Abstract
The marine nitrogen (N) cycle is a complex network of biological transformations in different N pools. The linkages among these different reservoirs are often poorly understood. Traditional methods for measuring N uptake rely on bulk community properties and cannot provide taxonomic information. 15N-based stable isotope probing (SIP), however, is a technique that allows detection of uptake of individual N sources by specific microorganisms. In this study we used 15N SIP methodology to assess the use of different nitrogen substrates by Synechococcus spp. and diatoms on the west Florida shelf. Seawater was incubated in the presence of 15N-labeled ammonium, nitrate, urea, glutamic acid, and a mixture of 16 amino acids. DNA was extracted and fractionated using CsCl density gradient centrifugation. Quantitative PCR was used to quantify the amounts of Synechococcus and diatom DNA as a function of density, and 15N tracer techniques were used to measure rates of N uptake by the microbial community. The ammonium, nitrate, urea, and dissolved primary amine uptake rates were 0.077, 0.065, 0.013, and 0.055 μmol N liter−1 h−1, respectively. SIP data indicated that diatoms and Synechococcus spp. actively incorporated N from [15N]nitrate, [15N]ammonium, and [15N]urea. Synechococcus also incorporated nitrogen from [15N]glutamate and 15N-amino acids, but no evidence indicating uptake of labeled amino acids by diatoms was detected. These data suggest that N flow in communities containing Synechococcus spp. and diatoms has more plasticity than the new-versus-recycled production paradigm suggests and that these phytoplankters should not be viewed strictly as recycled and new producers, respectively.
The marine nitrogen (N) cycle is a complex network of biological transformations in different inorganic and organic N reservoirs (58). Processes related to the N cycle can at times limit productivity in marine systems (47) and influence the rate at which carbon (C) is exported from the euphotic zone to the deep ocean and marine sediments, where it can be sequestered (21). The historical paradigm with respect to the marine N and C cycles is deeply interwoven with the concepts of new and regenerated primary production in the euphotic zone (17, 20). New and export production have traditionally been equated with large nutrient influxes, particularly influxes of nitrate, which lead to diatom productivity. When high levels of nitrate are present, diatoms often dominate and exhibit high sinking rates due to aggregation and/or packaging into fecal pellets (18, 48). By contrast, the subtropical and tropical oligotrophic surface oceans have been viewed primarily as areas where recycled productivity dominates.
In recent years, however, our view of the linkages between the marine N and C cycles has become increasingly complex (58). For example, geochemical rate estimates have suggested that N fixation rates in surface waters of the tropical and subtropical oceans may be many times greater than previously thought (13, 34). The divergence between in situ observations and data obtained using the geochemical mass balance approach is attributed, among other things, to meso-scale physical forcing (38) and to diazotrophic activity of planktonic cyanobacteria (14, 57). Furthermore, it is now appreciated that the ability to use nitrate is more widely distributed among the marine bacteria than previously thought and that bacteria are capable of competing with phytoplankton for both ammonium and nitrate (29, 31). Despite these advances, direct measurements of uptake of specific forms of N by individual populations of phyto- and bacterioplankton are scarce. This is primarily due to the fact that most measurements of N uptake are made using glass fiber filters that collect autotrophs and some variable fraction of heterotrophic bacteria (3). Uptake rates thus represent bulk uptake by hundreds of different phytoplankton and bacterial species. Methods that could be used to investigate uptake of N by specific species (e.g., Synechococcus spp.) or groups of species (e.g., diatoms) would therefore greatly improve our ability to elucidate N fluxes in marine systems.
DNA stable isotope probing (SIP) is a technique that is based on the observation that DNA molecules with different densities can be separated by ultracentrifugation in a concentrated solution of cesium chloride (CsCl). CsCl density gradient centrifugation has a long history in biological research and was first used to demonstrate the semiconservative nature of DNA replication (39). In their experiments, Meselson and Stahl grew Escherichia coli in medium in which all available forms of N contained the heavy, stable isotope 15N. Fully labeled with 15N, DNA has an average density of 1.722 g cm−3, whereas 14N-containing DNA has an average density of 1.700 g cm−3 (10, 11). This small, yet significant difference in density is enough to allow separation of 14N-containing DNA from 15N-containing DNA. DNA SIP has been used to study the dynamics of microbial communities (46). Radajewski et al. (46) used DNA SIP to identify the microbial species involved in the biotransformation of specific 13C-labeled substrates among the large pool of bacterial species that typically are present in environmental communities. These authors demonstrated that 13C-labeled DNA could be recovered from microbial populations after incubation. 13C-labeled DNA was then taxonomically characterized using routine molecular ecology methods to identify active community members, demonstrating that SIP can be a powerful technique for taxonomic identification of microbes performing specific metabolic processes under in situ conditions. A series of studies have since been performed using the 13C-based technique to examine microbial communities in different environments (for reviews, see references 19 and 40).
More recently, 15N-based SIP techniques have been developed to facilitate identification of the free-living diazotrophs responsible for in situ N fixation in soil (10). This work demonstrated that 15N-based SIP techniques could be used to study N flow in environmental communities. In the present study, 15N-based SIP techniques (10, 11) were employed to assess the use of a suite of inorganic and organic nitrogen substrates by Synechococcus spp. and diatoms in a coastal marine system. Our goal was to investigate the traditional characterization of Synechococcus spp. as recycled producers (mainly ammonium uptake) and diatoms as new producers (nitrate uptake). To do this, seawater was incubated with a series of 15N-labeled N substrates. DNA was then extracted at the end of the incubation period, and quantitative PCR (qPCR) was used to determine the amounts of Synechococcus and diatom DNA as a function of density in fractionated gradients. Shifts in the densities of Synechococcus and diatom DNA as the result of incubation with 15N-labeled N substrates were interpreted as evidence of uptake. Our data indicate that Synechococcus spp. and diatoms both actively incorporated [15N]ammonium, [15N]nitrate, and [15N]urea. Synechococcus spp. appeared to also incorporate N from [15N]glutamate and 15N-amino acids. These data suggest that N flow in communities containing Synechococcus spp. and diatoms has more plasticity than the new-versus-regenerated production paradigm suggests and that these two types of phytoplankton should not be viewed strictly as recycled and new producers, respectively.
MATERIALS AND METHODS
Strains and isolates.
Synechococcus sp. strain WH7803 (CCMP1334) was obtained from John Paul (University of South Florida), who initially obtained the strain from the CCMP culture collection. Phaeodactylum tricornutum CCMP1327 was obtained from CCMP directly. WH7803 and P. tricornutum stock cultures are maintained in SN (52) and F/2 (24) media, respectively.
Field site and incubation experiments.
