viability of spores after different treatments, with the spore control kept in PBS at 4°C (time zero).
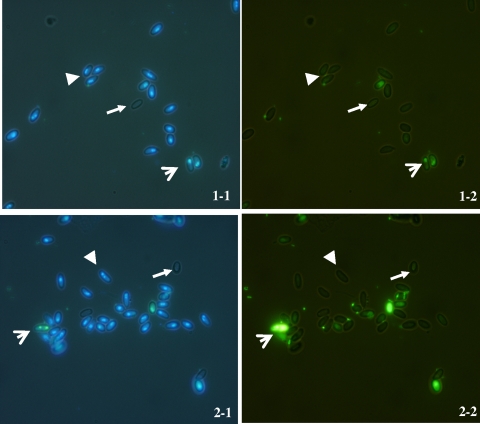
The procedure protocol allowed us to observe differences among live, dead, and extruded spores, which could be quantified in all cases (Fig. 1). The correlation between dual staining and infectivity of spores showed no statistical differences (P = 0.903).
FIG. 1.
N. ceranae spores, shown by dual staining with DAPI (1-1 and 2-1) and Sytox green (1-2 and 2-2) at ×100. Live spores, ; extruded spores,
; extruded spores, ; dead spores
; dead spores .
.
The autoclave treatment completely inactivated N. ceranae spores, as expected. However, the treatment at 60°C and 35°C for 2 h was not able to reduce N. ceranae viability. The same results were observed when the same treatment was applied to the same stock of spores 1 month later (Table 1). Moreover, treatment for 2, 3, 4, and 6 h at 60°C did not reduce the viability of N. ceranae spores significantly (Table 1).
TABLE 1.
Viability of Nosema spores after different treatments
| Microsporidiana | Medium or condition | Temp (°C) | Time | Mean % spore viability (SD)b |
||
|---|---|---|---|---|---|---|
| Live | Dead | Extruded | ||||
| N. ceranae (S1) | PBS | 4 | 0 h | 94.4 (0.06) | 5.6 (0.06) | 0.0 (0.00) |
| 1 wk | 92.4 (0.30) | 7.6 (0.70) | 0.0 (0.00) | |||
| 1 mo | 92.4 (0.36) | 7.6 (0.36) | 0.0 (0.00) | |||
| 3 mo | 87.9* (1.06) | 12.1 (1.06) | 0.0 (0.00) | |||
| 35 | 2 h | 93.9 (0.56) | 6.1 (1.10) | 0.0 (0.00) | ||
| 1 mo | 92.0 (1.80) | 7.5 (0.91) | 0.5 (0.00) | |||
| 60 | 1 h | 93.8 (0.16) | 6.2 (0.16) | 0.0 (0.00) | ||
| 1 mo | 94.0 (0.66) | 6.0 (0.66) | 0.0 (0.00) | |||
| 2 h | 94.1 (1.00) | 5.9 (1.00) | 0.0 (0.00) | |||
| 1 mo | 94.0 (2.40) | 6.0 (2.40) | 0.0 (0.00) | |||
| 4 h | 93.3 (0.33) | 6.7 (0.33) | 0.0 (0.00) | |||
| 1 mo | 93.2 (1.06) | 6.6 (1.26) | 0.2 (0.00) | |||
| 6 h | 92.1 (1.31) | 7.9 (1.31) | 0.0 (0.00) | |||
| 1 mo | 92.3 (0.81) | 7.7 (1.04) | 0.9 (0.20) | |||
| Autoclaved | 30 min | 0.0 (0.00) | 96.6 (0.64) | 3.4 (0.64) | ||
| RPMI | Freezing | 1 wk | 87.5* (0.52) | 12.5 (0.05) | 0.0 (0.00) | |
| 3 wk | 84.1* (0.18) | 15.9 (0.18) | 0.0 (0.00) | |||
| FBS | 1 wk | 89.4* (1.55) | 10.6 (1.55) | 0.0 (0.00) | ||
| 3 wk | 88.4* (4.81) | 11.6 (4.81) | 0.0 (0.00) | |||
| Air dried | 1 wk | 82.5* (0.14) | 17.5 (0.07) | 0.3 (0.20) | ||
| 3 wk | 81.6* (0.93) | 18.3 (0.93) | 0.1 (0.00) | |||
| RT | 12 h | 94.5 (1.10) | 5.5 (1.10) | 0.0 (0.00) | ||
| 48 h | 93.7 (0.66) | 6.3 (0.73) | 0.0 (0.00) | |||
| 1 wk | 93.5 (2.40) | 6.5 (3.60) | 0.0 (0.00) | |||
| N. apis | PBS | 4 | 0 h | 95.2 (1.10) | 4.8 (1.10) | 0.0 (0.00) |
