Abstract
The dlt operon encodes proteins that alanylate teichoic acids, the major components of cell walls of gram-positive bacteria. This generates a net positive charge on bacterial cell walls, repulsing positively charged molecules and conferring resistance to animal and human cationic antimicrobial peptides (AMPs) in gram-positive pathogenic bacteria. AMPs damage the bacterial membrane and are the most effective components of the humoral immune response against bacteria. We investigated the role of the dlt operon in insect virulence by inactivating this operon in Bacillus cereus, which is both an opportunistic human pathogen and an insect pathogen. The ΔdltBc mutant displayed several morphological alterations but grew at a rate similar to that for the wild-type strain. This mutant was less resistant to protamine and several bacterial cationic AMPs, such as nisin, polymyxin B, and colistin, in vitro. It was also less resistant to molecules from the insect humoral immune system, lysozyme, and cationic AMP cecropin B from Spodoptera frugiperda. ΔdltBc was as pathogenic as the wild-type strain in oral infections of Galleria mellonella but much less virulent when injected into the hemocoels of G. mellonella and Spodoptera littoralis. We detected the dlt operon in three gram-negative genera: Erwinia (Erwinia carotovora), Bordetella (Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica), and Photorhabdus (the entomopathogenic bacterium Photorhabdus luminescens TT01, the dlt operon of which did not restore cationic AMP resistance in ΔdltBc). We suggest that the dlt operon protects B. cereus against insect humoral immune mediators, including hemolymph cationic AMPs, and may be critical for the establishment of lethal septicemia in insects and in nosocomial infections in humans.
Gram-positive bacteria are generally enclosed by cell walls consisting of macromolecular assemblies of cross-linked peptidoglycan (murein), polyanionic teichoic acids (TAs), and surface proteins (69). TAs are polymers of repeating glycerophosphate residues. They may be covalently anchored to either peptidoglycan (wall-associated TAs) or the cytoplasmic membrane via glycolipids (lipoteichoic acids [LTAs]). TAs may be involved in controlling cell shape, autolytic enzyme activity, and cation homeostasis (69). They make a significant contribution to the overall negative charge of the bacterial cell wall, attracting negatively charged compounds, including the cationic antimicrobial peptides (AMPs) of the innate humoral immune systems of higher organisms (69).
Many of the gram-positive bacterial species pathogenic to humans display resistance to cationic AMPs because of a decrease in the net negative charge of bacterial cell envelopes (75). Modifications to the TAs at the bacterial surface involving the incorporation of positively charged residues, such as d-alanine, prevent cationic AMPs from reaching their target, thereby protecting the organism against these compounds. This process involves the Dlt proteins encoded by the dltABCD operon present in most of the genome sequences established to date for gram-positive bacteria (44, 58, 74). d-Alanine is incorporated into LTAs through a two-step reaction involving a d-alanine-d-alanyl carrier protein ligase (Dcl) and a d-alanyl carrier protein (Dcp), encoded by the dltA and dltC genes, respectively (18, 44, 45, 70). The dltB and dltD genes encode other proteins required for the d-alanylation of LTAs. DltD is involved in selection of the Dcp carrier protein for ligation with d-alanine (19), whereas DltB is thought to be involved in d-alanyl-Dcp secretion (69). d-Alanine may be transferred from d-alanylated LTAs to wall-associated TAs by transacylation. For many human gram-positive bacterial pathogens, dlt operon inactivation has been shown to affect bacterial resistance to cationic AMPs and virulence. Indeed, Listeria monocytogenes, Bacillus anthracis, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Lactobacillus reuteri, and group B streptococci harboring mutations in dlt genes all have a higher negative charge on the cell surface and are more susceptible to cationic AMPs of various origins (1, 34, 56, 58, 59, 77, 78, 89). The inactivation of dlt genes in these pathogenic bacterial species also decreases interactions with phagocytic and nonphagocytic cells (1, 13, 34, 78).
