Abstract
The opportunistic pathogen Staphylococcus epidermidis colonizes indwelling medical devices by biofilm formation but is primarily a skin resident. In many S. epidermidis strains biofilm formation is mediated by a cell wall-anchored protein, the accumulation-associated protein (Aap). Here, we investigate the role of Aap in skin adhesion. Aap is an LPXTG protein with a domain architecture including a terminal A domain and a B-repeat region. S. epidermidis NCTC 11047 expresses Aap as localized, lateral tufts of fibrils on one subpopulation of cells (Fib+), whereas a second subpopulation does not express these fibrils of Aap (Fib−). Flow cytometry showed that 72% of NCTC 11047 cells expressed Aap and that 28% of cells did not. Aap is involved in the adhesion of Fib+ cells to squamous epithelial cells from the hand (corneocytes), as the recombinant A-domain protein partially blocked binding to corneocytes. To confirm the role of the Aap A domain in corneocyte attachment, Aap was expressed on the surface of Lactococcus lactis MG1363 as sparsely distributed, peritrichous fibrils. The expression of Aap increased corneocyte adhesion 20-fold compared to L. lactis carrying Aap without an A domain. S. epidermidis isolates from catheters, artificial joints, skin, and the nose also used the A domain of Aap to adhere to corneocytes, emphasizing the role of Aap in skin adhesion. In addition, L. lactis expressing Aap with different numbers of B repeats revealed a positive correlation between the number of B repeats and adhesion to corneocytes, suggesting an additional function for the B region in enhancing A-domain-dependent attachment to skin. Therefore, in addition to its established role in biofilm formation, Aap can also promote adhesion to corneocytes and is likely to be an important adhesin in S. epidermidis skin colonization.
Staphylococcus epidermidis is the leading cause of nosocomial infections associated with indwelling medical devices including intravascular catheters, cardiac pacemakers, and artificial joints (16, 46). The main virulence mechanism is biofilm formation, which promotes persistence in the host, leading to infections such as bacteremia or endocarditis (1). S. epidermidis is also a common commensal resident on the skin all over the human body and may be a transient member of the oral microflora (31, 38). Clinical evidence shows that commensal strains from the skin and mucous membranes can translocate to cause bacteremia (12). In addition, there have been recent reports of linezolid resistance in skin-commensal strains of S. epidermidis (33, 41). It is therefore important to study the bacterial factors involved in S. epidermidis colonization of the skin, as this is likely to provide a reservoir for contaminating medical devices.
Very little is known about how S. epidermidis colonizes the skin, although many cell wall-associated adhesins that are involved with adhesion, mainly to host matrix proteins, have been identified. The S. epidermidis RP62A genome contains 11 putative LPXTG cell wall-anchored proteins (4), a class of proteins common on gram-positive cocci that often mediate adhesion to host proteins (49). So far, only three of these have prescribed functions: the Bap homology protein (Bhp) and the accumulation-associated protein (Aap) are involved in biofilm formation (13, 30, 45), and SdrG mediates adhesion to fibrinogen. In addition, S. epidermidis is known to express a variety of other non-LPXTG proteins such as the autolysins Aae, which promotes adhesion to vitronectin and the β-chain of fibrinogen (26, 47), and AltE, which promotes adhesion to vitronectin (25). Elastin binding protein (Ebp) (40, 59), extracellular lipase (GehD) (5), extracellular matrix binding protein (Embp) (57), and staphylococcal surface protein 1 (Ssp-1) and Ssp-2 (53) mediate adhesion to elastin, collagen, fibronectin, and polystyrene, respectively. Furthermore, teichoic acids have been shown to promote adhesion to fibronectin (29), and a polysaccharide termed PS/A or PIA (35) promotes adhesion to a plastic used to make catheters (52). To date, no work has been published linking any of these adhesins to the colonization of the skin.
We recently showed that one of the LPXTG cell wall-anchored proteins, Aap on S. epidermidis NCTC 11047, is a thin, fibrillar protein that projects 120 nm away from the cell wall in localized tufts (3); this study investigated the possible role of Aap in mediating adhesion to human skin cells. Aap is an archetypal LPXTG protein with a Sec-dependent signal sequence and a 556-amino-acid (aa) N-terminal A domain, which comprises 10 imperfect repeats of 16 aa and a nonrepetitive region. Proximal to the A domain are several 128-aa B repeats, the number of which varies between strains. For example, S. epidermidis strains RP62A and NCTC 11047 have 12 full and 1 partial B repeat (3, 20) compared to 5.5 B repeats in strain 5179 (45). Aap is important in biofilm formation, but the A domain must be cleaved for the B repeats to promote intercellular adhesion in the accumulation phase of biofilm formation (45). Cell-to-cell adhesion is thought to rely on the Zn2+-dependent dimerization of B-repeat regions (9). In addition, Aap has been indirectly implicated in adhesion to nasal epithelial cells (NECs) (43), as the Aap homolog SasG from Staphylococcus aureus was found to mediate adhesion to NECs, and a recombinant protein derived from the A domain of Aap (rAapA-Dom) was able to block the adhesion of a surrogate host expressing SasG to NECs. It was therefore suggested that both SasG and Aap share a receptor on the host cell surface of NECs (43).
Not all cells in a wild-type (WT) population of S. epidermidis NCTC 11047 express fibrillar tufts of Aap, as stationary-phase cells contain a subpopulation of cells with Aap fibrils (Fib+ cells) and a second subpopulation of cells that have no tufts of fibrils and no Aap on the cell surfaces (Fib− cells) (3). The subpopulations were separated by 36 cycles of hexadecane enrichment to yield two stable populations (Fib+ and Fib−). The Fib− subpopulation expressed only Aap mRNA but no Aap protein, and it was previously proposed that fibril expression is regulated at the posttranscriptional level by an unknown mechanism (3). Fib+ cells, expressing Aap, are also more hydrophobic and have greater affinity for polystyrene than do Fib− cells (3). Therefore, strain NCTC 11047 produces some cells that have the potential to form biofilms due to the presence of Aap and some that may lack the ability. Any adhesive functions mediated by Aap on the Fib+ subpopulation would be absent in the Fib− subpopulation.