| 6 mo | 64.0* (3.00) | 27.0 (5.00) | 90.0 (1.00) | |||
| 12 mo | 25.0* (2.42) | 65.0 (5.43) | 10.0 (2.96) | |||
| N. ceranae (S2) | 0 h | 94.4 (1.06) | 5.6 (1.06) | 0.0 (0.00) | ||
| 6 mo | 67.0* (2.50) | 33.0 (2.25) | 0.0 (0.00) | |||
| 12 mo | 25.0* (2.71) | 71.0 (3.05) | 4.0 (0.89) | |||
As a negative control, purified spores of N. ceranae were kept at 4°C in PBS during the experiment.
*, significant difference compared with 4°C for 0 h.
In the study of the effect of freezing and the air-dried treatment, the main variations were observed with freezing treatment, in which significant differences were detected in both cases. However, higher differences were observed in the case of freezing in RPMI medium for 1 week and 3 months (P < 0.005, F = 21.92, and P < 0.005, F = 9.4, respectively [chi-square test, SPSS 15.0]). When freezing in FBS medium, better viability results were obtained, although significant differences from the control spores were observed at 1 week and 3 months of treatment (P < 0.005, F = 10.51, and P < 0.015, F = 6.96, respectively [chi-square test, SPSS 15.0]). In the case of directly freezing air-dried spores for 1 week and 3 months, significant reductions of viability to 82.5 and 81.6% were observed (P < 0.005, F = 9.80, and P < 0.005, F = 30.14, respectively [chi-square test, SPSS 15.0]) (Table 1).
When the stability of purified N. ceranae spores in PBS at 4°C was studied (S1), a significant reduction to 87.9% was observed in the third month (P < 0.005, F = 7.727 [chi-square test, SPSS 15.0]) (Table 1). In addition, the viability of N. ceranae (S2) and N. apis spores kept at 4°C for 6 months was also studied, resulting in reductions to 67% and 64% viability, respectively. N. apis showed a higher tendency to extrude the spores kept at 4°C, not exceeding 20% at any time tested, with significant differences at 8.5 months (P < 0.005, F = 17.068) (Table 1).
Methods of measuring the viability of microsporidia spores are scarce, and the majority are slow and complex. In this study, the viability of Nosema spores was measured using a modification of dual staining described by Green et al. (5), which combines the nucleic acid stain Sytox green and the chitin stain calcofluor M2R to assess the viability of Encephalitozoon cuniculi microsporidia spores. The original method does not allow visualization of the presence of extruded spores, as calcofluor stains live and extruded spores. Here, the procedure developed allowed us to differentiate extruded spores not visible by either the Sytox green or DAPI stains. For this purpose, white-light microscopy was employed throughout the viewing (Fig. 1).