Field samples were collected aboard the R/V Pelican on 10 October 2008 as part of an Ecology of Harmful Algal Blooms (ECOHAB) project. All samples in this study originated from a station located ca. 2 km west of Narrows Key outside Charlotte Harbor (26.6183N, −82.2455W). Samples were collected from a depth of ca. 2 m at 1100 using 20-liter Niskin bottles. Incubation experiments were performed in 500-ml acid-cleaned polycarbonate bottles under two layers of neutral density screening to simulate the light intensity at a depth of ca. 2 m. Decktop incubation under ambient light was performed with a continuous flow of seawater through the incubator to maintain temperatures similar to those of the sampled seawater. Seawater (400 ml) was incubated in duplicate and contained no added N source (no-treatment control [NTC]) or 2 μmol N liter−1 of either 15NH4Cl, Na15NO3, [15N]urea, sodium [15N]glutamate, or 15N-labeled amino acids (an algal extract consisting of 16 amino acids; Cambridge Isotope Laboratories, Andover, MA). All preparations were incubated for 24 h, which was deemed sufficient time for phytoplankton to replicate their genomes and divide. The incubated samples were then filtered through 25-mm 0.45-μm Supor filters, which were stored in 1× STE buffer (10 mM Tris-HCl [pH 8.0], 0.1 M NaCl, 1 mM EDTA [pH 8.0]) and frozen.
15N-labeled DNA.
Synechococcus sp. strain WH7803 was grown on N-free artificial seawater medium (32, 56) supplemented with 10 mM Na215NO3 and VA vitamins (15). In order to maintain 14N-free conditions, the trace metal mixture was modified by replacing ferric ammonium citrate with ferric chloride and cobalt nitrate with cobalt chloride. Cultures were grown in artificial seawater medium through at least two transfers (with 0.1-volume inocula) before DNA extraction was performed to ensure complete labeling of Synechococcus DNA with 15N. All cultures were grown in acid-leached 500-ml Erlenmeyer flasks on a shaking table at 145 rpm. Cultures were maintained at 20°C and illuminated using cool white fluorescent tubes and cycles consisting of 12 h of light and 12 h of darkness. 15N-labeled DNA was extracted as described below.
DNA extraction.
Culture pellets of Synechococcus sp. and P. tricornutum were obtained by spinning 25-ml portions of cultures in 40-ml Oak Ridge tubes at 10,000 × g for 10 min and removing the supernatants. Seawater (400 ml) was filtered onto 0.45-μm Supor filters (Pall Life Sciences). Pellets and filters were then stored in 0.75 ml STE buffer and frozen in liquid N in the field. Filters were stored at −80°C in the lab until extraction. For extraction, 75 μl of 5% sodium dodecyl sulfate and 20 μl of 20-mg ml−1 proteinase K were added to tubes. After 30 min of digestion at 37°C, 0.2 g of muffled glass beads was added, and the tubes were bead beaten for 2 min. The tube contents were extracted twice with phenol-chloroform-isoamyl alcohol (25:24:1), and DNA was precipitated with 0.1 volume of 3 M sodium acetate (pH 5.3) and 0.6 volume of isopropanol. DNA was resuspended in 50 μl TE buffer (1 mM Tris [pH 9.0], 10 mM EDTA [pH 8.0]).
CsCl gradient ultracentrifugation.
Centrifugation and fractionation were performed as described by Buckley et al. (11). CsCl gradients were established in 4.7-ml polyallomer Optiseal tubes (Beckman) by adding 4.35 ml of 1.701-g ml−1 CsCl in gradient buffer A (15 mM Tris-HCl [pH 8.0], 15 mM KCl, 15 mM EDTA [pH 8.0], 2 mg ml−1 ethidium bromide) and 5 to 10 μg of DNA in 0.40 ml TE buffer. The tubes were centrifuged in a Beckman VTI65.2 rotor at ca. 140,000 × g for 48 h. CsCl gradients were immediately fractionated after centrifugation using a Beckman fraction recovery system. The bottoms of the polyallomer tubes were punctured, and mineral oil was pumped into the top of the tubes at a constant rate using a peristaltic pump (36, 37). Forty 100-μl fractions were collected from each tube, and their densities were determined by measurement of their refractive indices with a digital, handheld Reichert AR200 refractometer. Densities (ρ) were calculated as described previously (2) by using the following formula: ρ = anc − b, where a and b are coefficients with values of 10.927 and 13.593 at 20°C, respectively, and nc is the corrected refractive index, which is calculated by subtracting the refractive index of the gradient buffer from the observed value [nc = nobserved − (nbuffer − nwater)]. A small, empirically determined correction factor, 0.0038 (derived from Fig. 1), was applied to nc in order to account for the effect of ethidium bromide. Ethidium bromide intercalates into DNA and increases its hydration state, resulting in a decrease in the observed DNA density. DNA from each fraction was then precipitated by adding 2 volumes of gradient buffer, 1 μl of molecular grade glycogen (20 mg ml−1), and 2 volumes of isopropanol. After centrifugation, the pellets were washed with 70% ethanol, dried, and resuspended in 30 μl of water.
FIG. 1.
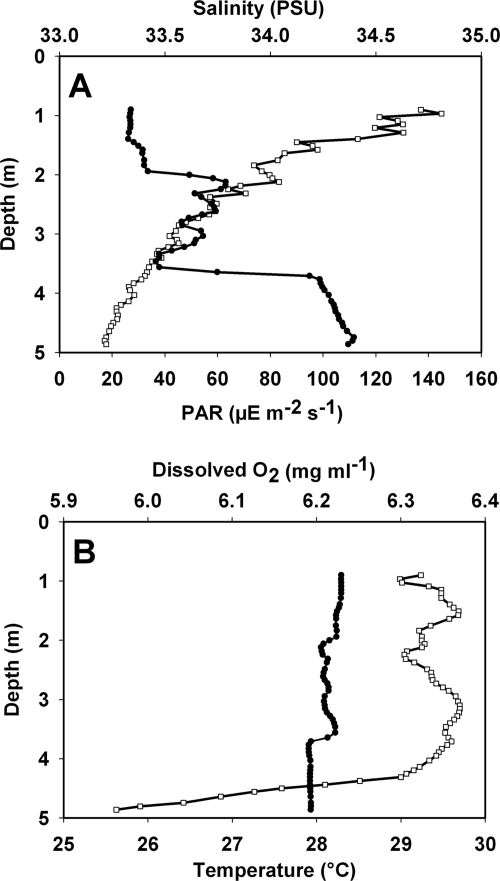
Physical and chemical data collected at the study site on the west Florida shelf, including (A) photosynthetically active radiation (PAR) (□) and salinity (in practical salinity units [PSU]) (•) and (B) temperature (•) and dissolved oxygen concentration (□). All parameters are expressed as a function of depth.
qPCR.
qPCR for Synechococcus spp. and diatoms was performed as previously described (27) by targeting rbcL, the ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit gene. Two microliters of DNA from each fraction was added to 28 μl of PCR master mixture prepared using 2× PCR TaqMan master mixture (PE Applied Biosystems, Foster City, CA) containing each primer at a concentration of 1 μM, 2 mM MgCl2, and 100 nM probe. Synechococcus-specific rbcL primers (forward primer CATCAAGCTGTCCGAG and reverse primer TGTTGGCYGTGAAGCC) and a TaqMan probe (FAM-TCACTACCTCAACGTGACCGC-TAMRA [where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine]) were used as previously described (27). The cycle parameters for the Synechococcus assay were 10 min at 95°C, followed by 40 cycles of 20 s at 95°C, 30 s at 52°C, and 1 min at 72°C. Diatom-specific TaqMan PCR was performed using the previously described diatom probe FAM-TGCGTTGGAGAGARCGTTTCTTA-TAMRA (55) and primers modified by John et al. (27) (forward primers GATGATGARAAYATTAACTCW, GATGACGARAAYATTAACTC, and GATGAYGARAACATCAACTC and reverse primers TAWGAACCTTTWACTTCWCC, TAWGAACCTTTWGTTTCACC, and TAAGAACCCTTAACYTCACC). The diatom thermocycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 20 s, 52°C for 60 s, and 72°C for 60 s. All reactions were performed and analyzed using an ABI 7300 real-time PCR system. Standard curves were produced by generating dilutions (1 ng to 10 fg) of genomic DNA extracted from cultures of Synechococcus sp. strain WH7803 and P. tricornutum maintained in our lab.