Here, we present data which shows that the A domain of Aap on S. epidermidis NCTC 11047 mediates adhesion to corneocytes from the uppermost layer of the skin epidermis. The results suggest that Aap could play an important role in the colonization of human skin by S. epidermidis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains and plasmids are listed in Table 1. S. epidermidis JB strains were isolated from the anterior nares (JBN strains) or from the skin of the forehead (JBS strains) of healthy volunteers or were donated by the University of Manchester Medical Microbiology Culture Collection, having been isolated from intravenous catheters (JBC strains) or cases of hip joint infection (JBJ strains). Isolates were confirmed as being S. epidermidis isolates by both API 20 Staph tests (Biomerieux Industry) and 16S rRNA gene sequence determination using the primers shown in Table 1 (34). S. epidermidis strains were cultured statically in tryptic soya broth (Oxoid) at 37°C. L. lactis MG1363 (17) cells were cultured statically at 30°C in M17 broth (Oxoid) supplemented with 0.5% (wt/vol) glucose (GM17). Streptococcus gordonii DL1 cells were cultured statically at 37°C in brain heart infusion broth (Oxoid) supplemented with 0.5% yeast extract. Erythromycin (5 μg/ml) (Sigma) was added to medium when strains containing pUB1000 were cultured.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain(s), plasmid(s), or primer | Descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| S. epidermidis | ||
| NCTC 11047 WT | Nasal isolate; Aap+ | |
| NCTC 11047 Fib+ | Subpopulation; Aap+ | 3 |
| NCTC 11047 Fib− | Subpopulation; Aap− | 3 |
| RP62A | Intravenous catheter isolate; Aap+ | 7 |
| JBN3, JBN8, JBN9, and JBN10 | Nasal isolates; Aap+ | This study |
| JBJ1, JBJ4, and JBJ5 | Joint infection isolates; Aap+ | This study |
| JBC7, JBC9, and JBC13 | Catheter infection isolates; Aap+ | This study |
| JBS4, JBS5, and JBS14 | Skin isolates; Aap+ | This study |
| L. lactis MG1363 | Surrogate host for Aap expression | 17 |
| S. gordonii DL1 (NCTC 7868) | Intermediate cloning host | |
| Plasmids | ||
| pUB1000 | L. lactis cell wall expression vector carrying erythromycin resistance | 24 |
| pUB100aap6high | pUB1000 carrying the aap gene with 6 B repeats giving a high level of expression | This study |
| pUB1000aap6highT | pUB1000 carrying a truncated version of aap6 with no A domain giving a high level of expression | This study |
| pUB1000aap2, pUB1000aap4, pUB1000aap5, pUB1000aap6, and pUB1000aap7 | pUB1000 carrying aap genes with 2, 4, 5, 6, or 7 B repeats giving a lower level of expression | This study |
| Primers | ||
| 16SR | 16S RNA gene sequencing of S. epidermidis isolates (CCGTCAATTCGTTT CAGTTT) | 34 |
| raap157-857F | Cloning region of short repeats within the A domain (CCGGGATCCGCAG AAGAAAAACAAGTTGATC) | 43 |
| raap157-857R | Cloning region of short repeats within the A domain (CGGAAGCTTGATAG TTGGAACATTCGGTGCTTC) | This study |
| aapFSalI | Cloning of aap into pUB1000 (TACGCTGTCGACCCAATTACACAAG CTAATCAAAATGATAG) | This study |
| aapRBamHI | Cloning of aap into pUB1000 (TGTCGGATCCAAATTATTTTT CATTACCTTTTTTACGACG) | This study |
| pUB1000F | Sequencing of aap inserts (CCGTTGTCAGGTGTTTACGCT) | This study |
| pUB1000R | Sequencing of aap inserts (CTTTTGGTGTCTCAGGTTTGT) | This study |
| aapTFSalI | Cloning of truncated aap into pUB1000 (TACGCTGTCGACAGAGCTGA TTTAGATGGTGC) | This study |
| aapTRSalI | Cloning of truncated aap into pUB1000 (TACGCTGTCGACAGCGTAAA CACCTG) | This study |
| Aap53-608 r.c. | Checking size of the B region (CATTGACATACACTCCTAAGC) | 43 |
| aapR | Checking size of the B region (CCAAATATGAACAATGATCCG) | This study |
Aap+ and Aap− indicate strains that express or do not express the Aap protein, respectively. Underlining in primer sequences indicates restriction sites.
Flow cytometry.
Fluorescent labeling of bacteria and flow cytometry were performed based on a method described previously by Humphries et al. (28). Bacteria (∼5 × 108 cells) from 18-h stationary-phase cultures were washed three times in phosphate-buffered saline (PBS; Sigma) containing 0.02% gelatin (PBS-gel) and then incubated in PBS-gel containing 0.2% normal goat serum (Sigma) for 30 min. Rabbit anti-Aap A-domain antiserum (3) was then added to the suspension at a dilution of 1:250 and incubated for a further hour. Cells were washed in PBS-gel three times and incubated in PBS-gel containing 0.2% normal goat serum and a 1:250 dilution of R-phycoerythrin-conjugated donkey anti-rabbit immunoglobulin G antibody (Abcam) for 1 h. Cells were washed and resuspended to ∼5 × 106 cells ml−1 in PBS. A Beckman Coulter Cyan ADP flow cytometer and Summit V4.3 software (Dako, Denmark) were used to analyze 30,000 events (bacteria). For statistical analysis, flow cytometry was repeated with samples from three independent experiments.
Quantification of bacterial adhesion to corneocytes.
Corneocytes were harvested from both hands of up to four healthy volunteers by gentle agitation of the hand inside a laboratory glove (nitrile powder-free exam glove; Kimberly Clark) containing 20 ml PBS for 2 min. The collection of corneocytes had full ethical approval from the University of Manchester ethics board. The corneocytes were washed three times and resuspended to an optical density at 440 nm (OD440) of 0.35 (∼7.0 ×104 corneocytes ml−1). Stationary-phase bacterial cultures (18 h) were washed three times and resuspended to an OD490 of 0.08 (7.0 × 106 CFU ml−1) for S. epidermidis strains and an OD490 of 0.6 (4.2 × 107 CFU ml−1) for L. lactis strains. Equal volumes (2.5 ml) of bacteria and corneocytes were then mixed in a Falcon tube (50 ml; Corning) and rotated (200 rpm, to avoid cell settling) at 37°C for 2 h for adhesion to occur.
The suspension of bacteria and corneocytes was immediately applied to the top of 5 ml of a solution of 6% (wt/vol) dextran (∼100,000 Da; Sigma) and 0.9% NaCl in a 15-ml Falcon tube (Corning). The tube was centrifuged at 1,200 × g for 5 min to pellet the corneocytes and leave the unbound bacteria in a band higher up in the dextran solution. The top 5 ml was discarded from the tube, and the remaining solution was centrifuged at 3,300 × g for 5 min. The resulting pellet was resuspended in 1 ml PBS and applied to the top of another dextran solution. This process was performed three times in order to remove all unbound bacteria. Finally, the pellet containing corneocytes and bound bacteria was resuspended in water (100 μl), and samples (50 μl) were applied onto microscope slides for counting of the attached bacteria. After Gram staining, the number of bacteria per corneocyte was counted for 30 corneocytes. To test reproducibility, three batches of cells were tested in the assay, and two slides were counted (2 × 30 corneocytes) for each batch.
For blocking experiments, the corneocytes were preincubated with 40 μl of each recombinant Aap domain protein (see below) to give final concentrations of 0.05, 0.2, or 1.0 μM for each protein. Corneocytes were preincubated for 20 min at 37°C at 200 rpm before 2.5 ml of bacterial suspension was added, and the assay was performed as described above.
Construction of recombinant His6-tagged fusion proteins.
Recombinant proteins corresponding to a single B repeat and the full A domain of Aap were constructed, expressed, and purified as described previously (3). The region of short repeats within the A domain of Aap (nucleotides 157 to 857) was cloned, expressed, and purified using primers raap157-857F and raap157-857R (Table 1) and the techniques described previously (3).
Cloning of Aap into L. lactis MG1363.