To ascertain the survival capability of N. ceranae, different studies of resistance to temperature and desiccation were carried out. A control consisting of spores kept at 4°C was used to compare the evolution of viability. Our data showed a moderate preservation of the viability of the spores (around 90%) after treatment at 60°C for 6 h, which inactivates another intracellular parasite (Cryptosporidium). Similar results were observed 1 month later. The maintenance of the viability of the spores of N. ceranae after treatments at 60°C and 35°C suggests good resistance to high temperatures. However, heating N. apis spores for at least 15 min at 60°C completely kills the parasite (2). This is in agreement with previous studies, in which a better adaptation of N. ceranae than N. apis to temperature has been observed. Martín-Hernández et al. (12) and Higes et al. (7) described the higher prevalence of N. ceranae-infected honeybees in all four seasons, compared with N. apis infection, which is more prevalent in milder seasons such as the spring and autumn. Moreover, Martín-Hernández et al. (13) confirmed the different epidemiological patterns between the two species. They found a better adaptation of N. ceranae than N. apis to temperature.
Studies related to the viability of microsporidial spores are scarce (1, 17), and this study, carried out to determine the viability of N. ceranae spores, provides a valuable contribution with multiple applications. It is notable that 60°C is a temperature regularly used to remove wax from old combs, to be reused later on. With our results, it may not be assumed that this practice will inactivate the possible N. ceranae spores that may be in the wax of the old combs from infected colonies. Once melted and installed in hives, this wax may contribute to the infection of new colonies. However, further experiments are needed to clarify this matter. Likewise, the treatment at 35°C, the normal temperature inside the beehives needed by immature bees to complete metamorphosis, didn't produce a decrease in viability of spores. This finding should be borne in mind, as this thermoresistance may facilitate the transmission among stages.
The effect of freezing with different media was also studied, showing a significant reduction in viability with all freezing media in the two time periods studied. Similarly, a marked decrease of infectivity of frozen spores of Encephalitozoon intestinalis (16) and of E. cuniculi was observed (10, 17). These results should be considered, since this temperature is regularly used to preserve organisms for experimental studies. The decrease of viability observed would be due to the cytotoxic effect of the cryopreservative DMSO used for freezing, although it was used at the regularly recommended concentration for eukaryotic cell cryopreservation.
Finally, the effect of RT on the viability of air-dried N. ceranae spores was measured to ascertain their resistance during drought. A good viability was observed after 12 h, 48 h, and 1 week (Table 1). Nevertheless, previous studies with N. apis spores showed a loss of viability of air-dried spores after 3, 5, and 45 days at 40, 45 or 49°C (11). Although new studies on the viability of N. ceranae should be carried out, such as the study of the effects of various temperatures with desiccation studies, these data must be borne in mind when control measures need to be established.
The study, carried out with N. apis and N. ceranae spores maintained at 4°C, showed significant degeneration of spores, with a 30% decrease in viability during the first 6 months of study, making them unacceptable for culturing purposes or drug effectiveness studies. Moreover, the decrease in viability of these spores after freezing makes it necessary to explore new methods of optimizing the conservation of N. ceranae spores in the laboratory, to avoid the continuous necessity of renewing the stock of infective spores and to make the related experiments seasonable (Table 1).
The thermotolerance of N. ceranae spores observed in our study shows the need not only to standardize the laboratory protocols, but also to review measures related to adequate disinfection of laboratory and field tools, as well as to control honey-related food that may be ingested by potentially susceptible individuals.
Acknowledgments
We are grateful to L. Hamalainen for help with the preparation of the manuscript.
This work was supported by API06-009 from the Ministerio de Agricultura, Pesca y Alimentación and by USP-PC 04/07 from the Fundacion Universitaria San Pablo-CEU.
Footnotes
Published ahead of print on 4 September 2009.