Nutrient analysis.
Total dissolved N and total dissolved phosphorus were measured after persulfate oxidation (7, 51). Dissolved organic carbon was measured after high-temperature combustion using a Shimadzu TOC-5000 (44). Concentrations of NH4+ were analyzed manually by the phenol hypochlorite method (23). Concentrations of NO3−, NO2−, and phosphate were measured with a Lachat autoanalyzer (42), and urea concentrations were measured using a manual monoxime method (45). Amino acid concentrations were measured by measuring the concentrations of dissolved primary amines using a fluorometric technique (42). In waters with low concentrations of NH4+, such as waters from the west Florida shelf, the values for free amino acids and dissolved primary amines are roughly equal (30); in this paper we refer to these compounds as amino acids.
Nitrogen uptake rates.
The rates of uptake of NH4+, NO3−, urea, glutamate, and a mixture of 16 amino acids were measured in duplicate using 15N tracer techniques and 6-h incubations (6). Samples were incubated under simulated in situ light and temperature conditions as described above. At the end of each incubation, samples were filtered through precombusted GF/F filters. The filters were subsequently dried and analyzed with a Europa isotope ratio mass spectrometer. The filtrates from the NH4+ and NO3− incubations were collected and frozen for later determination of the atom% enrichment of the pools. The NH4+ pool was isolated using the solid-phase extraction technique (9, 16). The NO3− pool was isolated using the denitrifier technique (49). The rates of uptake of NH4+ and NO3− were corrected for isotope dilution, and regeneration rates were calculated as described by Glibert et al. (22).
rbcL clone libraries.
In order to survey the composition of the phytoplankton community at our sampling site, two primer sets were used to amplify and clone partial rbcL genes as previously described (53, 54). The rbcL gene codes for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase, which is the key enzyme in the Calvin-Benson-Bassham cycle (the key pathway for carbon fixation in marine photoautotrophs). The rbcL gene is a good phylogenetic marker for marine phytoplankton (53, 54), but two primer sets are required to capture the bulk of marine phytoplankton diversity. The form IA/B primer set (forward primer TCIGCITGRAACTAYGGTCG and reverse primer CTGAGIGGIAARAACTACGG) amplifies rbcL from green algae, picocyanobacteria, and some chemolithotrophic bacteria, whereas the form ID primer set (forward primer GATGATGARAAYATTAACTC and reverse primer ATTTGDCCACAGTGDATACCA) amplifies rbcL from the majority of the remaining marine eukaryotic algae, including haptophyte and heterokont algae, as well as dinoflagellates. For PCR, each primer at a final concentration of 1 μM was added to PCR SuperMix (Invitrogen, Carlsbad, CA). The PCR conditions were as follows: 3 min at 95°C, followed by 40 cycles of 1 min at 95°C, 1 min at 52°C, and 1.5 min at 72°C. This was followed by a 15-min elongation step at 72°C. PCR products were purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA). Amplicons were then cloned into the PCR II vector (Invitrogen) according to the manufacturer's protocol. Ninety-six colonies (one microtiter plate) were picked from each transformation. Plasmids were purified using a Qiagen plasmid extraction kit, and inserts were sequenced.
Phylogenetic analysis.
rbcL sequences were trimmed to remove vector and primer sequences and then assembled into operational taxonomic units with >95% DNA sequence identity. These contigs were translated into amino acid sequences and aligned with a representative set of rbcL sequences obtained from GenBank, as well as their closest match in the database as determined by BlastX. Alignments were manually corrected for obvious misalignments and exported to Mega 4 (50) for tree building (data not shown) in order to establish phylogenetic affiliations.
Nucleotide sequence accession numbers.
The GenBank accession numbers for rbcL sequences cloned as part of this study are FJ981916 to FJ981974.
RESULTS
Field measurements, nutrient analysis, and nitrogen uptake rates.
Experiments were conducted in the context of an ECOHAB project targeting episodic blooms of the toxic alga Karenia brevis on the west Florida shelf. The study site was located 2 km west of Narrows Key and was sampled intensely, because there was a small bloom of K. brevis (∼123,000 K. brevis cells liter−1). Flow cytometric analysis of surface water indicated the presence of ca. 5.38 × 105 cells liter−1 of Synechococcus spp. (L. A. Procise, personal communication). Direct microscopic observations during the time of sampling indicated that there were large populations of diatoms belonging to the genera Rhizosolenia, Pseudonitzschia, Cetoceros, and Thalassionema. The salinity was ca. 33.3 practical salinity units near the surface (where samples were collected) and slightly higher (34.4 practical salinity units) near the bottom (Fig. 1A). The most likely reason for lower salinity at the surface was freshwater input into the coastal environment near Narrows Key from Charlotte Harbor. Eighty-five percent of the photosynthetically active radiation was absorbed in the upper 5 m of the water column (Fig. 1A). The temperature throughout the water column was ca. 28°C (Fig. 1B). The O2 levels decreased near the bottom, indicating that there was some suspension of bottom material. Urea was the dominant nitrogenous nutrient present, and the urea concentrations were approximately threefold greater than the concentrations of ammonium and nitrate (Table 1). Despite the greater concentrations of urea and amino acids, the nitrate and ammonium uptake rates were the highest uptake rates measured (Table 1). The ammonium regeneration values were approximately equal to the ammonium uptake rates, but nitrate regeneration was 2.3-fold greater than nitrate uptake (Table 1). The concentrations of amino acids were surprisingly high and suggested that there were high rates of amino acid regeneration. The same was observed for urea. However, none of the rates of uptake of the organic substrates were corrected for isotope dilution. Therefore, the organic N uptake rates should be considered conservative estimates of uptake.
TABLE 1.