Routine cloning techniques were performed as described previously by Sambrook et al. (48). The aap gene of S. epidermidis NCTC 11047 was amplified using high-fidelity DNA polymerase (Roche). Primers aapFSalI and aapRBamHI (Table 1) were used to amplify a 7,195-bp fragment of the aap gene from immediately downstream of the N-terminal signal sequence to the stop codon. The single PCR product was purified and digested with SalI and BamHI and ligated into the SalI/BamHI site of the lactococcal surface expression vector pUB1000 (24) to give pUB1000aap. pUB1000 contains a cell wall-associated expression cassette comprising a constitutive lactococcal promoter fused to the N-terminal signal sequence of the cell wall-associated protein sspA from S. gordonii. The cloning of aap into the BamHI/SalI cloning site of pUB1000 generated a fusion between the signal sequence of sspA and the aap gene such that the fusion protein would be correctly directed for export through the Sec pathway. pUB1000aap plasmids were transformed into competent S. gordonii DL1 cells as described previously (21), and transformants were selected on brain heart infusion broth (Oxoid) supplemented with 0.5% yeast extract with erythromycin. Transformants were screened for Aap expression by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of cell wall proteins (see below). Plasmids were purified from clones expressing five different sizes of Aap using a miniprep kit (Qiagen), and these were electroporated into electrocompetent L. lactis MG1363 cells as described previously (23, 56). The number of B repeats in each aap gene was determined by PCR of the insert region using primers Aap53-608 r.c. and aapR (Table 1), which anneal on either side of the B-repeat region. Primers pUB1000F and pUB1000R (Table 1), which anneal either side of the BamHI/SalI cloning site, were used to confirm the sequence of the 5′ and 3′ ends of the aap gene.
pUB1000aap6highT, a truncated derivative of pUB1000aap6high with the entire A domain removed, was generated using primers aapTFSalI and aapTRSalI (Table 1) and pUB1000aap6high as template DNA with Expand high-fidelity long-template DNA polymerase (Roche). The forward primer annealed at the start of the B-repeat region, and the reverse primer annealed immediately upstream of the A domain. The PCR product, comprising the full pUB1000 backbone and the B region of aap with a SalI cut site at either end (8.8 kb), was digested with SalI and self-ligated. It was then electroporated directly into L. lactis MG1363 cells as described above. Primers pUB1000F and pUB1000R (Table 1) were used to confirm the correct insert sequence.
Western blotting of cell wall proteins.
Cell wall proteins were extracted from stationary-phase cultures (20 ml; OD600 of 2.5) using mutanolysin (Sigma) as described previously (15). Stationary-phase cultures (20 ml) were washed once in PBS and resuspended in spheroplasting buffer (50 μl) (20 mM Tris-HCl [pH 6.8], 10 mM MgCl2, 26% [wt/vol] raffinose·5H2O). Mutanolysin (final concentration of 500 U ml−1; Sigma) was added along with 1 mM phenylmethylsulfonyl fluoride (Sigma). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8% gel) and blotted onto a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked with 6% (wt/vol) skim milk (Oxoid) in PBS containing Tween 20 (0.05%; Sigma) overnight at 4°C. Rabbit anti-Aap A-domain antiserum at a dilution of 1:5,000 or anti-Aap B-repeat antiserum at a dilution of 1:1,000 (3) and a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody (1:35,000; Sigma) were used to probe the blot. Proteins were visualized using Western Lightning chemiluminescence reagent (Perkin-Elmer, United Kingdom).
TEM.
Cell surface structures were analyzed by negative staining with 2% methylamine tungstate (Agar Scientific, United Kingdom) at pH 6.5 by transmission electron microscopy (TEM) as described previously (3, 22). Immunogold negative staining was performed with anti-Aap A-domain antiserum and secondary anti-rabbit immunoglobulin G conjugated to 10-nm gold particles (Agar Scientific, United Kingdom) (3, 36). Cells were visualized using an FEI Tecnai 12 electron microscope (FEI Co., Eindhoven, The Netherlands) at 100 kV.
Whole-cell immunoblotting.
A method for whole-cell immunoblotting described previously by Corrigan et al. (11) was used. Stationary-phase cells were washed and resuspended to an OD600 of 2 in PBS. Doubling dilutions of bacterial suspensions (10 μl) were then spotted onto a PVDF membrane (Bio-Rad). The membrane was blocked, probed with anti-Aap A-domain antiserum, and developed as described above for Western blotting.
Statistical analysis.
Statistical analysis was performed using SPSS software (version 11.5). One-way analysis of variance with the Tukey post hoc test was used to determine statistical differences at the 0.05 level.
RESULTS
S. epidermidis NCTC 11047 stationary-phase cultures comprise two subpopulations defined on the basis of Aap expression.
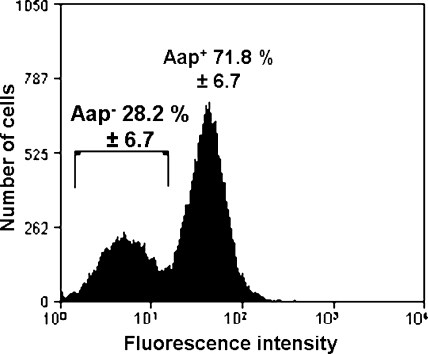
Our previous finding that the S. epidermidis NCTC 11047 WT population is comprised of two subpopulations (3) was confirmed by using flow cytometry (Fig. 1). WT NCTC 11047 cells were labeled with anti-Aap A-domain antiserum and a secondary fluorescent antibody. Cells were found to have a low, background level of fluorescence with the same low intensity shown with preimmune antiserum or a higher level of fluorescence (Fig. 1) that indicated the presence of Aap. These results indicated that WT NCTC 11047 cells comprised 72% Aap-expressing cells and 28% Aap-negative cells. The presence of these two subpopulations was also confirmed using anti-Aap B-repeat antiserum (data not shown), and the same ratio of the two cell types was detected.
FIG. 1.
Histogram showing flow cytometry results from one representative batch of WT NCTC 11047 cells labeled with anti-Aap A-domain antiserum and phycoerythrin-conjugated secondary antibody. The mean percentages (± standard deviations) of Aap-negative Fib− cells (left peak) and Aap-positive Fib+ cells (right peak) are shown for three batches of WT NCTC 11047 cells.
To determine whether other Aap-expressing S. epidermidis strains also contained similar Aap-negative subpopulations, RP62A and 13 other S. epidermidis strains isolated from catheters, hip joints, anterior nares, and the skin (Table 1) were analyzed by flow cytometry with anti-Aap A-domain antiserum. Only two strains (JBN3 and JBC7) contained Aap-negative subpopulations, and the remaining strains all comprised a single Aap-expressing population (data not shown). In contrast to NCTC 11047, the majority of cells in stationary-phase cultures of JBN3 and JBC7 did not express Aap, with cultures containing 90% and 75% Aap-negative cells, respectively.
The A domain of Aap contributes to the adhesion of S. epidermidis NCTC 11047 WT/Fib+ cells to corneocytes.
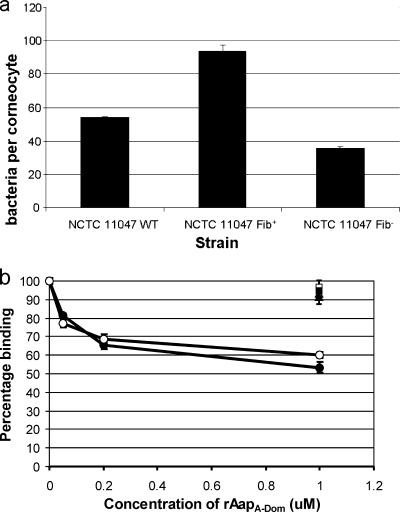
The two subpopulations of NCTC 11047 were previously found to have different cell surface properties, with the Fib+ cells being more adherent to polystyrene and having higher cell surface hydrophobicity than the Fib− cells (3). To compare the abilities of the two subpopulations to colonize skin, their respective affinities for corneocytes were determined (Fig. 2a).
