Abstract
Previously it has been demonstrated that Staphylococcus aureus is sensitive toward Pseudomonas-secreted exotoxins, which preferentially target the electron transport chain in staphylococci. Here it is shown that a subpopulation of S. aureus survives these respiratory toxins of Pseudomonas aeruginosa by selection of the small-colony variant (SCV) phenotype. Purified pyocyanin alone causes the same effect. A hemB mutant of S. aureus survives cocultivation with P. aeruginosa without a decrease in CFU.
Pseudomonas aeruginosa and Staphylococcus aureus are opportunistic pathogens and frequently coinfect the lungs of patients with cystic fibrosis (CF). P. aeruginosa excretes an arsenal of small respiratory inhibitors, like pyocyanin (5), hydrogen cyanide (2), or quinoline N-oxides (9), that may act against the commensal microbiota as well as host cells. Previously it has been demonstrated that S. aureus is sensitive toward the toxic products generated by P. aeruginosa and that these exotoxins preferentially target the electron transport chain (17).
Staphylococcal species can be divided into two groups: the sensitive group, comprising pathogenic species such as S. aureus and S. epidermidis, and the resistant group, represented by nonpathogenic species such as S. carnosus, S. piscifermentans, and S. gallinarum. The resistance in the latter group was due to cydAB genes, which encode a pyocyanin- and cyanide-resistant cytochrome bd quinol oxidase (17). It has also been shown that the resistant or sensitive phenotype is determined by the CydB subunit, which is part of the cytochrome bd quinol oxidase of S. aureus. Despite its sensitivity to these exotoxins, S. aureus has frequently been coisolated with P. aeruginosa from the skin, eyes, and catheter infections and from the lungs of patients with CF. The aim of this study is to elucidate the escape mechanism of S. aureus by cocultivating S. aureus and P. aeruginosa. The findings indicate that a subpopulation of the staphylococcal community can survive in the presence of P. aeruginosa by the selection of small-colony variants (SCVs), which usually are defective in the electron transport chain. SCVs grow as tiny, nonpigmented colonies and are auxotrophic to hemin, menadione, or thymidine (14). Here we show that both a culture supernatant of P. aeruginosa and purified pyocyanin select for the SCV phenotype in S. aureus.
Cocultivation of S. aureus and P. aeruginosa can select for S. aureus SCVs.
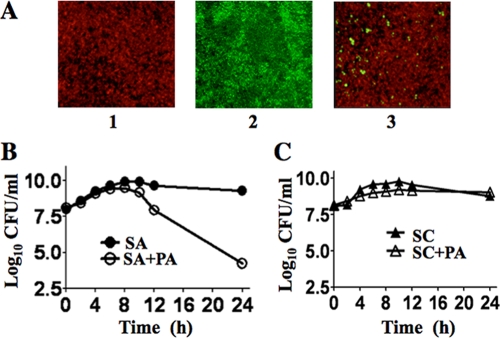
S. aureus was grown in monoculture or in coculture with P. aeruginosa (1:1, optical density at 578 nm) in tryptic soy broth (TSB) medium under biofilm or planktonic conditions. Biofilm studies using S. aureus(pCtuf-gfp) and P. aeruginosa::pUT-tell-rfp grown in TSB medium supplemented with 0.5% glucose under static conditions for 36 h showed that both S. aureus and P. aeruginosa form thicker biofilm in monocultures, while in a mixed biofilm with P. aeruginosa only few S. aureus cells were visible (Fig. 1A).
FIG. 1.
(A) Confocal laser scanning microscopy analysis of biofilm formation by S. aureus(pCtuf-gfp) and P. aeruginosa::pUT-tell-rfp, expressing red fluorescent protein (RFP) and green fluorescent protein (GFP), respectively, after 24 h. (1) P. aeruginosa. (2) S. aureus. (3) S. aureus and P. aeruginosa. (B) Planktonic growth of S. aureus in monoculture (SA) and in coculture with P. aeruginosa (SA+PA). (C) Growth of S. carnosus in monoculture (SC) and in coculture with P. aeruginosa (SC+PA).
