Abstract
Protective cultures can be used successfully as an additional hurdle together with phages to reduce growth of Listeria monocytogenes on sliced cooked ham. Addition of phages resulted in a rapid 10-fold reduction of L. monocytogenes. After 14 to 28 days of storage, a 100-fold reduction was observed in samples with phages and protective culture compared to results for samples with phages alone.
Listeriosis in Europe has an average incidence between 2 and 10 reported cases per million population per year (7). Listeria monocytogenes is found in raw and ready-to-eat (RTE) products, poultry, seafood, and dairy products. A review of the incidence and transmission of L. monocytogenes in RTE products has been published by Lianou and Sofos (11). The USDA has implemented a “zero-tolerance” policy for L. monocytogenes in RTE products (2). In the European Union, the limit for common RTE foods is 100 CFU/g (1). Recently, Codex Alimentarius adopted new standards for L. monocytogenes in RTE foods, with a limit of 100 CFU/g in foods where L. monocytogenes cannot grow and absence in foods where the bacterium can grow. However, an alternative approach is accepted. Competent authorities may choose to establish and implement other validated limits (http://www.codexalimentarius.net/web/archives.jsp?lang=en, Alinorm 09/32/REP and Alinorm 09/32/13). L. monocytogenes in cooked products is connected with cross-contamination after heat treatment (11, 12). Bacteriophages have been successfully applied to a number of food products to reduce the level of contaminating L. monocytogenes (6, 8-10, 13, 14). The effect of phages varies with the type of product and is strongly dose dependent (6, 8). Active phages can be recovered from foods after long storage, but the phage particles appear to become immobilized soon after addition to nonliquid foods and therefore, due to limited diffusion, cannot infect bacteria (8). Bacteria surviving phage treatment can later grow in the product. Additional hurdles should therefore be present to inhibit later outgrowth of L. monocytogenes.
We have previously employed Lactobacillus sakei TH1 as a protective culture against L. monocytogenes (4, 5). Here we examine the combined use of phages and protective culture to reduce outgrowth of L. monocytogenes on cooked ham.
Rifampin (rifampicin)-resistant mutants of L. monocytogenes 2230/92 serotype 1, implicated in a listeriosis outbreak in Norway (12), and L. monocytogenes 167 serotype 4b were grown overnight in brain heart infusion (Difco Laboratories, Detroit, MI) at 37°C without shaking and stored at 4°C for 24 h (3-5). Cells were diluted in 0.9% NaCl and plated on brain heart infusion agar with 200 μg/ml rifampin. L. sakei TH1 was grown at 30°C in MRS (de Man, Rogosa, Sharpe) broth (CM 359; Oxoid, Hampshire, England) (pH 6.2) and plated on MRS agar (5). Listex P100 phages, 2 × 1011 PFU/ml, were from EBI Food Safety (Wageningen, The Netherlands).
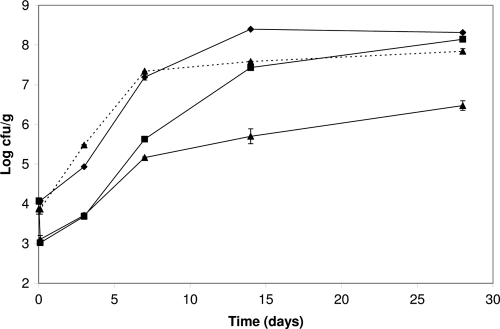
Ten-gram slices of hams with 2.3% NaCl and 0.01% disodium diphosphate (pH 6.2; aw > 0.97), made at Nofima's pilot plant (Aas, Norway), were inoculated with a cold-adapted 1:1 mixture of L. monocytogenes 2230/92 and 167. Bacteria were spread in 100 μl 0.9% NaCl over the 80-cm2 surface area of each slice to 103 CFU/cm2 using a bent glass rod. After 1 h at 20°C, phages (5 × 107 PFU/cm2 in a total volume of 100 μl) were spread over the same surface. After one additional hour, 103 CFU/cm2 L. sakei TH1 in 100 μl 0.9% NaCl was added where appropriate. The slices were vacuum packed and stored at 10°C. Growth was measured before and after spiking and at 0, 3, 7, 14, and 28 days after homogenizing the slices in 100 ml 0.9% NaCl in a Stomacher homogenizer. No lactic acid bacteria were detected in uninoculated samples. Experiments were performed with three parallel samples. L. monocytogenes alone grew from 104 CFU/g at the onset of the experiment to 107 CFU/g the first 7 days, reached 2 × 108 CFU/g after 14 days, and remained unchanged thereafter (Fig. 1). In samples with both L. monocytogenes and phages, a rapid 1-log reduction in L. monocytogenes was observed. Surviving L. monocytogenes, however, grew as well as that in the phage-free controls, reaching >107 CFU/g after 14 days. In samples where both P100 phages and L. sakei TH1 were added, the same initial reduction of L. monocytogenes was observed, but the later outgrowth was reduced by the fast-growing lactic acid bacteria and the L. monocytogenes levels were 2 logs lower than those with P100 phages alone after 28 days of incubation. The phages did not influence the growth and survival of L. sakei TH1. During the 28 days of storage, the pH changed from 6.20 to 6.05 in samples with L. monocytogenes and to 6.00 in samples with both L. monocytogenes and L. sakei TH1. The results were reproduced in a separate repetition of the experiment at 10°C (not shown).