FIG. 2.
Comparison of corneocyte binding of WT, Fib+, and Fib− cells and contribution of the Aap A domain to adhesion of NCTC 11047 to corneocytes. (a) Mean number of NCTC 11047 WT, Fib+, and Fib− cells attached to corneocytes after 2 h of incubation. (b) Blocking of adhesion of NCTC 11047 WT (filled symbols) and Fib+ (hollow symbols) cells to corneocytes using specific recombinant Aap domains. Corneocytes were preincubated with increasing concentrations of rAapA-Dom (circles), 1 μM rAapB-rep (squares), or 1 μM rAapA-reps (triangles), and the adhesion of NCTC 11047 WT and Fib+ cells is shown as a percentage of original binding. Results represent the means and standard errors for at least two experiments.
The Aap-expressing Fib+ subpopulation showed almost a twofold enhancement in adhesion compared to WT cells, whereas adhesion of the Aap-negative Fib− subpopulation was two-thirds of the WT level. This suggests that Aap may be a contributory factor in adhesion to corneocytes. However, other factors must also mediate adhesion, as Fib− cells did attach to corneocytes. To determine whether the A domain of Aap contributes to corneocyte adhesion, the ability of rAapA-Dom to block adhesion was tested (Fig. 2b). rAapA-Dom inhibited the adhesion of NCTC 11047 WT and Fib+ cells to corneocytes in a concentration-dependent manner, whereas rAapB-rep and rAapA-reps were unable to block adhesion. In addition, the adhesion of NCTC 11047 Fib− cells could not be significantly blocked by 1 μM rAapA-Dom (91% ± 4% binding [mean and standard deviation for two experiments]). Therefore, the nonrepetitive region of the A domain of Aap specifically contributes to the adhesion of NCTC 11047 WT and Fib+ cells to corneocytes.
However, it is not clear from these results whether Aap merely enhances adhesion or can promote adhesion to corneocytes independently of other adhesive factors.
Aap is expressed on the surface of Lactococcus lactis MG1363 pUB1000aap6high as sparse peritrichous fibrils.
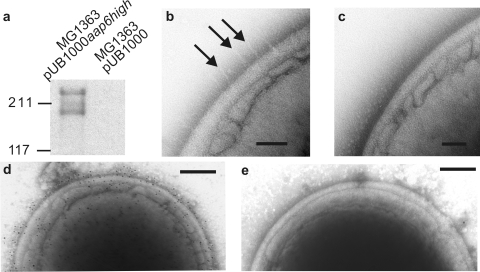
To determine the ability of Aap to mediate the adhesion of bacteria to corneocytes independently of other adhesins, Aap was expressed on the surface of L. lactis MG1363, a bacterium with very low affinity for epithelial cells (43). The aap gene was amplified from S. epidermidis NCTC 11047 genomic DNA and inserted into the lactococcal surface expression vector pUB1000. The number of B repeats resulting from this procedure was reduced from the WT number of 12.2 (3) to 6, and recombinant Aap was expressed in L. lactis MG1363 cells (termed MG1363 pUB1000aap6high) (Table 1). The level of surface expression of Aap was demonstrated by Western blotting and by negative staining (Fig. 3).
FIG. 3.
Surface expression of fibrillar Aap on L. lactis MG1363 pUB1000aap6high cells. (a) Western blot of cell wall proteins probed with anti-Aap A-domain antiserum. (b and c) TEM of L. lactis MG1363 strains negatively stained with 2% methylamine tungstate. (d and e) TEM of L. lactis MG1363 strains negatively stained and immunogold labeled with anti-Aap A-domain antiserum and 10-nm gold-conjugated secondary antibody. (b) MG1363 pUB1000aap6high cell showing fibrils projecting from the cell wall. (c) MG1363 pUB1000 cell showing a smooth cell surface. (d) MG1363 pUB1000aap6high cell showing gold labeling evenly distributed over the cell. (e) MG1363 pUB1000 cell showing only a small amount of nonspecific gold labeling.
A Western blot of cell wall proteins showed two anti-Aap A-domain antiserum-reactive bands at ∼270 and 200 kDa, whereas no antibody-reactive bands were seen for MG1363 pUB1000, indicating that Aap was present in the cell wall of MG1363 pUB1000aap6high (Fig. 3a). Negative staining showed sparse peritrichous fibrils on the surface of MG1363 pUB1000aap6high, in contrast to the smooth cell wall of MG1363 pUB1000 (Fig. 3b and c). Finally, immunogold labeling with anti-Aap A-domain antiserum also showed gold particles sparsely distributed over the surface of MG1363 pUB1000aap6high but not on MG1363 pUB1000 cells (Fig. 3d and e), proving that Aap is exposed on the cell surface when pUB1000aap6high is present. Together, these results show that the fibrillar structure of Aap was maintained in an L. lactis background, but Aap fibrils were peritrichous rather than localized in a tuft as on NCTC 11047 WT and Fib+ cells (3).
The A domain of Aap independently mediates adhesion of L. lactis MG1363 pUB1000aap6high to corneocytes.
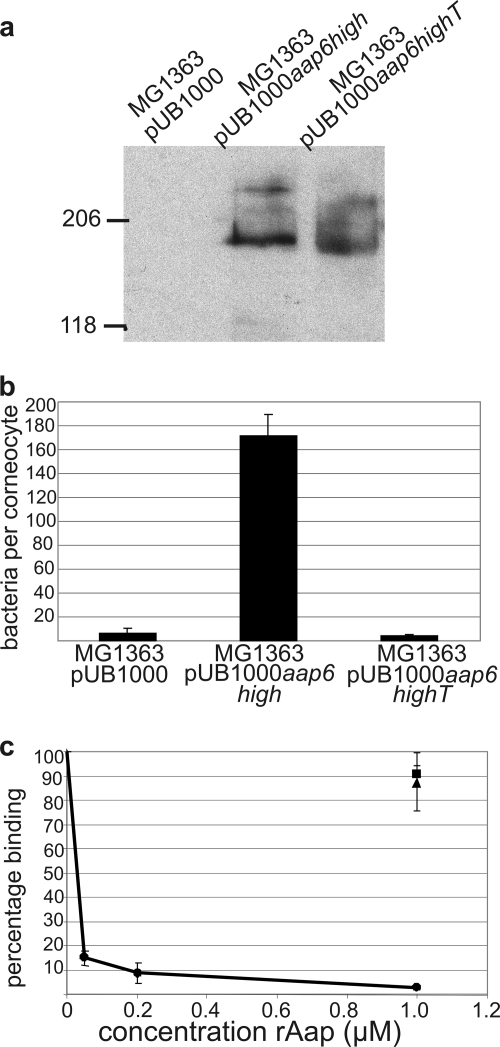
The ability of Aap to mediate adhesion to corneocytes independently of other adhesins was investigated by measuring the adhesion of L. lactis MG1363 pUB1000aap6high cells. In order to prove the role of the A domain in adhesion, MG1363 expressing truncated Aap with six B repeats but no A domain (MG1363 pUB1000aap6highT) (Table 1) was generated. The surface expression of truncated Aap was proven by Western blotting of cell wall proteins using anti-Aap B-repeat antiserum (Fig. 4a), and the presence of Aap fibrils was confirmed by negative staining by TEM (data not shown). An intact A domain was found to be required for Aap-mediated adhesion to corneocytes (Fig. 4b). The adhesion of MG1363 pUB1000aap6high to corneocytes was over 30 times that of MG1363 pUB1000, showing that Aap can mediate adhesion to corneocytes independently of other adhesins. MG1363 pUB1000aap6highT, expressing the same amount of Aap as MG1363 pUB1000aap6high (Fig. 4a) but lacking the A domain, adhered at the same very low level as the MG1363 pUB1000 control (Fig. 4b). Furthermore, rAapA-Dom blocked the adhesion of MG1363 pUB1000aap6high to corneocytes in a concentration-dependent manner, with almost a complete inhibition of adhesion at 1 μM rAapA-Dom (Fig. 4c). In contrast, the addition of rAapA-reps and rAapB-rep to the adhesion assay mixture did not significantly reduce adhesion, even at a concentration of 1 μM. These results prove that only the A domain of Aap adheres to a ligand on the surface of human corneocytes.