Ambient substrate concentrations and uptake and regeneration rates in surface water
| Substrate | Concn (μmol N liter−1) | Uptake rate (μmol N liter−1 h−1) | Regeneration rate (μmol N liter−1 h−1) |
|---|---|---|---|
| NH4+ (ammonium) | 0.135 ± 0.036 | 0.077 ± 0.012 | 0.082 ± 0.008 |
| NO3− (nitrate) | 0.118 ± 0.012 | 0.065 ± 0.014 | 0.147 ± 0.035 |
| Urea | 0.328 ± 0.067 | 0.013 ± 0.004 | NDa |
| Dissolved primary amines (approximately amino acids) | 0.854 ± 0.220 | 0.055 ± 0.001 | ND |
| DON | 16.2 ± 0.271 | ND | ND |
| PO4−3 (phosphate) | 0.028 ± 0.002 | ND | ND |
| Dissolved organic phosphate | 0.635 ± 0.021 | ND | ND |
| Biogenic silicate | 5.543 ± 0.374 | ND | ND |
ND, not determined.
rbcL clone libraries.
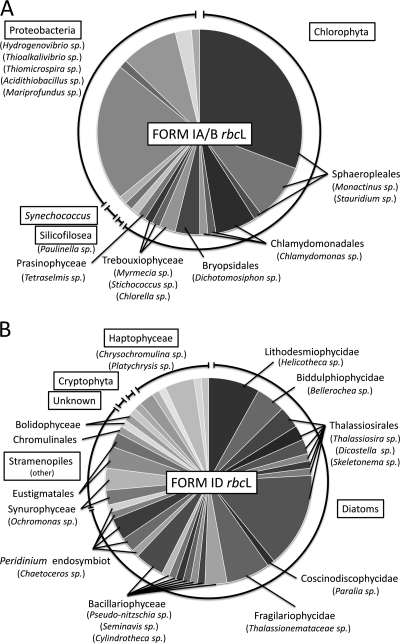
To obtain a more detailed phylogenetic description of the composition of the phytoplankton community at the study site, culture-independent methods were used. More than 60% of the sequences in the form IA/B library (Fig. 2A) were affiliated with the chlorophyte lineage, mainly the genera Monactinus, Stauridium, Chlamydomonas, and Dichotomosiphon. Three Trebouxiophyceae species (most similar to Mymecia sp., Stichococcus sp., and Chlorella sp.), one Prasinophyceae species (most similar to Tetraselmis sp.), and one Silicofilosea species (similar to Paulinella sp.) were also detected. Among the prokaryotes detected in the library were Synechococcus spp., as well as several operational taxonomic units that were most similar to proteobacterial lineages most similar to the genera Hydrogenovibrio, Thioalkalivibrio, Thiomicrospira, Acidithiobacillus, and Mariprofundus. The form ID library (Fig. 2B) was primarily (>71%) composed of sequences affiliated with the diatom lineage and confirmed the presence of the genera Pseudonitzschia, Chaetoceros, and Thalassionema. Rhizosolenia sp., although observed by microscopy, was not detected in the library, but sequences most similar to Helicotheca, Bellerochea, Thalassiosira, Dicostella, Skeletonema, Paralia, Seminavis, and Cylindrotheca sequences were detected. Several other stramenopiles, notably the genera Ochromonas, Nannochloropsis, Chrysamoeba, and Bolidomonas, were also detected. Within the haptophyte lineage sequences most similar to the prymnesiophytes, Chrysochromulina sp., and Platychrysis sp. were observed.
FIG. 2.
Phytoplankton community composition as determined by culture-independent methods. rbcL genes were amplified using two different (form IA/B- and form ID-specific) broad-range PCR primer sets, cloned, and sequenced. Sequences were grouped into operational taxonomic units with >95% sequence identity and compared to the GenBank database by means of BlastX searches. The charts show the phylogenetic distribution of the best BlastX matches for each of the operational taxonomic units recovered from station 9C. Phylum-level groups are enclosed in boxes. Class- and order-level groups are indicated by bold type. Genus-level taxonomic groups are in parentheses. (A) Pie chart indicating the frequencies of different types of form IA/B-like rbcL genes in the clone library. (B) Pie chart indicating the frequencies of different types of form ID-like rbcL genes in the clone library.
DNA SIP.
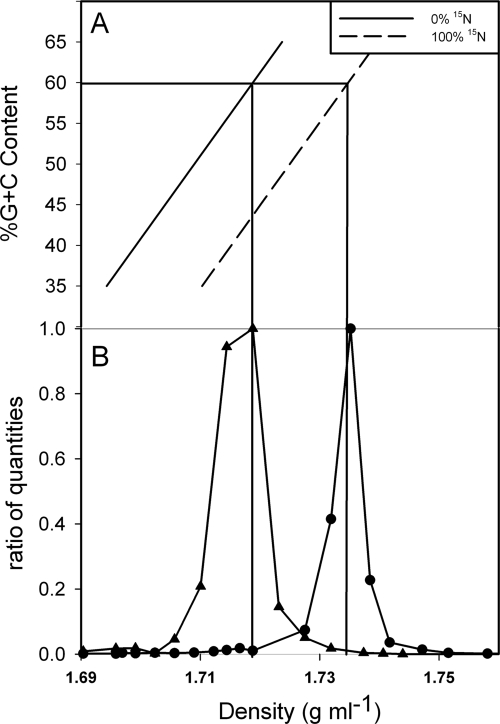
A “proof-of-concept” experiment was conducted (using the same experimental approach used later in the field) to test whether N-based SIP methodology could be applied to marine phytoplankton species (Fig. 3). Synechococcus sp. strain WH7803 was grown on 14N- and 15N-containing medium. DNA was extracted from the cultures, fractionated on CsCl gradients, and quantified as a function of density by performing qPCR. The resulting data demonstrate that 14N- and/or 15N-containing Synechococcus DNA can be clearly separated under optimal conditions (100% 14N-containing DNA and/or 100% 15N-containing DNA) (Fig. 3B). The genome of Synechococcus sp. strain WH7803 has a G+C content of 60%, and the peak density of 14N-labeled Synechococcus DNA was determined to be 1.7188 g ml−1 based on the expected relationship between density and G+C content (see Materials and Methods). 15N-containing Synechococcus DNA is expected to have a density of ca. 1.7348 g ml−1, whereas the observed density was 1.7352 g ml−1 (Fig. 3). The difference (0.0004 g ml−1) between the observed and expected densities of 15N-labeled Synechococcus DNA was less than 2.5% of the total expected difference (0.0164 g ml−1) between the values for unlabeled and labeled DNA.
FIG. 3.
Stable isotope incorporation showing 15NO3 uptake in a culture of Synechococcus sp. strain WH7803. (A) Relationship between buoyant density of DNA in a CsCl gradient, G+C content, and percentage of 15N incorporated. The solid diagonal line indicates the predicted buoyant density of DNA containing only 14N. The dashed line indicates the predicted buoyant density of DNA with 100% 15N incorporation. The horizontal line indicates the G+C content of WH7803, and the vertical lines indicate the predicted buoyant densities of WH7803 with 0% and 100% 15N incorporation. (B) Two cultures of Synechococcus sp. strain WH7803 were grown on 14NO3 and 15NO3 as N sources. DNA was extracted from both cultures, and both cultures were fractionated on CsCl gradients. The WH7803 DNA in each fraction was quantified by qPCR by determining the rbcL gene copy number in each 100-μl fraction. The data are the ratios of quantities, which were calculated by dividing the measured amount of Synechococcus rbcL DNA in each fraction by the highest value measured in any of the fractions collected from a CsCl column. ▴, unlabeled WH7803 DNA (0% 15N); •, labeled WH7803 DNA (100% 15N).
