Abstract
The disease chytridiomycosis, which is caused by the chytrid fungus Batrachochytrium dendrobatidis, is associated with recent declines in amphibian populations. Susceptibility to this disease varies among amphibian populations and species, and resistance appears to be attributable in part to the presence of antifungal microbial species associated with the skin of amphibians. The betaproteobacterium Janthinobacterium lividum has been isolated from the skins of several amphibian species and produces the antifungal metabolite violacein, which inhibits B. dendrobatidis. In this study, we added J. lividum to red-backed salamanders (Plethodon cinereus) to obtain an increased range of violacein concentrations on the skin. Adding J. lividum to the skin of the salamander increased the concentration of violacein on the skin, which was strongly associated with survival after experimental exposure to B. dendrobatidis. As expected from previous work, some individuals that did not receive J. lividum and were exposed to B. dendrobatidis survived. These individuals had concentrations of bacterially produced violacein on their skins that were predicted to kill B. dendrobatidis. Our study suggests that a threshold violacein concentration of about 18 μM on a salamander's skin prevents mortality and morbidity caused by B. dendrobatidis. In addition, we show that over one-half of individuals in nature support antifungal bacteria that produce violacein, which suggests that there is a mutualism between violacein-producing bacteria and P. cinereus and that adding J. lividum is effective for protecting individuals that lack violacein-producing skin bacteria.
The amphibian fungal pathogen Batrachochytrium dendrobatidis causes a lethal skin disease that has caused substantial declines in amphibian populations (18). However, some species, such as the bullfrog (Rana catesbeiana) and the tiger salamander (Ambystoma tigrinum), are relatively asymptomatic when they are infected with this pathogen (4, 5). Variation in survival among species has been attributed to differences in innate immune factors, such as antimicrobial peptides (20) and skin-associated microbial species (8-11), as well as behavior (16). The presence of antifungal microbes is of particular interest because it suggests that these organisms are mutualistic associates of amphibian species. In addition, augmentation of the cutaneous microbial community by adding species of bacteria that inhibit B. dendrobatidis has the potential to provide resistance to chytridiomycosis (9).
We have identified a number of bacteria associated with the skin of amphibians that inhibit B. dendrobatidis in vitro via secretion of antifungal metabolites (2, 3, 10, 11). The bacterial species used in this study, Janthinobacterium lividum, produces the anti-B. dendrobatidis metabolites violacein and indole-3-carboxaldehyde (MIC, 1.82 μM and 69 μM, respectively) (3). We have shown that violacein inhibits B. dendrobatidis in laboratory assays (3) and is strongly correlated with survival in vivo of the frog species Rana muscosa (9). Violacein was also present on three of seven wild-collected red-backed salamanders (Plethodon cinereus) at concentrations that inhibit B. dendrobatidis in vitro (3), suggesting that this salamander species has a mutualistic community of violacein-producing bacteria on its skin. In this study, we added J. lividum to salamander skins to generate a wide range of violacein concentrations in order to determine what concentration is needed to prevent mortality caused by chytridiomycosis in vivo.
MATERIALS AND METHODS
Sixty-two adult red-backed salamanders were collected from the George Washington National Forest in Rockingham County, Virginia, on 16 May 2008. Salamanders were placed in individual sterile plastic containers containing autoclaved filter paper moistened with autoclaved Provosoli medium (artificial pond water) (22) and immediately taken to a laboratory. All salamanders were individually weighed and rinsed twice in sterile Provosoli medium to remove transient bacteria (11). Individuals were formally, randomly assigned to one of the following four treatment groups: control group (n = 16) (animals exposed to 5 ml of sterile Provosoli medium), bacterium group (n = 15) (animals exposed to 5 ml of Provosoli medium containing 6.7 × 107 J. lividum cells isolated from the salamander Hemidactylium scutatum), bacterium-B. dendrobatidis group (n = 15) (animals exposed to 6.7 × 107 J. lividum cells 3 days prior to exposure to 6 × 106 B. dendrobatidis strain JEL 310 zoospores suspended in 5 ml of Provosoli medium), and B. dendrobatidis group (n = 16) (animals exposed to Provosoli medium containing 6 × 106 B. dendrobatidis zoospores). All individuals were handled to the same extent to control for handling effects. Details of the exposure protocol and culturing of B. dendrobatidis have been described previously by Harris et al. (9).