FIG. 4.
Role of the A domain of Aap in adhesion to corneocytes. (a) Western blot of cell wall proteins from MG1363 containing pUB1000, pUB1000aap6high, and pUB1000aap6highT and probed with anti-Aap B-repeat antiserum. (b) Mean numbers of L. lactis MG1363 pUB1000aap6high, pUB1000aap6highT, and pUB1000 control cells that adhered to corneocytes. (c) Corneocytes were preincubated with increasing concentrations of rAapA-Dom (circles), 1 μM rAapA-reps (triangles), or 1 μM rAapB-rep (squares), and the mean numbers of MG1363 pUB1000aap6high cells that adhered to corneocytes are shown. The results represent the means and standard errors for three experiments.
The A domain of Aap mediates adhesion of S. epidermidis clinical isolates to corneocytes.
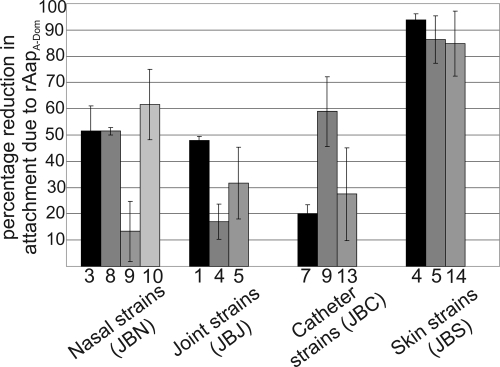
The role of the Aap A domain in adhesion to corneocytes was investigated for different S. epidermidis strains isolated from intravenous catheters, hip joint infections, anterior nares, and the skin of different individuals (Table 1). For each isolate, between 40 and 114 bacteria were attached per corneocyte, and there was no apparent correlation between numbers attached and isolate origin (data not shown). In order to determine whether the Aap A domain mediated the attachment of the strains, rAapA-Dom was used to block the adhesion of all the strains. The percentage of reduction in numbers of bacteria attaching after blocking with rAapA-Dom was calculated (Fig. 5). The percentage of reduction in attachment after blocking with rAapA-Dom varied between strains, with some strains being blocked almost completely (JBS4, JBS5, and JBS14), indicating that Aap may be the major means of adhesion in these strains. However, other strains (JBN9, JBJ4, and JBC7) showed only a small reduction in adhesion after blocking with rAapA-Dom, indicating that these strains were not heavily dependent on Aap for attachment to corneocytes.
FIG. 5.
Contribution of the A domain of Aap to corneocyte adhesion for clinical isolates of S. epidermidis. Corneocyte adhesion of S. epidermidis isolates was blocked by pretreating corneocytes with 1 μM rAapA-Dom. The percentage of the reduction in the number of attached bacteria due to blocking is shown. Results are the means and standard errors for two experiments.
Although only 10% of JBN3 cells expressed Aap, as shown by flow cytometry (data not shown), blocking with rAapA-Dom reduced adhesion by 50% (Fig. 5). This suggests that ∼50% of cells that did attach to unblocked corneocytes were cells expressing Aap, and their attachment was dependent on the Aap A domain. Therefore, the subpopulation (90%) that did not express Aap may have attached in fewer numbers due to relatively weak attachment via an as-yet-unidentified adhesin, and this attachment would not have been affected by rAapA-Dom. A detailed interpretation of the role of Aap in the adhesion of each of these fresh strains is complex; however, the blocking experiment (Fig. 5) showed that the reliance on Aap as a means of attachment to corneocytes varies form strain to strain and that some strains are likely to express other molecules to promote corneocyte adhesion. It should be noted that the strains shown in Table 1 were selected for this study due to their ability to express Aap on the cell surface, and not all S. epidermidis strains isolated from these sites in this study contained the aap gene.
The number of Aap B repeats influences the level of adhesion of L. lactis MG1363 expressing Aap to corneocytes.
Although the B-repeat region of Aap is not directly involved in corneocyte binding, the number of B repeats in S. epidermidis strains varies from 3 to up to 17 (44). Therefore, the influence of the length of the B-repeat region on corneocyte adhesion was investigated. Aap proteins with 2, 4, 5, 6, and 7 B repeats were expressed on the surface of L. lactis MG1363 clones using the pUB1000 expression vector (Fig. 6a and b). These strains (MG1363 pUB1000aap2, MG1363 pUB1000aap4, MG1363 pUB1000aap5, MG1363 pUB1000aap6, and MG1363 pUB1000aap7, respectively) all expressed Aap at a lower level than the original MG1363 pUB1000aap6high strain (Fig. 6b).
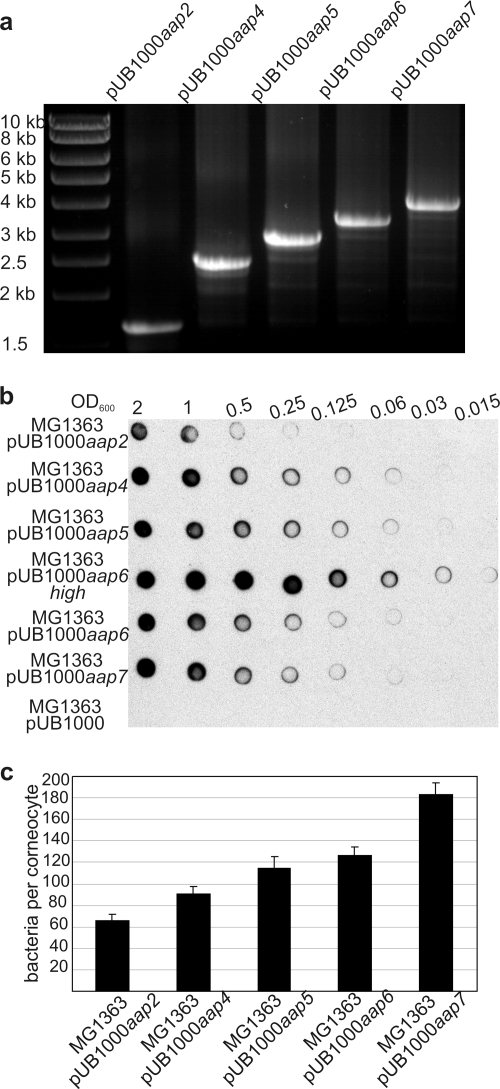
FIG. 6.