Synechococcus spp. and diatoms were the dominant phytoplankton at the study site during the sampling time based on microscopy (data not shown), flow cytometry, and clone library analysis. These two important marine algal groups were therefore chosen for further characterization by 15N-based SIP. Our goal was to investigate the traditional characterization of diatoms as new producers (nitrate uptake) and Synechococcus spp. as recycled producers (mainly ammonium uptake).
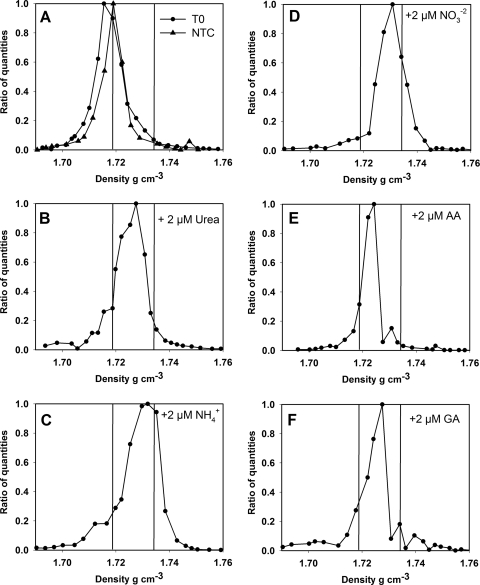
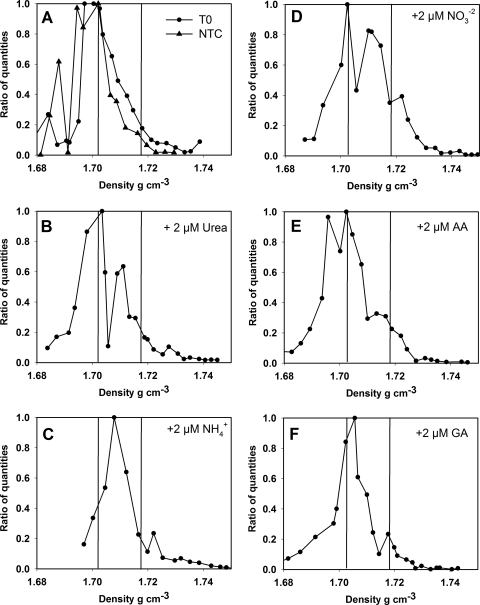
DNA extracted from seawater that was not incubated (T0) and seawater incubated without addition of any labeled or unlabeled N source (NTC) formed Synechococcus DNA peaks at 1.7155 g ml−1 and 1.7190 g ml−1, respectively, which was in good agreement with the density measured for the 14N-labeled WH7803 DNA peak (Fig. 4). Significant shifts in Synechococcus DNA density were observed for all 15N-labeled substrates used in this study. The greatest differences compared to controls were the differences for ammonium and nitrate (Fig. 4C and 4D), for which there were peaks at 1.7319 g ml−1 and 1.7308 g ml−1, respectively, corresponding to ca. 78% and 71% labeling of Synechococcus DNA with 15N during the incubation period. Uptake of the organic N substrates by Synechococcus spp. was less pronounced but was supported by SIP data. For the urea and glutamic acid incubations, the peak Synechococcus DNA densities were 1.7275 g ml−1 and 1.7264 g ml−1, corresponding to ca. 53% and 46% label incorporation, respectively (Fig. 4B and 4F). The lowest proportion of uptake was observed for mixed 15N-amino acids, for which there was a peak at 1.7243 g ml−1, corresponding to only about 34% labeling of Synechococcus DNA with 15N (Fig. 4E).
FIG. 4.
Amount of Synechococcus DNA as a function of density as determined by CsCl centrifugation and quantitative PCR, using the Synechococcus rbcL gene as a proxy in field samples. The vertical lines correspond to the vertical lines in Fig. 3 and indicate the density of Synechococcus DNA containing only 14N or only 15N. (A) Data for the ambient population (T0) and for incubation without addition of a 15N-labeled nitrogen source (NTC). (B to F) Data obtained after incubation in the presence of 2 μmol N liter−1 of (B) [15N]urea, (C) 15NH4+, (D) 15NO3−, (E) 15N-amino acids (AA), and (F) [15N]glutamic acid (GA). The data are ratios of quantities, which were calculated by dividing the measured amount of Synechococcus rbcL DNA in each fraction by the highest value measured for any of the fractions collected from a CsCl column.
qPCR data indicated the distributions (peaks) for diatom DNA were somewhat wider across the gradients (Fig. 5) than the distributions for Synechococcus DNA, which typically displayed very defined peaks and for which most of the signal was detected in only three or four fractions. For the T0 treatment and the NTC there were maxima at 1.7040 g ml−1 and 1.7021 g ml−1, respectively (Fig. 5A). Significant shifts in diatom DNA density were observed for treatments with 15N-labeled ammonium and nitrate. The maximum diatom DNA density in the ammonium treatment was 1.7079 g ml−1, corresponding to approximately 35% label incorporation (assuming a difference of 0.0164 g ml−1 between labeled DNA and unlabeled DNA) (Fig. 5C). Nitrate and urea treatments produced peak DNA concentrations at densities of 1.7024 g ml−1 and 1.7035 g ml−1, which correspond to little or no label uptake (Fig. 5B and 5D). However, the density distributions of diatom DNA for the nitrate and urea treatments also exhibited secondary peaks at 1.7100 g ml−1 and 1.7111 g ml−1, corresponding to 37% and 43% labeling of DNA with 15N, respectively (using the T0 measurement as a baseline). Treatments with 15N-amino acids and [15N]glutamic acid produced diatom DNA peaks at densities of 1.7024 g ml−1 and 1.7054 g ml−1, which were less than 2% and 15% greater than the control measurements, respectively (Fig. 5E and 5F).
FIG. 5.
Amount of diatom DNA as a function of density as determined by CsCl centrifugation and qPCR, using diatom rbcL genes as a proxy. The vertical lines correspond to the vertical lines in Fig. 1 and indicate the density of diatom DNA containing only 14N or only 15N. (A) Data for the ambient population (T0) and for incubation without addition of a 15N-labeled nitrogen source (NTC). (B to F) Data obtained after incubation in the presence of 2 μmol N liter−1 of (B) [15N]urea, (C) 15NH4+, (D) 15NO3−, (E) 15N-amino acids (AA), and (F) [15N]glutamic acid (GA). The data are ratios of quantities, which were calculated by dividing the measured amount of diatom rbcL DNA in each fraction by the highest value measured for any of the fractions collected from a CsCl column.
DISCUSSION
The objective of this work was to apply 15N-based SIP methodology to an investigation of N uptake in marine phytoplankton populations. Specifically, we targeted Synechococcus spp. and diatoms, which typically are important phytoplankton groups in near-shore environments along the west Florida shelf. We aimed to determine whether Synechococcus spp. and diatoms actively incorporated different inorganic and organic N compounds. The power of the technique described here lies in its ability to specifically link the uptake of an N source to DNA sequence data, which provides direct evidence for incorporation of a given substrate. Traditional methods for measuring N uptake rely on bulk community properties and cannot provide taxonomic information.