FIG. 1.
Inhibition of L. monocytogenes in cooked ham with bacteriophages and protective culture at 10°C. Sliced ham was inoculated with 103 CFU/cm2 (corresponding to approximately 104 CFU/g) L. monocytogenes (⧫), L. monocytogenes and 5 × 107 PFU/cm2 P100 phages (▪), or L. monocytogenes, 5 × 107 PFU/cm2 P100 phages, and 103 CFU/cm2 (approximately 104 CFU/g) protective-culture L. sakei TH1 (▴) and stored at 10°C. Growth of L. sakei TH1 is shown by the broken line.
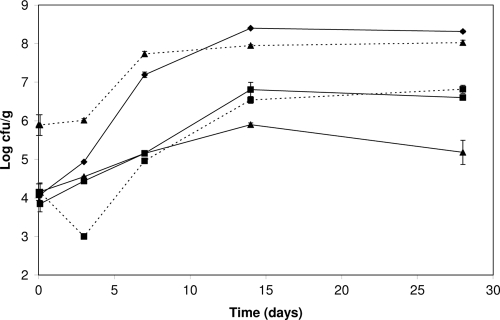
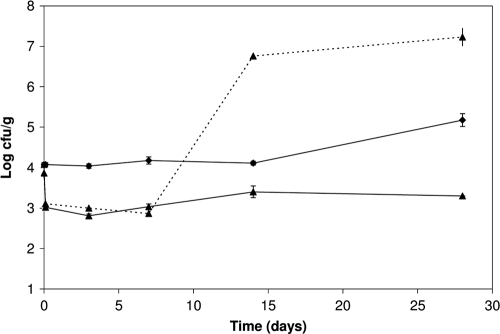
The effect of the protective culture was dose dependent when 104 CFU/g and 106 CFU/g of L. sakei TH1 were added to slices of ham (Fig. 2). L. monocytogenes alone grew to 2 × 108 CFU/g after 14 days. When L. sakei TH1 was added at a low concentration (104 CFU/g), L. monocytogenes grew to approximately 4 × 106 CFU/g, while when L. sakei TH1 was added at a high concentration, L. monocytogenes levels were 1 to 2 logs lower. The pHs in the low- and high-inoculum hams were reduced from the initial 6.20 to 6.16 and 6.02, respectively, at day 28. For hams stored at 4°C, slow growth of L. monocytogenes occurred between days 14 and 28 from 104 to 105 CFU/g (P = 0.003) (Fig. 3). With phages and L. sakei TH1 added, a rapid 1-log reduction of L. monocytogenes was observed due to the phage attack, and no growth was observed during the 28-day storage period. The L. sakei TH1 strain showed a longer lag phase at this low temperature but nevertheless reached 107 CFU/g at day 14 and thereby inhibited any growth of L. monocytogenes.
FIG. 2.
Inhibition of L. monocytogenes in cooked ham inoculated with large or small amounts of protective culture at 10°C. Sliced ham was inoculated with 103 CFU/cm2 (corresponding to approximately 104 CFU/g) L. monocytogenes (⧫), L. monocytogenes and 106 CFU/g (105 CFU/cm2) L. sakei TH1 (▴), or L. monocytogenes and 104 CFU/g (103 CFU/cm2) L. sakei TH1 (▪) and stored at 10°C. Growth of L. sakei TH1 is shown by broken lines. The L. monocytogenes control is the same control as in Fig. 1.
FIG. 3.
Inhibition of L. monocytogenes in cooked ham with bacteriophages and protective culture at 4°C. Sliced ham was inoculated with 103 CFU/cm2 (corresponding to approximately 104 CFU/g) L. monocytogenes (⧫) or L. monocytogenes, 5 × 107 PFU/cm2 P100 phages, and 104 CFU/g (103 CFU/cm2) protective-culture, L. sakei TH1 (▴) and stored at 4°C. Growth of L. sakei TH1 is shown by the broken line.
Since L. sakei TH1 grows well at low temperatures, prevents growth of L. monocytogenes, and has no negative influence on the organoleptic properties of ham (4, 5), it can successfully be employed as an additional hurdle together with phages.