Influence of numbers of B repeats in Aap on adhesion to corneocytes. (a) Agarose gel showing sizes of aap in pUB1000aap2, pUB1000aap4, pUB1000aap5, pUB1000aap6, and pUB1000aap7. The expected sizes of PCR products for pUB1000aap with 2, 4, 5, 6, and 7 B repeats are 1,652, 2,420, 2,804, 3,188 and 3,572 bp, respectively. PCR was performed using primers Aap53-608rc and aapR (Table 1). (b) Whole-cell dot immunoblot of MG1363 pUB1000 expressing different lengths of Aap showing surface expression levels of Aap. Serial dilutions of cells were applied onto a PVDF membrane and probed with anti-Aap A-domain antiserum. (c) Mean number of MG1363 pUB1000aap cells expressing different lengths of Aap that adhered to corneocytes.
Aap expression on the surfaces of MG1363 pUB1000aap4, MG1363 pUB1000aap5, MG1363 pUB1000aap6, and MG1363 pUB1000aap7 cells was approximately 25% of that of the original strain MG1363 pUB1000aap6high (Fig. 6b). The expression of Aap with two B repeats was reduced by a further fourfold, and an MG1363 pUB1000aap2 transformant with greater Aap expression could not be identified. The reason for the relatively low level of expression of Aap in these transformants is not clear, but the promoter sequences, N-terminal signal sequences, and LPXTG cell wall-anchoring motifs were all found to be identical to that of pUB1000aap6high (data not shown).
Adhesion to corneocytes was tested using MG1363 pUB1000aap variants that expressed Aap at similar levels. A clear correlation was observed between the number of B repeats and the number of bacteria adhering to corneocytes (Fig. 6c), with three times more aap7 cells than aap2 cells being attached per corneocyte. MG1363 pUB1000aap2 gave the lowest adhesion to corneocytes, although it expressed Aap at fourfold-lower levels than did MG1363 pUB1000aap4. However, it would appear that the length of Aap is more important than cell surface density because, in the case of MG1363 expressing low and high levels of Aap with six B repeats, a fourfold difference in Aap expression gave only a 1.4-fold reduction in adhesion (Fig. 4a and 6). These results demonstrate that longer fibrils, with a higher number of B repeats, resulted in higher numbers of bacteria attaching to the corneocytes.
DISCUSSION
Banner et al. (3) previously used negative staining to show that the ratio of Aap-expressing Fib+ cells to Aap-negative Fib− cells in an NCTC 11047 WT population was 25% Fib+ to 75% Fib− cells (3). However, flow cytometry with fluorescent antibodies has given a much more accurate assessment of the relative numbers of the two subpopulations, revealing that WT NCTC 11047 is comprised of 72% cells that express Aap and 28% that do not. The previously reported counting method resulted in an underestimation of the number of Fib+ cells due to difficulties in detecting fibrillar tufts by negative staining by TEM. An as-yet-unknown mechanism must exist to control the ratio of the two subpopulations in stationary-phase cultures, as the ratio is consistent from batch to batch. New cultures of NCTC 11047 obtained from the NCTC collection always produced the same stable ratio after the first batch culture grown from the ampoules (data not presented). This consistently stable ratio of Fib+ to Fib− cells in different batches of WT NCTC 11047 cells cannot be explained by a loss of Aap expression resulting from a mutation. A random mutation occurring during cell division would not give reproducible subpopulation ratios. The mechanism of control of the subpopulation ratios is currently under investigation, as is the mechanism by which stable subpopulations were previously generated by repeated hexadecane enrichment (3).
Analysis of 13 other Aap-expressing S. epidermidis isolates showed that two subpopulations were not unique to strain NCTC 11047, and in the strains that we tested, 15% (2 of 13 strains) contained a subpopulation that did not express Aap. The presence within a WT population of a subpopulation of cells expressing a surface protein is not commonly reported in the literature, but S. aureus cells in the early exponential phase are known to contain a subpopulation of cells expressing the fibronectin binding protein FnBP (37). However, the number of cells in this subpopulation was found to decrease to zero in the late exponential phase.
Aap-positive cells of NCTC 11047 have a greater affinity for corneocytes than do Aap-negative cells, implying distinct roles for the two subpopulations in skin colonization. It is thought that commensal strains can translocate from the skin and other sites to cause infection (12). The weaker attachment of the Fib− subpopulations (if present) could aid detachment from the skin, leading to reattachment on another surface (a catheter, for example) via a different surface adhesin.
Heterologous expression of surface proteins on L. lactis is a common technique used to determine the function of a wide variety of surface proteins (2, 23, 27). Aap fibrils were observed over the whole cell surface of MG1363 pUB1000aap6high, and there was no localization of fibrils, in direct contrast to the native asymmetrical tuft distribution observed in WT S. epidermidis NCTC 11047 cells (3). In staphylococci the targeting of surface proteins to specific sites in the cell wall is at least partially dependent on the N-terminal signal sequence (14). The aap gene in the pUB1000 constructs had the N-terminal signal sequence of the S. gordonii protein SspA in place of the native Aap signal sequence. However, as the Aap protein was expressed in a heterologous background, this study suggests only that the tuft phenotype observed in NCTC 11047 is not intrinsic to the mature Aap fibril or to the LPXTG sortase recognition sequence.
Measurements of lengths of cell surface fibrils from TEM images with negative staining are possible, and tufts of Aap fibrils on S. epidermidis NCTC 11047 were 122.2 ± 10.8 nm from cell surface to tip (3). Measurements of 28 individual fibrils on seven different MG1363 pUB1000aap6high cells gave a mean fibril length of 42.5 ± 6.7 nm (data not shown), suggesting that these fibrils are shorter than those of NCTC 11047, which comprise 12.2 B repeats. Although the value of 42.5 nm is likely to be an underestimate due to a lack of resolution toward the end of the fibrils, it is consistent with the idea that fewer B repeats give shorter fibrils.
The adhesion of NCTC 11047 and all other Aap-expressing S. epidermidis strains tested in this study was at least partially dependent on the A domain of Aap. Previous studies have shown the aap gene to be present in between 77 and 89% of isolates (44, 58). Therefore, it is likely that Aap is widely used to mediate attachment to the skin, in addition to other unidentified adhesins. The expression of a range of different adhesins would ensure that bacteria could adhere to any given host regardless of possible variations in the expression of host receptors. The S. epidermidis RP62A genome contains several surface adhesins (4), in addition to Aap, that could potentially promote adhesion to corneocytes, such as members of the serine aspartate repeat (Srd) family, which have homologs in S. aureus. Three Sdr proteins in S. aureus (ClfB, SdrC, and SdrD) as well as the Aap homolog SasG are known to contribute to adhesion to nasal epithelial cells (10, 43), raising the possibility that the Sdr proteins from S. epidermidis (SdrF, SdrH, and Fbe) may have a role in adhesion to corneocytes. Also, in S. aureus, cell wall teichoic acids were previously found to have a role in the colonization of cotton rat nares and adhesion to nasal epithelial cells (55). However, integral components of the cell wall such as teichoic acids are unlikely to have major roles in the adhesion of S. epidermidis to corneocytes, as rAapA-Dom almost completely blocked the adhesion of the three WT S. epidermidis strains JBS4, JBS5, and JBS14.