Synechococcus spp. are found throughout the world's oceans and have been characterized as important contributors to global C fixation, particularly in the oligotrophic oceans (33). Their abundance in nutrient-depleted regions has often led to the interpretation that Synechococcus spp. obtain their N primarily from recycled sources. Synechococcus spp. are, however, also often very abundant in coastal environments and upwelling regions, where nutrient concentrations are elevated (43). Furthermore, there is no unambiguous experimental evidence demonstrating sole use of recycled N by Synechococcus spp. (33). Our data indicate that Synechococcus spp. are, in fact, very versatile in terms of satisfying their N demand. All forms of labeled N presented to the ambient population of Synechococcus spp. at the study site were rapidly incorporated into DNA (within 24 h). These data therefore support the interpretation that Synechococcus spp. use recycled forms of N, including dissolved organic N (DON) forms such as amino acids and urea. Additionally, there was little or no difference between ammonium incorporation and nitrate incorporation (Fig. 4), which invalidates the notion that Synechococcus uses only recycled forms of nitrogen. The use of nitrate by Synechococcus spp. along the west Florida coast is particularly noteworthy in view of the high concentrations of regenerated DON forms available (Table 1). DON is composed of a large number of chemical structures that are often poorly characterized but are present in seawater at concentrations of 3 to 7 μM (4, 12, 57); thus, the levels of DON often far exceed the levels of dissolved inorganic N. DON concentrations in coastal systems can at times be even higher (28), and our study site was no exception, since the DON levels exceeded 16 μM (137-fold greater than the concentration of nitrate).
It is possible that some of the 15N label in the [15N]nitrate treatments was converted to [15N]ammonium during the course of the experiment via remineralization of particulate organic nitrogen (PON). If 15N-labeled ammonium was released as a result of nitrate uptake, the effect of this conversion on SIP measurements would likely be small. The mean atom% enrichment of the PON at the end of the experiment was 1.39. This means that a maximum of 1.39% of the N in the PON pool, which was available for remineralization to ammonium, was labeled with 15N. Although it is true that some of the labeled nitrogen atoms could be released as ammonium and then reincorporated by other cells, quantitatively the effect on measured ammonium uptake rates would be very small.
The use of urea by natural populations of Synechococcus spp. is consistent with data from genome sequencing. While the genome of strain WH7803 (a marine strain) does not contain genes annotated as encoding urease, many of the other sequenced Synechococcus genomes (freshwater or marine) in the database do contain such genes, including strains BL107, CC9311, CC9605, CC9902, JA-2-3Ba(2-13), JA-3-3Ab, RCC307, PCC 7002, RS9916, RS9917, WH5701, WH7805, and WH8102. Although urea has been recognized as an important source of N in marine systems (8, 35), relatively little is known about the microbiology of urea transformation in the oceans (58). Data presented here suggest that urea should be considered more carefully when the N dynamics in marine phytoplankton communities is studied.
Free amino acids are an important component of DON and have been shown in laboratory experiments to be a viable N source for some phytoplankton (5, 41, 58). Experimental evidence from field studies, however, is scarce, and most of it indicates that amino acids are not significantly used by phytoplankton (25, 26, 41). Our data contradict these previous observations and represent some of the first direct evidence of utilization of amino acids as an N source by a marine phytoplankton species in the environment (3, 59).
SIP data from our study site were consistent with a more restrictive N diet for diatoms (Fig. 5) than for Synechococcus spp. (Fig. 4). No evidence was detected to support the idea that mixed 15N-labeled amino acids or [15N]glutamate was incorporated into diatom DNA. This is somewhat contrary to recent evidence from genomic sequencing of the diatom Thalassiosira pseudonana, which included evidence for the uptake and use of organic forms of N, including amino acids and purines (1). However, several explanations for this discrepancy are plausible. Sufficient quantities of inorganic nutrients may have been present at the study site, and the relevant amino acid transporter genes may not have been expressed. Alternatively, observations for the T. pseudonana genome (1) may not reflect the genetic properties of the bulk diatom populations on the west Florida shelf. It is also possible that diatoms supplement their N needs by using only low levels of free amino acids, so that amino acid uptake accounts for only a small proportion of their total N demand. The average density increment of a 100-μl fraction collected in our experiments was ca. 0.0030 g ml−1. This corresponds to ca. 18% label incorporation, indicating that any uptake accounting for less than 18% of N nutrition would not have been resolved. Ammonium appeared to be the preferred N source for diatoms (Fig. 5C), which is consistent with the notion that ammonium requires the smallest amount of energy for assimilation. Both nitrate and urea treatments, however, produced bimodal distributions of diatom DNA in CsCl gradients, indicating that there is a more complex N nutritional profile for diatoms, which includes uptake of N from ammonium, nitrate, and urea. Bimodal distributions of diatom DNA in our gradients also indicate the presence of multiple populations of diatoms with different N nutritional strategies. The study site contained at least 24 different diatom lineages (at >95% rbcL gene sequence identity) (Fig. 2B). Different species may not have behaved as a homogeneous population.
The bimodal distribution of diatom DNA obtained with [15N]urea and [15N]nitrate treatments highlights an important limitation of the SIP technique. The diatom qPCR assay targets diatoms as a whole (55). It is possible that individual subpopulations within a larger phylogenetic group such as diatoms behave quite differently, making it difficult to resolve their behavior in CsCl gradients. Further, when a large and diverse phylogenetic group, such as diatoms, is targeted, there may be considerable variability in the DNA G+C content. G+C content significantly affects the apparent density of DNA during CsCl centrifugation (11). In our experiments, diatom DNA formed a peak that was somewhat broader and less well defined (Fig. 5A) than the peak observed in the phylogenetically more restricted Synechococcus qPCR assay (Fig. 4A). The utility of 15N SIP therefore increases with increasing phylogenetic specificity. Increased specificity, however, is accompanied by increases in signal-to-noise ratios during quantification. Using DNA extracted from cultures, we were able to resolve approximately 1 ng of Synechococcus DNA in a CsCl gradient (data not shown). This corresponds to approximately 106 genome equivalents and was the smallest amount of DNA that we could resolve and quantify reliably, even under optimal conditions. In the context of this study, for example, an attempt was made to study N uptake in K. brevis. K. brevis was present at a concentration of about 1.3 × 105 cells liter−1, and 400 ml of seawater was incubated and filtered. Each CsCl column therefore contained only approximately ca. 105 gene targets (assuming 100% extraction efficiency and diploid genomes), which produced inconclusive results during qPCR quantification.
Our data demonstrate that 15N SIP can be a powerful tool for investigation of N nutrition in marine phytoplankton communities. The data demonstrate that Synechococcus spp. are extremely versatile in satisfying their N demand and use both organic and inorganic N sources, while diatom N nutrition appears to be more complex. The uptake rates and SIP data also indicate that urea is an important N source for Synechococcus spp. and diatoms on the west Florida shelf. These data imply that traditional notions of new production versus recycled production in the context of Synechococcus spp. and diatoms should be applied with caution.