We here chose to perform the storage experiments under “worst-case” conditions. Generally, the contamination levels of L. monocytogenes are lower than in our setup, in the range of 10 to 100 CFU/g (see reference 11 and references therein). Since L. sakei TH1 grows well at low temperatures (Fig. 3), its selective advantage will be greater at 4°C than at abuse temperatures. From the above, it is evident that it is possible to optimize L. monocytogenes inhibition by increasing both the phage titer and the starting amount of protective culture. An enhanced effect may also be experienced by modifying phage application, e.g., by using larger liquid volumes (6, 8).
Emergence of resistant L. monocytogenes may be a potential problem when treating foods with phages. No emergence of resistance has been detected after phage treatment (6, 8). Such strategies as use of phage mixtures, phage rotation schemes, and treatment of products immediately prior to packaging may reduce eventual resistance problems (8). Some L. monocytogenes strains are naturally phage resistant (6). In these cases, a protective culture still constitutes a powerful hurdle.
In conclusion, we have shown here that by applying phages and protective culture as two independent hurdles, it is possible to both reduce the number of L. monocytogenes bacteria on a product and inhibit outgrowth of eventual remaining surviving cells. This is a general method that can potentially be applied to different foods where there is a potential risk for growth of L. monocytogenes, provided a suitable protective culture is available.
Acknowledgments
We thank Steven Hagens and Mark Offerhaus, EBI Food Safety, Wageningen, The Netherlands, for providing P100 phages and for fruitful discussions and Tom C. Johannessen, Nofima mat, for making the hams.
Footnotes
Published ahead of print on 28 August 2009.
REFERENCES
- 1.Anonymous. 2005. Commission regulation (EC) no. 2073/2005 of November 2000 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005:L338. [Google Scholar]
- 2.Anonymous. 1989. Revised policy for controlling Listeria monocytogenes. Fed. Regist. 54:22345-22346. [Google Scholar]
- 3.Blom, H., E. Nerbrink, R. Dainty, T. Hagtvedt, E. Borch, H. Nissen, and T. Nesbakken. 1997. Addition of 2.5% lactate and 0.25% acetate controls growth of Listeria monocytogenes in vacuum-packed, sensory-acceptable servelat sausage and cooked ham stored at 4 degrees C. Int. J. Food Microbiol. 38:71-76. [DOI] [PubMed] [Google Scholar]
- 4.Bredholt, S., T. Nesbakken, and A. Holck. 2001. Industrial application of an antilisterial strain of Lactobacillus sakei as a protective culture and its effect on the sensory acceptability of cooked, sliced, vacuum-packaged meats. Int. J. Food Microbiol. 66:191-196. [DOI] [PubMed] [Google Scholar]
- 5.Bredholt, S., T. Nesbakken, and A. Holck. 1999. Protective cultures inhibit growth of Listeria monocytogenes and Escherichia coli O157:H7 in cooked, sliced, vacuum- and gas-packaged meat. Int. J. Food Microbiol. 53:43-52. [DOI] [PubMed] [Google Scholar]
- 6.Carlton, R. M., W. H. Noordman, B. Biswas, E. D. de Meester, and M. J. Loessner. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43:301-312. [DOI] [PubMed] [Google Scholar]
- 7.de Valk, H., C. Jacquet, V. Goulet, V. Vaillant, A. Perra, F. Simon, J. C. Desenclos, and P. Martin. 2005. Surveillance of Listeria infections in Europe. Euro Surveill. 10:251-255. [PubMed] [Google Scholar]
- 8.Guenther, S., D. Huwyler, S. Richard, and M. J. Loessner. 2009. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 75:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leverentz, B., W. S. Conway, M. J. Camp, W. J. Janisiewicz, T. Abuladze, M. Yang, R. Saftner, and A. Sulakvelidze. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leverentz, B., W. S. Conway, W. Janisiewicz, and M. J. Camp. 2004. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67:1682-1686. [DOI] [PubMed] [Google Scholar]
- 11.Lianou, A., and J. N. Sofos. 2007. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J. Food Prot. 70:2172-2198. [DOI] [PubMed] [Google Scholar]
- 12.Nesbakken, T. 1995. Listeria monocytogenes in the food chain—a foodborne infection problem common to the Nordic countries. TemaNord 635. Nordic Council of Ministers, Copenhagen, Denmark.
- 13.Roy, B., H. W. Ackermann, S. Pandian, G. Picard, and J. Goulet. 1993. Biological inactivation of adhering Listeria monocytogenes by Listeria phages and a quaternary ammonium compound. Appl. Environ. Microbiol. 59:2914-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schellekens, M. M., J. Wouters, S. Hagens, and J. Hugenholtz. 2007. Bacteriophage P100 application to control Listeria monocytogenes on smeared cheese. Milk Sci. Int. 62:284-287. [Google Scholar]