Salamanders were individually weighed and swabbed using the protocol described by Harris et al. (9) prior to experimental treatment and 13 days after exposure to B. dendrobatidis. Individuals who died prior to day 13 were swabbed immediately after they died. DNA was extracted from swabs using a DNeasy blood and tissue kit (Qiagen, Germantown, MD) by following the manufacturer's protocol. DNA obtained from the swabs was amplified in triplicate using real-time PCR with the J. lividum-specific primers described by Harris et al. (9). Twenty-five-microliter PCR mixtures contained 5 μl of DNA template, 0.2 μM of each primer, and 12.5 μl of 2× SYBR green PCR master mixture (Applied Biosystems, Warrington, United Kingdom). Amplification of each sample was completed using a DNA Engine Opticon 2 system (MJ Research, Waltham, MA). The amplification conditions were as follows: an initial cycle consisting of 10 min at 94°C, followed by 40 cycles of 30 s at 94°C, 20 s at 58°C, and 30 s at 72°C and then a final cycle consisting of 10 min at 72°C. DNA was extracted from pure cultures of J. lividum with an UltraClean microbial DNA isolation kit (MoBio, Carlsbad, CA) to create standards containing 105, 104, 103, 102, and 10 J. lividum cell genome equivalents. Standards were amplified along with extracted bacterial swabs. A standard curve was generated for each 96-well plate to estimate the number of J. lividum cell equivalents.
The experiment ended 14 days after exposure to B. dendrobatidis, when all surviving individuals were euthanized. This day was chosen so that we could accurately analyze violacein concentrations for surviving and dead salamanders when the death rate was high. Immediately following mortality during the experiment or upon euthanasia, a portion of skin between the shoulders and hips of each individual was excised, measured, and extracted with methanol to determine the concentration of violacein on the skin using high-pressure liquid chromatography and comparison to a standard curve for the metabolite. Details of this protocol have been described by Brucker et al. (3).
Data that were not normally distributed were transformed, and if transformation failed, an appropriate nonparametric test was performed to test for differences between treatments or correlation between variables.
RESULTS
J. lividum successfully established on the skins of salamanders. By use of real-time PCR and primers specific for J. lividum, we determined that 93% (28/30) of individuals in the bacterium and bacterium-B. dendrobatidis treatment groups were negative for the presence of J. lividum prior to treatment with J. lividum and that all 30 salamanders were positive 2 weeks after treatment.
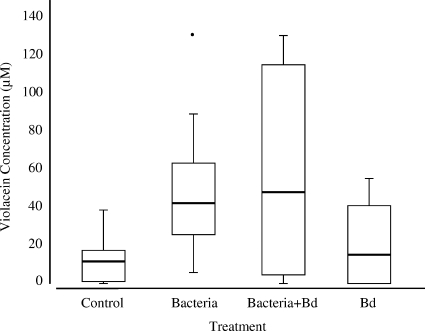
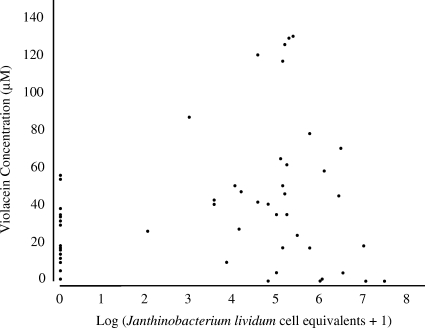
Treating salamanders with J. lividum resulted in a wide range of violacein concentrations (up to 129 μM). The violacein concentrations were higher on salamander skins treated with J. lividum than on salamander skins not treated with J. lividum (B. dendrobatidis and control groups) (Fig. 1) (df = 3, χ2 = 16.381, and P = 0.001, Kruskal-Wallis test). Surprisingly, 63% (20/32) of the salamanders in the nonaugmented B. dendrobatidis and control treatment groups had detectable quantities of violacein on their skins. However, only 10% (2/20) of salamanders with detectable levels of violacein on their skins were positive for J. lividum, indicating that other violacein-producing bacteria were present. Overall, there was a significant positive correlation between quantitative PCR estimates of the number of J. lividum equivalents and violacein concentrations, suggesting that J. lividum was a component of the violacein-producing bacterial community (Fig. 2) (df = 60, r = 0.332, and P = 0.0084, Spearman correlation analysis).
FIG. 1.