REFERENCES
- 1.Amigo, J. M., M. P. Gracia, M. Rius, H. Salvado, P. A. Maillo, and C. P. Vivares. 1996. Longevity and effects of temperature on the viability and polar-tube extrusion of spores of Glugea stephani, a microsporidian parasite of commercial flatfish. Parasitol. Res. 82:211-214. [DOI] [PubMed] [Google Scholar]
- 2.Cantwell, G. E., and H. Shimanuki. 1970. The use of heat to control Nosema and increase production for the commercial beekeeper. Am. Bee J. 110:263. [Google Scholar]
- 3.Chen, Y., J. D. Evans, I. B. Smith, and J. S. Pettis. 2008. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 97:186-188. [DOI] [PubMed] [Google Scholar]
- 4.Fries, I., R. Martín, A. Meana, P. García-Palencia, and M. Higes. 1996. Nosema ceranae n. sp. (Microspora, Nosematidae). Morphological and molecular characterization of microsporidian parasite of the Asian honey bees Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 32:356-365. [Google Scholar]
- 5.Green, L. C., P. J. LeBlanc, and E. S. Didier. 2000. Discrimination between viable and dead Encephalitozoon cuniculi (microsporidian) spores by dual staining with Sytox Green and Calcofluor White M2R. J. Clin. Microbiol. 38:3811-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higes, M., P. Garcia-Palencia, R. Martin-Hernandez, and A. Meana. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94:211-217. [DOI] [PubMed] [Google Scholar]
- 7.Higes, M., R. Martin-Hernandez, C. Botias, E. G. Bailon, A. V. Gonzalez-Porto, L. Barrios, M. J. Del Nozal, J. L. Bernal, J. J. Jimenez, P. G. Palencia, and A. Meana. 2008. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 10:2659-2669. [DOI] [PubMed] [Google Scholar]
- 8.Higes, M., R. Martin-Hernandez, E. Garrido-Bailon, C. Botias, P. Garcia-Palencia, and A. Meana. 2008. Regurgitated pellets of Merops apiaster as fomites of infective Nosema ceranae (Microsporidia) spores. Environ. Microbiol. 10:1374-1379. [DOI] [PubMed] [Google Scholar]
- 9.Higes, M., R. Martin, and A. Meana. 2006. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 92:93-95. [DOI] [PubMed] [Google Scholar]
- 10.Koudela, B., S. Kucerova, and T. Hudcovic. 1999. Effect of low and high temperatures on infectivity of Encephalitozoon cuniculi spores suspended in water. Folia Parasitol. (Praha) 46:171-174. [PubMed] [Google Scholar]
- 11.Malone, L. A., H. S. Gatehouse, and E. L. Tregidga. 2001. Effects of time, temperature, and honey on Nosema apis (Microsporidia: Nosematidae), a parasite of the honeybee, Apis mellifera (Hymenoptera: Apidae). J. Invertebr. Pathol. 77:258-268. [DOI] [PubMed] [Google Scholar]
- 12.Martín-Hernández, R., A. Meana, L. Prieto, A. M. Salvador, E. Garrido-Bailon, and M. Higes. 2007. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 73:6331-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín-Hernández, R., A. Meana, P. García-Palenci, P. Marín, C. Botías, E. Garrido-Bailón, L. Barrios, and M. Higes. 2009. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 75:2554-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldroyd, B. P. 2007. What's killing American honey bees? PLoS Biol. 5:1195-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxton, R. J. K., J. S. Korpela, and I. Fries. 2007. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38:1-9. [Google Scholar]
- 16.Santillana-Hayat, M., C. Sarfati, S. Fournier, F. Chau, R. Porcher, J. M. Molina, and F. Derouin. 2002. Effects of chemical and physical agents on viability and infectivity of Encephalitozoon intestinalis determined by cell culture and flow cytometry. Antimicrob. Agents Chemother. 46:2049-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shadduck, J. A., and M. B. Polley. 1978. Some factors influencing the in vitro infectivity and replication of Encephalitozoon cuniculi. J. Protozool. 25:491-496. [DOI] [PubMed] [Google Scholar]
- 18.Zander, E. 1909. Tierische Parasiten als Krankheitserreger bei der Biene. Leipziger Bienenztg. 24:147-150, 163-166. [Google Scholar]