Acknowledgments
We thank the crew of the R/V Pelican for providing excellent shipboard support, C. Heil for ship access, M. Garrett for conductivity, temperature, and depth data, M. Mulholland and L. A. Procise for Synechococcus counts, P. Bernhardt for mass spectrometric analysis, and Q. Roberts and L. Killberg for field assistance and analytical support. We acknowledge L. D. McDaniel, D. John, and J. H. Paul for cultures and fruitful discussions. Lastly, we thank D. H. Buckley for technical advice and suggestions about 15N SIP methodology.
Funding was provided by the NOAA ECOHAB program via grant NA 06 NOS4780246 to D. A. Bronk and by a Junior Faculty Summer Research Fellowship to B. Wawrik through the University of Oklahoma.
Footnotes
Published ahead of print on 4 September 2009.
VIMS contribution 3042 from the Virginia Institute of Marine Science, The College of William and Mary.
REFERENCES
- 1.Armbrust, E. V., J. A. Berges, C. Bowler, B. R. Green, D. Martinez, N. H. Putnam, S. Zhou, A. E. Allen, K. E. Apt, M. Bechner, M. A. Brzezinski, B. K. Chaal, A. Chiovitti, A. K. Davis, M. S. Demarest, J. C. Detter, T. Glavina, D. Goodstein, M. Z. Hadi, U. Hellsten, M. Hildebrand, B. D. Jenkins, J. Jurka, V. V. Kapitonov, N. Kroger, W. W. Lau, T. W. Lane, F. W. Larimer, J. C. Lippmeier, S. Lucas, M. Medina, A. Montsant, M. Obornik, M. S. Parker, B. Palenik, G. J. Pazour, P. M. Richardson, T. A. Rynearson, M. A. Saito, D. C. Schwartz, K. Thamatrakoln, K. Valentin, A. Vardi, F. P. Wilkerson, and D. S. Rokhsar. 2004. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Birnie, G. D., and D. Rickwood (ed.). 1978. Centrifugal separations in molecular and cell biology. Butterworth-Heinemann Publishers, Boston, MA.
- 3.Bradley, P., and D. A. Bronk. Inorganic and organic nitrogen uptake by phytoplankton and heterotrophic bacteria in a stratified Mid-Atlantic Bight upwelling region. Estuar. Coast. Shelf Sci., in press.
- 4.Bronk, D. A. 2002. Dynamics of DON, p. 153-249. In D. A. Hansell and C. A. Carlson (ed.), Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego, CA.
- 5.Bronk, D. A., and K. J. Flynn. 2006. Algal cultures as a tool to study the cycling of dissolved organic nitrogen, p. 301-341. In S. R. V. Durvasula (ed.), Algal cultures, analogues of blooms and applications. Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi, India.
- 6.Bronk, D. A., P. M. Glibert, T. C. Malone, S. Banahan, and E. Sahlsten. 1998. Inorganic and organic nitrogen cycling in Chesapeake Bay: autotrophic versus heterotrophic processes and relationships to carbon flux. Aquat. Microb. Ecol. 15:177-189. [Google Scholar]
- 7.Bronk, D. A., M. Lomas, P. M. Glibert, K. J. Schukert, and M. P. Sanderson. 2000. Total dissolved nitrogen analysis: comparisons between the persulfate, UV and high temperature oxidation methods. Mar. Chem. 69:163-178. [Google Scholar]
- 8.Bronk, D. A., J. H. See, P. Bradley, and L. Killberg. 2007. DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences 4:283-296. [Google Scholar]
- 9.Brzezinski, M. A. 1987. Colorimetric determination of nanomolar concentrations of ammonium in seawater using solvent extractions. Mar. Chem. 20:277-288. [Google Scholar]
- 10.Buckley, D. H., V. Huangyutitham, S. F. Hsu, and T. A. Nelson. 2007. Stable isotope probing with 15N2 reveals novel noncultivated diazotrophs in soil. Appl. Environ. Microbiol. 73:3196-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley, D. H., V. Huangyutitham, S. F. Hsu, and T. A. Nelson. 2007. Stable isotope probing with 15N achieved by disentangling the effects of genome G+C content and isotope enrichment on DNA density. Appl. Environ. Microbiol. 73:3189-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capone, D. G. 2000. The marine microbial nitrogen cycle, p. 455-494. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, NY.
- 13.Carpenter, E. J., and D. G. Capone. 2008. Nitrogen fixation in the marine environment, p. 141-199. In D. G. Capone, D. A. Bronk, M. Mulholland, and E. J. Carpenter (ed.), Nitrogen in the marine environment. Elsevier, Academic Press, San Diego, CA.
- 14.Carpenter, E. J., H. R. Harvey, B. Fry, and D. G. Capone. 1997. Biogeochemical tracers of the marine cyanobacterium Trichodesmium. Deep-Sea Res. Part I 44:27-38. [Google Scholar]
- 15.Davis, H. C., and R. R. L. Guillard. 1958. Relative value of ten genera of micro-organisms as foods for oyster and clam larvae. U.S. Fish Wildl. Serv. Fish. Bull. 136:293-304. [Google Scholar]
- 16.Dudek, N., M. A. Brzezinski, and P. A. Wheeler. 1986. Recovery of ammonium nitrogen by solvent extraction for the determination of relative 15N abundance in regeneration experiments. Mar. Chem. 18:59-69. [Google Scholar]
- 17.Dugdale, R. C., and J. J. Goering. 1967. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol. Oceanogr. 12:196-206. [Google Scholar]
- 18.Dugdale, R. C., and F. P. Wilkerson. 1998. Silicate regulation of new production in the equatorial Pacific upwelling. Nature 391:270-273. [Google Scholar]
- 19.Dumont, M. G., and J. C. Murrell. 2005. Stable isotope probing—linking microbial identity to function. Nat. Rev. Microbiol. 3:499-504. [DOI] [PubMed] [Google Scholar]
- 20.Eppley, R. W., and B. J. Peterson. 1979. Particulate organic matter flux and planktonic new production in the deep ocean. Nature 282:677-680. [Google Scholar]
- 21.Falkowski, P. G., R. T. Barber, and V. V. Smetacek. 1998. Biogeochemical controls and feedbacks on ocean primary production. Science 281:200-207. [DOI] [PubMed] [Google Scholar]
- 22.Glibert, P. M., F. Lipschultz, J. J. McCarthy, and M. A. Altabet. 1982. Isotope dilution models of uptake and remineralization of ammonium by marine plankton. Limnol. Oceanogr. 27:639-650. [Google Scholar]
- 23.Grasshoff, K., K. Kremling, and M. Ehrhardt. 1999. Methods of seawater analysis, p. 599. Wiley-VCH, Weinheim, Germany.
- 24.Guillard, R. R. L., and J. H. Ryther. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 8:229-239. [DOI] [PubMed] [Google Scholar]
- 25.Hollibaugh, J. T. 1976. The biological degradation of argninine and glutamic acid in seawater in relation to the growth of phytoplankton. Mar. Biol. 36:.