The rAapB-rep protein was unable to inhibit Aap-dependent adhesion to corneocytes. A recent study suggested that recombinant B repeats of Aap are fully folded only when capped at the C terminus by an additional half-repeat (9). For this reason it is unlikely that the rAapB-rep used in this study is completely folded. However, Fig. 4c demonstrated that the linear amino acid sequence of a single B repeat was unable to adhere to corneocytes. In addition, MG1363 pUB1000aap6highT, which contained the C-terminal-half B repeat, expressed Aap fibrils lacking the A domain that were visible by negative staining by TEM (Fig. 4a and data not shown), strongly suggesting that the B repeats are correctly folded when expressed on L. lactis MG1363 cells. In conclusion, the inability of the fibrillar, truncated Aap to mediate corneocyte adhesion proves that the B repeats have no ligand binding function.
Increasing numbers of B repeats in the Aap molecule promoted an increasingly enhanced adhesion of MG1363 pUB1000aap cells to corneocytes. As the B repeats have no innate receptor binding function, the length of the Aap fibril must influence adhesion. This demonstrates a function for the B region in projecting the terminal ligand binding A domain away from the cell to allow an enhanced attachment of bacteria to corneocytes. MG1363 does not produce a capsule (18, 19), and no surface proteins could be seen on MG1363 pUB1000 cells by TEM, suggesting that the masking of shorter Aap fibrils by other bacterial surface components does not occur in this strain background. Longer Aap fibrils may allow bacteria to reduce electrostatic repulsive forces between the bacterial cell surface and the surface of the corneocytes. Alternatively, longer fibrils would be expected to be more flexible, giving the A domain a larger range of movement, which may allow more A-domain-host ligand interactions to occur simultaneously.
The host receptor for Aap on corneocytes is currently unknown. The cornified cell envelope comprises a cross-linked network of proteins, the main constituent of which is loricrin (32, 50, 51), and beyond this is a layer of lipids (6). The precise molecular arrangement of these proteins and lipids and their respective accessibilities to bacteria are not known, but the S. aureus adhesin ClfB promotes adhesion to nasal epithelial cells via the envelope protein cytokeratin-10 (39, 54), as does the S. aureus protein IsdA, which also binds to loricrin and involucrin (8). As these inner components of the cornified envelope are accessible to bacteria, other components of the protein envelope as well as components of the lipid layer may also be possible receptors for Aap. Aap and SasG of S. aureus share an as-yet-unidentified corneocyte ligand, and SasG was previously shown not to adhere to fibrinogen, fibronectin, human epidermal keratin, collagen, von Willebrand factor, laminin, heparin sulfate, or submaxillary mucin (43). The A domains of Aap and SasG share a 212-aa region that is 59% identical in terms of amino acid sequence (42), and it is likely that this region contains a binding site for a corneocyte ligand. However, there is the possibility that Aap may adhere to more than one ligand, as in the case of IsdA (8). The Aap and SasG A domains contain unique regions (42) that could contain additional, as-yet-unidentified, ligand binding sites.
This study has confirmed that Aap is a fibrillar adhesin and has shown that the terminal A domain directly mediates adhesion to corneocytes, implying a role for Aap in the colonization of the skin. Aap is also known to promote biofilm formation, making this cell wall-anchored protein a bifunctional molecule important for both the commensal and pathogenic life-styles of S. epidermidis.
Acknowledgments
This study was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) CASE award supported by Novartis (Frimley Business Park, Frimley, Camberley, Surrey GU16 7SR, United Kingdom).
We thank Sarah Maddocks for pUB1000F/R primers. We thank the staff at the EM facility in the Facility of Life Sciences (University of Manchester) for their assistance and the Welcome Trust for equipment grant support to the EM facility.
Footnotes
Published ahead of print on 11 September 2009.
REFERENCES
- 1.Arber, N., E. Pras, Y. Copperman, J. M. Schapiro, V. Meiner, I. S. Lossos, A. Militianu, D. Hassin, A. Shai, M. Moshkowitz, and Y. Sidi. 1994. Pacemaker endocarditis—report of 44 cases and review of the literature. Medicine 73:299-305. [DOI] [PubMed] [Google Scholar]
- 2.Avall-Jaaskelainen, S., A. Lindholm, and A. Palva. 2003. Surface display of the receptor-binding region of the Lactobacillus brevis S-layer protein in Lactococcus lactis provides nonadhesive lactococci with the ability to adhere to intestinal epithelial cells. Appl. Environ. Microbiol. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banner, M. A., J. G. Cunniffe, R. L. Macintosh, T. J. Foster, H. Rohde, D. Mack, E. Hoyes, J. Derrick, M. Upton, and P. S. Handley. 2007. Localized tufts of fibrils on Staphylococcus epidermidis NCTC 11047 are comprised of the accumulation-associated protein. J. Bacteriol. 189:2793-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowden, M. G., W. Chen, J. Singvall, Y. Xu, S. J. Peacock, V. Valtulina, P. Speziale, and M. Hook. 2005. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology 151:1453-1464. [DOI] [PubMed] [Google Scholar]
- 5.Bowden, M. G., L. Visai, C. M. Longshaw, K. T. Holland, P. Speziale, and M. Hook. 2002. Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin? J. Biol. Chem. 277:43017-43023. [DOI] [PubMed] [Google Scholar]
- 6.Candi, E., R. Schmidt, and G. Melino. 2005. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6:328-340. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke, S. R., G. Andre, E. J. Walsh, Y. F. Dufrene, T. J. Foster, and S. J. Foster. 2009. Iron-regulated surface determinant protein A mediates adhesion of Staphylococcus aureus to human corneocyte envelope proteins. Infect. Immun. 77:2408-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrady, D. G., C. C. Brescia, K. Horii, A. A. Weiss, D. J. Hassett, and A. B. Herr. 2008. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. USA 105:19456-19461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrigan, R. M., H. Miajlovic, and T. J. Foster. 2009. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrigan, R. M., D. Rigby, P. Handley, and T. J. Foster. 2007. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 153:2435-2446. [DOI] [PubMed] [Google Scholar]
- 12.Costa, S. F., M. H. Miceli, and E. J. Anaissie. 2004. Mucosa or skin as source of coagulase-negative staphylococcal bacteraemia? Lancet Infect. Dis. 4:278-286. [DOI] [PubMed] [Google Scholar]
- 13.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeDent, A. C., M. McAdow, and O. Schneewind. 2007. Distribution of protein A on the surface of Staphylococcus aureus. J. Bacteriol. 189:4473-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demuth, D. R., Y. Duan, W. Brooks, A. R. Holmes, R. McNab, and H. F. Jenkinson. 1996. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol. Microbiol. 20:403-413. [DOI] [PubMed] [Google Scholar]
- 16.Finch, R. G., P. Hill, and P. Williams. 1995. Staphylococci—the emerging threat. Chem. Ind. 1995:225-228. [Google Scholar]
- 17.Gasson, M. J. 1983. Genetic transfer systems in lactic acid bacteria. Antonie van Leeuwenhoek 49:275-282. [DOI] [PubMed] [Google Scholar]