Titers of S. aureus grown under planktonic conditions in monoculture and in coculture were determined by plating 0.1-ml samples on Chapman agar (selection medium for staphylococci) after diluting appropriately with TSB medium. The samples grew almost parallel up to 109 CFU for approximately 8 h. Then, the CFU of the S. aureus cells in coculture declined steadily for the next 24 h (Fig. 1B). This correlates exactly with the onset of pyocyanin production in P. aeruginosa, as it has been shown that P. aeruginosa produces pyocyanin in an aerobic medium when cells enter the stationary phase, at approximately 1.2 × 109 cells/ml in batch culture (15). In contrast to the growth of S. aureus, the growth of S. carnosus did not decline in cocultivation with P. aeruginosa, because of its pyocyanin- and cyanide-resistant cytochrome bd quinol oxidase (Fig. 1C). P. aeruginosa titers were determined by plating 0.1-ml samples on Cetrimid agar (selection medium for P. aeruginosa) after diluting appropriately. No difference in the CFU of P. aeruginosa grown in monoculture and coculture was observed (data not shown).
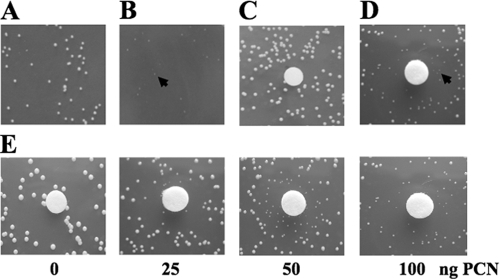
S. aureus colonies had the appearance of SCVs after 24 h of cocultivation (Fig. 2A and B). Typically, this phenotype disappeared upon subculture, indicating that the growth limitation is reversible. Equivalent results were obtained with P. aeruginosa PAO1 and SH1 strains and with different clinical isolates of S. aureus. The stationary-phase culture supernatant of P. aeruginosa can select for SCVs (Fig. 2D).
FIG. 2.
Pyocyanin induces SCV selection in S. aureus. Arrows indicate SCVs. (A) S. aureus grown in monoculture exhibits a normal phenotype. (B) The S. aureus SCV phenotype is observed after 24 h of growth in coculture with P. aeruginosa. (C to E) Agar disc diffusion assay with S. aureus. Filter discs contained buffer as a control (C), a filter-sterilized culture supernatant of P. aeruginosa (D), and various concentrations of purified pyocyanin (PCN) (E).
Pyocyanin is secreted into the medium by P. aeruginosa in copious amounts. Previously it has been shown that pyocyanin blocks the respiratory chain of S. aureus (17). To determine whether pyocyanin can induce electron transport-defective SCV selection, pyocyanin was isolated and purified and its purity was verified with high-performance liquid chromatography as described earlier (4, 9, 17). To see the effect of pyocyanin on S. aureus, we used a disc diffusion assay in which sterile filter discs were spotted with various concentrations of pyocyanin and placed on top of the soft agar containing S. aureus cells. S. aureus formed increasing numbers of smaller colonies with increasing pyocyanin concentrations. At the highest concentration (100 nM), most of the colonies appeared to be smaller and dispersed (Fig. 2E). At a concentration of more than 200 nM pyocyanin, a zone of inhibition surrounded by a zone of SCVs was observed (data not shown). The selection of electron transport-defective SCVs in the presence of pyocyanin allows S. aureus to coexist with P. aeruginosa.
Decreased pigment formation is one of the characteristics of SCV production of staphyloxanthin (3, 12) in S. aureus strains. Therefore, four different S. aureus strains (S. aureus Newman, S. aureus COL, S. aureus Mu50, and S. aureus USA300) were grown on tryptic soy agar (TSA) and TSA supplemented with a 25% (vol/vol) culture supernatant of P. aeruginosa. No staphyloxanthin production was observed in the TSA plates supplemented with the culture supernatant of P. aeruginosa; in addition, the colonies grew very slowly and had an SCV phenotype (data not shown).