- 26.Hoppe, H. G. 1976. Determination and properties of actively metabolizing heterotrophic bacteria in the sea, investigated by means of microautoradiography. Mar. Biol. 36:291-302. [Google Scholar]
- 27.John, D. E., S. S. Patterson, and J. H. Paul. 2007. Phytoplankton-group specific quantitative polymerase chain reaction assays for RuBisCO mRNA transcripts in seawater. Mar. Biotechnol. 9:747-759. [DOI] [PubMed] [Google Scholar]
- 28.Kirchman, D. L. 2000. Uptake and regeneration of inorganic nutrients by marine heterotrophic bacteria, p. 261-288. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, NY.
- 29.Kirchman, D. L. 1994. The uptake of inorganic nutrients by heterotrophic bacteria. Microb. Ecol. 28:255-271. [DOI] [PubMed] [Google Scholar]
- 30.Kirchman, D. L., P. G. Keil, and P. A. Wheeler. 1989. The effect of amino acids on ammonium utilization and regeneration by heterotrophic bacteria in the subarctic Pacific. Deep-Sea Res. 36:1763-1776. [Google Scholar]
- 31.Kirchman, D. L., and P. A. Wheeler. 1998. Uptake of ammonium and nitrate by hetertrophic bacteria and phytoplankton in the sub-Arctic Pacific. Deep-Sea Res. 45:347-365. [Google Scholar]
- 32.Lindell, D., E. Padan, and F. F. Post. 1998. Regulation of ntcA expression and nitrite uptake in the marine Synechococcus sp. strain WH 7803. J. Bacteriol. 180:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindell, D., and A. F. Post. 2001. Ecological aspects of ntcA gene expression and its use as an indicator of the nitrogen status of marine Synechococcus spp. Appl. Environ. Microbiol. 67:3340-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipschultz, F., N. R. Bates, C. A. Carlson, and D. A. Hansell. 2002. New production in the Sargasso Sea: history and current status. Global Biogeochem. Cycles 16:1-16. [Google Scholar]
- 35.Lomas, M. W., T. M. Trice, P. M. Glibert, D. A. Bronk, and J. J. McCarthy. 2002. Temporal and spatial dynamics of urea uptake and regeneration rates and concentrations in Chesapeake Bay. Estuaries 25:469-482. [Google Scholar]
- 36.Manefield, M., A. S. Whiteley, R. I. Griffiths, and M. J. Bailey. 2002. RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manefield, M., A. S. Whiteley, N. Ostle, P. Ineson, and M. J. Bailey. 2002. Technical considerations for RNA-based stable isotope probing: an approach to associating microbial diversity with microbial community function. Rapid Commun. Mass Spectrom. 16:2179-2183. [DOI] [PubMed] [Google Scholar]
- 38.McGillicuddy, D. J., A. R. Robinson, D. A. Siegel, H. W. Jannasch, R. Johnson, T. Dickeys, J. McNeil, A. F. Michaels, and A. H. Knap. 1998. Influence of mesoscale eddies on new production in the Sargasso Sea. Nature 394:263-266. [Google Scholar]
- 39.Meselson, M., and F. W. Stahl. 1958. The replication of DNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 44:671-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neufeld, J. D., M. G. Dumont, J. Vohra, and J. C. Murrell. 2007. Methodological considerations for the use of stable isotope probing in microbial ecology. Microb. Ecol. 53:435-442. [DOI] [PubMed] [Google Scholar]
- 41.Palenik, B., and F. M. M. Morel. 1990. Amino acid utilization by marine phytoplankton: a novel mechanism. Limnol. Oceanogr. 35:260-269. [Google Scholar]
- 42.Parsons, T. R., Y. Maita, and C. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, United Kingdom.
- 43.Partensky, F., J. Blanchot, and D. Vaulot. 1999. Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: a review, p. 457-475. In L. Charpy and A. W. D. Larkum (ed.), Marine cyanobacteria, vol. 19. Musee Oceanographique, Monaco. [Google Scholar]
- 44.Peltzer, E. T., B. Fry, P. H. Doering, J. H. McKenna, B. Norrman, and U. L. Zweifel. 1996. A comparison of methods for the measurement of dissolved organic carbon in natural waters. Mar. Chem. 54:85-96. [Google Scholar]
- 45.Price, N., and P. Harrison. 1987. Comparison of methods for the analysis of dissolved urea in seawater. Mar. Biol. 94:307-317. [Google Scholar]
- 46.Radajewski, S., P. Ineson, N. R. Parekh, and J. C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646-649. [DOI] [PubMed] [Google Scholar]
- 47.Ryther, J. H., and W. M. Dunstan. 1971. Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science 171:1008-1013. [DOI] [PubMed] [Google Scholar]
- 48.Scharek, R., L. M. Tupas, and D. M. Karl. 1999. Diatom fluxes to the deep sea in the oligotrophic North Pacific gyre at Station ALOHA. Mar. Ecol. Prog. Ser. 182:55-67. [Google Scholar]
- 49.Sigman, D. M., K. L. Casciotti, M. Andreani, C. Barford, M. Galanter, and J. K. Bohlke. 2001. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Ann. Chem. 73:4145-4153. [DOI] [PubMed] [Google Scholar]
- 50.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 51.Valderrama, J. C. 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 10:109-122. [Google Scholar]
- 52.Waterbury, J. B., S. W. Watson, F. W. Valois, and D. G. Franks. 1986. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can. Bull. Fish. Aquat. Sci. 217:71. [Google Scholar]
- 53.Wawrik, B., and J. H. Paul. 2004. Phytoplankton community structure and productivity along a transect of the Mississippi Plume. Aquat. Microb. Ecol. 35:175-184. [Google Scholar]
- 54.Wawrik, B., J. H. Paul, L. Campbell, D. Griffin, L. Houchin, A. Fuentes-Ortega, and F. Mueller-Karger. 2003. Vertical structure of rbcL-containing phytoplankton phylotypes associated with a coastal plume in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 251:87-101. [Google Scholar]
- 55.Wawrik, B., J. H. Paul, and F. R. Tabita. 2002. Real-time PCR quantification of rbcL (ribulose-1,5-bisphosphate carboxylase/oxygenase) mRNA in diatoms and pelagophytes. Appl. Environ. Microbiol. 68:3771-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyman, M., F. P. F. Gregory, and N. G. Carr. 1985. Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science 230:818-820. [DOI] [PubMed] [Google Scholar]
- 57.Zehr, J. P., M. T. Mellon, and S. Zani. 1998. New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase (nifH) genes Appl. Environ. Microbiol. 64:5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zehr, J. P., and B. B. Ward. 2002. Nitrogen cycling in the ocean: new perspectives on processes and paradigms. Appl. Environ. Microbiol. 68:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zubkov, M., and G. A. Tarran. 2005. Amino acid uptake of Prochlorococcus spp. in surface waters across the South Atlantic Subtropical Front. Aquat. Microb. Ecol. 40:241-249. [Google Scholar]