- 18.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO-712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germond, J. E., M. Delley, N. D'Amico, and S. J. F. Vincent. 2001. Heterologous expression and characterization of the exopolysaccharide from Streptococcus thermophilus Sfi39. Eur. J. Biochem. 268:5149-5156. [DOI] [PubMed] [Google Scholar]
- 20.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. DeBoy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. X. Jiang, H. Y. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haisman, R. J., and H. F. Jenkinson. 1991. Mutants of Streptococcus gordonii Challis over-producing glucosyl transferase. J. Gen. Microbiol. 137:483-489. [DOI] [PubMed] [Google Scholar]
- 22.Handley, P. S., P. L. Carter, J. E. Wyatt, and L. M. Hesketh. 1985. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect. Immun. 47:217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartford, O., L. O'Brien, K. Schofield, J. Wells, and T. J. Foster. 2001. The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogen. Microbiology 147:2545-2552. [DOI] [PubMed] [Google Scholar]
- 24.Heddle, C., A. H. Nobbs, N. S. Jakubovics, M. Gal, J. P. Mansell, D. Dymock, and H. F. Jenkinson. 2003. Host collagen signal induces antigen I/II adhesin and invasin gene expression in oral Streptococcus gordonii. Mol. Microbiol. 50:597-607. [DOI] [PubMed] [Google Scholar]
- 25.Heilmann, C., M. Hussain, G. Peters, and F. Gotz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 26.Heilmann, C., G. Thumm, G. S. Chhatwal, J. Hartleib, A. Uekotter, and G. Peters. 2003. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 149:2769-2778. [DOI] [PubMed] [Google Scholar]
- 27.Hirt, H., S. L. Erlandsen, and G. M. Dunny. 2000. Heterologous inducible expression of Enterococcus faecalis pCF10 aggregation substance Asc10 in Lactococcus lactis and Streptococcus gordonii contributes to cell hydrophobicity and adhesion to fibrin. J. Bacteriol. 182:2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphries, A. D., M. Raffatellu, S. Winter, E. H. Weening, R. A. Kingsley, R. Droleskey, S. P. Zhang, J. Figueiredo, S. Khare, J. Nunes, L. G. Adams, R. M. Tsolis, and A. J. Baumler. 2003. The use of flow cytometry to detect expression of subunits encoded by 11 Salmonella enterica serotype Typhimurium fimbrial operons. Mol. Microbiol. 48:1357-1376. [DOI] [PubMed] [Google Scholar]
- 29.Hussain, M., C. Heilmann, G. Peters, and M. Herrmann. 2001. Teichoic acid enhances adhesion of Staphylococcus epidermidis to immobilized fibronectin. Microb. Pathog. 31:261-270. [DOI] [PubMed] [Google Scholar]
- 30.Hussain, M., M. Herrmann, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson, M. S., J. Bagg, M. N. Gupta, and R. D. Sturrock. 1999. Oral carriage of staphylococci in patients with rheumatoid arthritis. Rheumatology 38:572-575. [DOI] [PubMed] [Google Scholar]
- 32.Kalinin, A., L. N. Marekov, and P. M. Steinert. 2001. Assembly of the epidermal cornified cell envelope. J. Cell Sci. 114:3069-3070. [DOI] [PubMed] [Google Scholar]
- 33.Kelly, S., J. Collins, M. Maguire, C. Gowing, M. Flanagan, M. Donnelly, and P. G. Murphy. 2008. An outbreak of colonization with linezolid-resistant Staphylococcus epidermidis in an intensive therapy unit. J. Antimicrob. Chemother. 61:901-907. [DOI] [PubMed] [Google Scholar]
- 34.Lane, D. J. 1991. 16/235 rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons Inc., Chichester, United Kingdom.
- 35.Mack, D., H. Rohde, L. G. Harris, A. P. Davies, M. A. Horstkotte, and J. K. Knobloch. 2006. Biofilm formation in medical device-related infection. Int. J. Artif. Organs 29:343-359. [DOI] [PubMed] [Google Scholar]
- 36.McNab, R., H. Forbes, P. S. Handley, D. M. Loach, G. W. Tannock, and H. F. Jenkinson. 1999. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J. Bacteriol. 181:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed, N., L. Visai, P. Speziale, and J. M. Ross. 2000. Quantification of Staphylococcus aureus cell surface adhesins using flow cytometry. Microb. Pathog. 29:357-361. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch, F. E., R. L. Sammons, and I. L. C. Chapple. 2004. Isolation and characterization of subgingival staphylococci from periodontitis patients and controls. Oral Dis. 10:155-162. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4:759-770. [DOI] [PubMed] [Google Scholar]
- 40.Park, P. W., J. Rosenbloom, W. R. Abrams, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 41.Potoski, B. A., J. Adams, L. Clarke, K. Shutt, P. K. Linden, C. Baxter, A. W. Pasculle, B. Capitano, A. Y. Peleg, D. Szabo, and D. L. Paterson. 2006. Epidemiological profile of linezolid-resistant coagulase-negative staphylococci. Clin. Infect. Dis. 43:165-171. [DOI] [PubMed] [Google Scholar]
- 42.Roche, F. M., R. Massey, S. J. Peacock, N. P. Day, L. Visai, P. Speziale, A. Lam, M. Pallen, and T. J. Foster. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149:643-654. [DOI] [PubMed] [Google Scholar]
- 43.Roche, F. M., M. Meehan, and T. J. Foster. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759-2767. [DOI] [PubMed] [Google Scholar]
- 44.Rohde, H., E. C. Burandt, N. Siemssen, L. Frommelt, C. Burdelski, S. Wurster, S. Scherpe, A. P. Davies, L. G. Harris, M. A. Horstkotte, J. K. M. Knobloch, C. Ragunath, J. B. Kaplan, and D. Mack. 2007. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 28:1711-1720. [DOI] [PubMed] [Google Scholar]
- 45.Rohde, H., C. Burdelski, K. Bartscht, M. Hussain, F. Buck, M. A. Horstkotte, J. K. Knobloch, C. Heilmann, M. Herrmann, and D. Mack. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55:1883-1895. [DOI] [PubMed] [Google Scholar]
- 46.Rupp, M. E., and G. L. Archer. 1994. Coagulase-negative staphylococci—pathogens associated with medical progress. Clin. Infect. Dis. 19:231-243. [DOI] [PubMed] [Google Scholar]
- 47.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Gotz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 49.Schneewind, O., A. Fowler, and K. F. Faull. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268:103-106. [DOI] [PubMed] [Google Scholar]
- 50.Steinert, P. M., T. Kartasova, and L. N. Marekov. 1998. Biochemical evidence that small proline-rich proteins and trichohyalin function in epithelia by modulation of the biomechanical properties of their cornified cell envelopes. J. Biol. Chem. 273:11758-11769. [DOI] [PubMed] [Google Scholar]
- 51.Steinert, P. M., and L. N. Marekov. 1995. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin and small proline-rich protein-1 and protein-2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J. Biol. Chem. 270:17702-17711. [DOI] [PubMed] [Google Scholar]
- 52.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 53.Veenstra, G. J., F. F. Cremers, H. van Dijk, and A. Fleer. 1996. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J. Bacteriol. 178:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh, E. J., L. M. O'Brien, X. W. Liang, M. Hook, and T. J. Foster. 2004. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J. Biol. Chem. 279:50691-50699. [DOI] [PubMed] [Google Scholar]
- 55.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 56.Wells, J. M., P. W. Wilson, and R. W. F. Lepage. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 57.Williams, R. J., B. Henderson, L. J. Sharp, and S. P. Nair. 2002. Identification of a fibronectin-binding protein from Staphylococcus epidermidis. Infect. Immun. 70:6805-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao, Y. F., D. E. Sturdevant, A. Villaruz, L. Xu, Q. Gao, and M. Otto. 2005. Factors characterizing Staphylococcus epidermidis invasiveness determined by comparative genomics. Infect. Immun. 73:1856-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]