Concentrations of violacein on the skins of salamanders in the control (n = 16), bacterium (n = 15), bacterium-B. dendrobatidis (n = 15), and B. dendrobatidis (n = 16) treatment groups. Salamanders treated with J. lividum prior to exposure to B. dendrobatidis had significantly higher violacein concentrations on their skins than nontreated individuals (df = 3, χ2 = 16.381, and P = 0.001, Kruskal-Wallis test). Bd, B. dendrobatidis. The interquartile ranges are indicated by the boxes, the medians are indicated by the horizontal lines in the boxes, the bars indicate the highest and lowest values in 1.5 interquartile ranges, and an outlier is indicated by a point.
FIG. 2.
Correlation between log10 number of J. lividum cell equivalents and violacein concentration on the skins of salamanders (df = 60, r = 0.332, and P = 0.0084, Spearman correlation analysis).
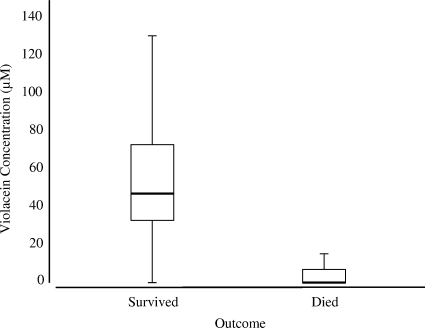
Survival was strongly associated with violacein concentration on the skin (Fig. 3). For the animals exposed to B. dendrobatidis, those that lived had significantly higher violacein concentrations than those that died (Z = −3.908 and P < 0.0001, Wilcoxon two-sample test). Eighty percent (8/10) of the salamanders that died had no detectable violacein concentration on their skins; the other two salamanders had concentrations less than 18.8 μM on their skins. In contrast, only 14% (3/21) of the salamanders that survived had no detectable concentration of violacein on their skins; the other 18 individuals had violacein concentrations on their skins ranging from 18.3 μM to 129 μM. The proportion of salamanders that survived in the control and bacterium treatment groups was 100% (16/16 and 15/15, respectively). In the bacterium-B. dendrobatidis and B. dendrobatidis treatment groups, the levels of survival were 73% (11/15) and 56% (9/16), respectively. The salamanders that died did so between days 9 and 13.
FIG. 3.
Violacein concentrations on salamanders that died (n = 10) and survived (n = 21). Salamanders that died had significantly lower violacein concentrations on their skins (Z = −3.908 and P < 0.0001, Wilcoxon two-sample test). The interquartile range is indicated by the box, the median is indicated by the horizontal line in the box and the bars indicate the highest and lowest values in 1.5 interquartile ranges.
DISCUSSION
Adding J. lividum to salamanders led to significant increases in the violacein concentrations on their skins. Violacein concentration was strongly associated with whether an individual lived or died when it was exposed to the pathogen B. dendrobatidis. Our study suggests that a threshold concentration of violacein of about 18 μM on a salamander's skin prevents mortality and morbidity caused by B. dendrobatidis. It is possible that a longer experiment would have led to additional mortality; however, recent results with another amphibian species indicated that violacein concentration is correlated with longer survival (5 months) (9). Violacein was present on the skins of some salamanders that had no detectable J. lividum. Since amphibians are not known to produce violacein, the occurrence of violacein on skins of some salamanders without J. lividum strongly suggests that another violacein-producing microbial species was present on the skins. The statistically significant relationship between the number of J. lividum equivalents and violacein concentration had a fairly low correlation coefficient (Fig. 2), which also suggests that other violacein producers were present on the skins. Violacein is produced by three bacterial species in the betaproteobacterial group closely related to J. lividum: Duganella sp. (19), Chromobacterium violaceum, and Iodobacter fluviatile (7, 12). In addition, Pseudoalteromonas tunicata and Pseudoalteromonas luteoviolacea in the gammaproteobacterial group produce violacein, which protects P. tunicata from protozoan grazing and may protect its hosts, such as algae and tunicates, from fouling (13, 15, 23).