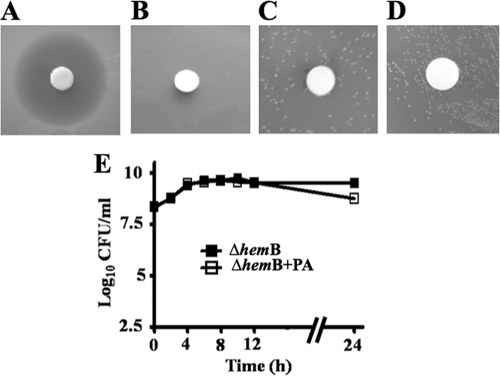
The persistence of S. aureus in CF has previously been associated with the isolation of a subpopulation of S. aureus with SCV phenotypes (11, 13). Therefore, a defined electron transport-deficient SCV mutant, S. aureus ΔhemB, which is auxotrophic for hemin (18), was tested for its sensitivity toward the culture supernatant of P. aeruginosa and purified pyocyanin. S. aureus ΔhemB was insensitive to both the culture supernatant and purified pyocyanin (Fig. 3A to D). The viability of S. aureus ΔhemB when grown in coculture with P. aeruginosa was found to be unaffected until 12 h; there was only a slight decrease of CFU after 24 h (Fig. 3E).
FIG. 3.
Sensitivity of a defined electron transport-deficient SCV mutant, S. aureus ΔhemB. (A to D) Agar disc diffusion assay with filter discs containing 25 μl culture supernatant of P. aeruginosa. The agar contains embedded cells of wild-type S. aureus (A) or S. aureus ΔhemB (B). The effect of pyocyanin on S. aureus ΔhemB is shown, with discs containing buffer as a control (C) and purified pyocyanin (D). (E) Growth of S. aureus ΔhemB in monoculture (ΔhemB) and in coculture with P. aeruginosa (ΔhemB+PA).
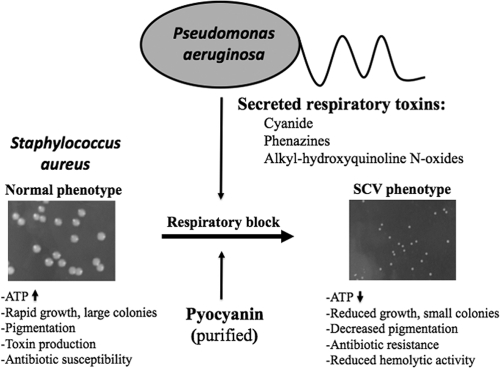
In patients with CF, S. aureus is often the initial pathogen colonizing the lungs (10, 16). Mainly, S. aureus infects the lungs of children up to 10 years; however, P. aeruginosa is the predominant pathogen in adolescents and adults (1, 8). It has been shown that S. aureus persists in the airways of patients with CF over extended periods by transforming into SCVs. P. aeruginosa produces an arsenal of virulence factors, which include respiratory inhibitors like pyocyanin, hydrogen cyanide, and alkyl-hydroxyquinoline N-oxides (HQNO). Pyocyanin is a blue redox-active secondary metabolite that is readily recovered in large quantities in sputum from patients with CF who are infected by P. aeruginosa. It is shown here that the culture supernatant of P. aeruginosa and purified pyocyanin can select for an electron transport-deficient SCV phenotype in S. aureus and that the defined electron transport-deficient SCV mutant S. aureus ΔhemB is resistant to both. HQNO have also been shown to target the electron transport chain (7) and have recently been shown to induce SCV selection in S. aureus (6). Our results have shown that the secreted respiratory inhibitors of P. aeruginosa suppress the aerobic metabolism and growth of S. aureus. However, S. aureus as a versatile pathogen can evade this suppression with the help of selection of a respiration-defective subpopulation that is characterized by the SCV phenotype. The blockage of the electron transport pathway drives S. aureus to fermentative growth, which is accompanied by a decreased ATP yield and slower growth that finally results in the formation of smaller colonies (Fig. 4). SCVs are known to persist better than their normal counterparts within host cells, are resistant to aminoglycoside antibiotics, and are difficult to detect. This study explains why SCV phenotypes of S. aureus are frequently isolated from patients with CF.