The amphibian species used in this study, P. cinereus, is not known to be experiencing population declines related to B. dendrobatidis infection. The presence of violacein on 63% of the skins of unmanipulated individuals in this study and on 43% (3/7) of individuals straight from the field (3) suggests that their anti-B. dendrobatidis microbiotas are a factor that protects P. cinereus salamanders from disease symptoms and are an important component of the innate immune system. Amphibians periodically shed their skins, so it is reasonable to expect that some proportion of the population lacks violacein on the skin at any given time. Species that attend their embryos in nests, such as P. cinereus, minimize the effects of pathogenic fungi on their embryos (1, 6, 14). We have shown that in another species of salamander, H. scutatum, females with culturable antifungal skin bacteria can minimize embryonic mortality due to fungi (1). We hypothesize that species that attend nests have undergone natural selection to support antifungal bacteria as a means to protect embryos from fungi and also protect adults (10).
The implication of our results is that violacein-producing bacteria form a mutualistic association with P. cinereus. The salamanders are protected from pathogens such as B. dendrobatidis and probably from other species of pathogenic fungi that attack their embryos in the nest. The skin bacteria have a food resource (mucus) and a substrate (the skin). We hypothesize that amphibians' own antimicrobial peptide secretions have selected for a group of microbes with the functional capacity to resist pathogenic fungi (21). A similar mutualism is found in bacteria and corals (17).
Assaying populations of amphibians prior to the arrival of B. dendrobatidis for anti-B. dendrobatidis, bacterially produced metabolites may provide an indication of which species are at greatest risk, although techniques are needed that allow sampling of small quantities of metabolites from amphibian skins using swab samples. We are developing such an assay using a more selective high-performance liquid chromatography-mass spectrometry protocol. Populations in which a high proportion of individuals have protective metabolites are likely to be at less risk from chytridiomycosis than populations that lack protective metabolites. Many individuals of the salamander species used in this study were protected from B. dendrobatidis without bioaugmentation, which can help explain the survival of individuals that were experimentally exposed to B. dendrobatidis and not to J. lividum. Other amphibian species are likely to be less well protected, and populations that lack anti-B. dendrobatidis, bacterially produced metabolites are candidates for a bioaugmentation approach. Adding antifungal skin bacteria to amphibians as a strategy to conserve threatened species could be effective if the added bacteria persist at population densities high enough to limit B. dendrobatidis persistence. We predict that bioaugmentation will limit the symptoms of chytridiomycosis, as shown recently in our laboratory study with the frog Rana muscosa (9). Other defensive mechanisms, such as antimicrobial peptides, behaviors, skin shedding rates, and adaptive immune responses, are important in patterns of species-specific survival in response to chytridiomycosis. However, these mechanisms are not amenable to manipulation as a management tool in conservation. The effects of bioaugmentation on nontarget species need to be assessed before bioaugmentation can be used in the field. Additional research is needed to understand the ecological interactions on amphibian skin and to lay the groundwork for preventing the devastating effects of B. dendrobatidis in nature.
Acknowledgments
We thank D. Flaherty for microscopy work and D. Woodhams, J. Walke, J. Becker, and B. Lam for valuable comments on the manuscript. Permits to collect were provided by the Virginia Department of Fish and Game. Animal care protocols were approved by JMU's Animal Care and Use Committee.
This research was supported by National Science Foundation grant 0640373 to R.N.H., by the Research Corporation (Cottrell College Science Award to K.P.C.M.), and by the Thomas F. Jeffress and Kate M. Jeffress Memorial Trust (K.P.C.M.).
Footnotes
Published ahead of print on 28 August 2009.