FIG. 4.
Illustration of P. aeruginosa-induced S. aureus SCV selection. (Left) Colony size of S. aureus under normal conditions. (Right) In the presence of respiratory toxins, like HQNO or the Pseudomonas quinolone signal, pyocyanin or cyanide produced by P. aeruginosa leads to selection of the electron transport-deficient SCV phenotype in S. aureus.
Acknowledgments
We thank Leo Eberl (Institute of Plant Biology, University of Zürich) for providing us with P. aeruginosa strains. We thank Hans-Peter Fiedler (Department of Microbiology/Biotechnology, University of Tübingen) for help with high-performance liquid chromatography analysis and Regine Stemmler for technical assistance.
This work was supported by the DFG (SPP1316), the University of Tübingen graduate college (GK685), and the BMBF PathoGenoMik (031U213B).
Footnotes
Published ahead of print on 28 August 2009.
REFERENCES
- 1.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 2.Castric, P. A. 1975. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 21:613-618. [DOI] [PubMed] [Google Scholar]
- 3.Clauditz, A., A. Resch, K. P. Wieland, A. Peschel, and F. Götz. 2006. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 74:4950-4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox, C. D. 1986. Role of pyocyanin in the acquisition of iron from transferrin. Infect. Immun. 52:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan, H. M., and I. Fridovich. 1980. Mechanism of the antibiotic action pyocyanine. J. Bacteriol. 141:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman, L. R., E. Deziel, D. A. D'Argenio, F. Lepine, J. Emerson, S. McNamara, R. L. Gibson, B. W. Ramsey, and S. I. Miller. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 103:19890-19895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kogut, M., and J. W. Lightbown. 1962. Selective inhibition by 2-heptyl-4-hydroxyquinoline N-oxide of certain oxidation-reduction reactions. Biochem. J. 84:368-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machan, Z. A., G. W. Taylor, T. L. Pitt, P. J. Cole, and R. Wilson. 1992. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J. Antimicrob. Chemother. 30:615-623. [DOI] [PubMed] [Google Scholar]
- 10.Marks, M. I. 1990. Clinical significance of Staphylococcus aureus in cystic fibrosis. Infection 18:53-56. [DOI] [PubMed] [Google Scholar]
- 11.McNamara, P. J., and R. A. Proctor. 2000. Staphylococcus aureus small colony variants, electron transport and persistent infections. Int. J. Antimicrob. Agents 14:117-122. [DOI] [PubMed] [Google Scholar]
- 12.Pelz, A., K. P. Wieland, K. Putzbach, P. Hentschel, K. Albert, and F. Götz. 2005. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 280:32493-32498. [DOI] [PubMed] [Google Scholar]
- 13.Proctor, R. A., P. van Langevelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95-102. [DOI] [PubMed] [Google Scholar]
- 14.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295-305. [DOI] [PubMed] [Google Scholar]
- 15.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO1 positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 16.Rubio, T. T. 1986. Infection in patients with cystic fibrosis. Am. J. Med. 81:73-77. [DOI] [PubMed] [Google Scholar]
- 17.Voggu, L., S. Schlag, R. Biswas, R. Rosenstein, C. Rausch, and F. Götz. 2006. Microevolution of cytochrome bd oxidase in staphylococci and its implication in resistance to respiratory toxins released by Pseudomonas. J. Bacteriol. 188:8079-8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, G. Peters, and F. Götz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]