REFERENCES
- 1.Banning, J. L., A. L. Weddle, G. W. Wahl III, M. A. Simon, A. Lauer, R. L. Walters, and R. N. Harris. 2008. Antifungal skin bacteria, embryonic survival, and communal nesting in four-toed salamanders, Hemidactylium scutatum. Oecologia 156:423-429. [DOI] [PubMed] [Google Scholar]
- 2.Brucker, R. M., C. M. Baylor, R. L. Walters, A. Lauer, R. N. Harris, and K. P. C. Minbiole. 2008. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 34:39-43. [DOI] [PubMed] [Google Scholar]
- 3.Brucker, R. M., R. N. Harris, C. R. Schwantes, T. N. Gallaher, D. C. Flaherty, B. A. Lam, and K. P. C. Mibiole. 2008. Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J. Chem. Ecol. 34:1422-1429. [DOI] [PubMed] [Google Scholar]
- 4.Daszak, P., A. Strieby, A. A. Cunningham, J. E. Longcore, C. C. Brown, and D. Porter. 2004. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J. 14:201-207. [Google Scholar]
- 5.Davidson, E. W., M. Parris, J. P. Collins, J. E. Longcore, A. P. Pessier, and J. Brunner. 2003. Pathogenicity and transmission of chytridiomycosis in tiger salamanders (Ambystoma tigrinum). Copeia 2003:601-607. [Google Scholar]
- 6.Forester, D. C. 1979. The adaptiveness of parental care in Desmognathus ochrophaeus (Urodela: Plethodontidae). Copeia 1979:332-341. [Google Scholar]
- 7.Gillis, M., and J. de Ley. 2006. The genera Chromobacterium and Janthinobacterium, p. 737-746. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, 3rd ed., vol. 5. Springer, New York, NY. [Google Scholar]
- 8.Harris, R. N., A. Lauer, M. A. Simon, J. L. Banning, and R. A. Alford. 2009. Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis. Aquat. Org. 83:11-16. [DOI] [PubMed] [Google Scholar]
- 9.Harris, R. N., R. M. Brucker, J. B. Walke, M. H. Becker, D. C. Woodhams, C. R. Schwantes, D. C. Flaherty, and K. P. C. Minbiole. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 3:818-824. [DOI] [PubMed] [Google Scholar]
- 10.Harris, R. N., T. Y. James, A. Lauer, M. A. Simon, and A. Patel. 2006. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3:53-56. [Google Scholar]
- 11.Lauer, A., M. A. Simon, J. L. Banning, E. Andre, K. Duncan, and R. N. Harris. 2007. Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 2007:630-640. [Google Scholar]
- 12.Logan, N. A. 1989. Numerical taxonomy of violet-pigmented, gram-negative bacteria and description of Iodobacter fluviatile gen. nov., comb. nov. Int. J. Syst. Bacteriol. 39:450-456. [Google Scholar]
- 13.Matz, C., J. S. Webb, P. J. Schupp, S. Y. Phang, A. Penesyan, S. Egan, P. Steinberg, and S. Kjelleberg. 2008. Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS ONE 3:e2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng, M. Y., and H. M. Wilbur. 1995. The cost of brooding in Plethodon cinereus. Herpetologica 51:1-8. [Google Scholar]
- 15.Rao, D., J. S. Webb, C. Holmström, R. Case, A. Low, P. Steinberg, and S. Kjelleberg. 2007. Low densities of epiphytic bacteria from the marine alga Ulva australis inhibit settlement of fouling organisms. Appl. Environ. Microbial. 73:7844-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley, J. J. L., and R. A. Alford. 2007. Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis. Aquat. Org. 77:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Schnit-Orland, M., and A. Kushmaro. 2009. Coral mucus-associated bacteria: a possible first line of defense. FEMS Microbiol. Ecol. 67:371-380. [DOI] [PubMed] [Google Scholar]
- 18.Skerratt, L. F., L. Berger, R. Speare, S. Cashins, K. R. McDonald, A. D. Phillott, H. B. Hines, and N. Kenyon. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125-134. [Google Scholar]
- 19.Wang, H., P. Jiang, Y. Lu, Z. Ruan, R. Jiang, X. Xing, K. Lou, and D. Wei. 2009. Optimization of culture conditions for violacein production by a new strain of Duganella sp. B2. Biochem. Eng. J. 44:119-124. [Google Scholar]
- 20.Woodhams, D. C., K. Ardipradja, R. A. Alford, G. Marantelli, L. K. Reinert, and L. A. Rollins-Smith. 2007. Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim. Conserv. 10:409-417. [Google Scholar]
- 21.Woodhams, D. C., L. A. Rollins-Smith, R. A. Alford, M. A. Simon, and R. N. Harris. 2007. Innate immune defenses of amphibian skin: antimicrobial peptides and more. Anim. Conserv. 10:425-428. [Google Scholar]
- 22.Wyngaard, G. A., and C. C. Chinnappa. 1982. General biology and cytology of cyclopoids, p. 485-533. In F. W. Harrison, and R. R. Cowden (ed.), Developmental biology of freshwater invertebrates. A.R. Liss, New York, NY.
- 23.Yada, S., Y. Wang, Y. Zou, K. Nagasaki, K. Hosokawa, I. Osaka, R. Arakawa, and K. Enomoto. 2008. Isolation and characterization of two groups of novel marine bacteria producing violacein. Mar. Biotechnol. 10:128-132. [DOI] [PubMed] [Google Scholar]