The impact of Dlt proteins on cationic AMP resistance and virulence in insect bacterial pathogens has never before been studied, despite the major role of cationic AMPs in insect humoral immunity (9, 61). Insect bacterial pathogens also termed entomopathogenic bacteria are able to multiply in the insect hemocoel from small inocula (<10,000 viable cells) and produce fatal septicemia (8, 57). Entomopathogenic bacteria entering the hemolymph are targeted by an array of immune system mediators of both cellular and humoral reactions. The cellular response results in bacterial phagocytosis or encapsulation by circulating hemocytes, whereas the humoral response generates cationic AMPs (61). These peptides are small, inducible molecules produced in large amounts in hemolymph by the fat body (9, 26). They participate to the insect antimicrobial defense in a systemic response. Many AMP have been reported to cause damage in microbial membranes, which may ultimately lead to bacterial cell lysis (94).
We investigated the role of the dlt operon in cationic AMP resistance and virulence in Bacillus cereus, a human opportunistic and insect facultative bacterial pathogen. B. cereus sensu stricto is a spore-forming gram-positive bacterium. The B. cereus sensu lato group of bacteria also includes the closely related insect pathogen Bacillus thuringiensis and the human pathogen B. anthracis. Genomic data have shown that B. thuringiensis and B. cereus have almost identical chromosomal genetic backgrounds (54, 55) but that B. thuringiensis carries a plasmid encoding entomopathogenic cytoplasmic crystalline δ-endotoxins (Cry proteins) specifically active against insect larvae upon ingestion (22, 23, 83). B. cereus can cause opportunistic food-borne gastroenteritis and local/systemic infections in immunocompromised humans (85). Both B. thuringiensis (with and without Cry toxins) and B. cereus strains are highly pathogenic when injected directly into the hemocoels of insect larvae, in which they cause lethal septicemia (46, 82, 86, 96). The occurrence, structure, and glycosylation of LTAs were studied for different Bacillus species, including B. cereus strains containing LTAs (built up of polyglycerol phosphate chains and hydrophobic anchors) and d-alanine (11, 50, 51, 62). Therefore, the presence of a dlt operon in the B. cereus 14579 genome suggests that the LTAs may be alanylated.
We report here that the dlt operon of B. cereus is required for resistance to cationic AMPs of bacterial or insect origin. The dlt operon is required for full B. cereus virulence following bacterial injection into two lepidopteran insects, the caterpillar Spodoptera littoralis and the wax moth Galleria mellonella. We also detected the dlt operon in three gram-negative bacterial genera: Erwinia (Erwinia carotovora), Bordetella (Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica), and Photorhabdus (the entomopathogenic bacterium Photorhabdus luminescens TT01).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Permanent stocks of all bacterial strains were maintained at −80°C in Luria-Bertani (LB) broth supplemented with 17% glycerol. The bacterial strains and plasmids used in this study are listed in Table 1. B. cereus ATCC 14579 and Escherichia coli strains were routinely grown in LB broth (Difco) or on LB agar (Difco) at 37°C for E. coli and 30°C or 37°C for B. cereus. E. coli K-12 TG1 and E. coli K-12 XL1Blue were used as hosts for cloning, and E. coli ET 12567 Dam− Dcm− was used to generate unmethylated DNA for the electrotransformation of B. cereus. For electrotransformation experiments, B. cereus was grown in brain heart infusion broth (Difco) at 37°C. E. coli and B. cereus strains were transformed by electroporation, as previously described (27, 64). When required, the final concentrations of the chemicals and antibiotics added were as follows: 40 μg·ml−1 X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), 100 μg·ml−1 ampicillin (Ap), and 20 μg·ml−1 kanamycin (Km) for E. coli and 120 μg·ml−1 X-Gal, 10 μg·ml−1 erythromycin (Em), and 200 μg·ml−1 Km for B. cereus.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or relevant characteristic | Reference or source |
|---|---|---|
| Strains | ||
| B. cereus | ||
| ATCC 14579 | Wild-type reference strain | Laboratory stock |
| ΔdltBc strain | ATCC 14579 dltXABCD::aphA3 Km | This study |
| P. luminescens TT01 | Wild type isolated from Heterorhabditis bacteriophora nematode TH01 | 33 |
| E. coli | ||
| XL1-Blue | Δ(mrcA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tetr)] | Stratagene |
| K-12 TG1 | Δlac-proAB) supE thi hsdD5 (F′ traD36 proA+proB+lacIqlacZDM15) | 36 |
| ET 12567 | dam-13::Tn9 dcm-6 hsdM hsdR recF143 zjj201::Tn10 galK2 galT22 ara-14 lacY1 xyl-5 leuB6 thi-1 tonA31 rpsL136 hisG4 tsx-78 mtlI glnV44 F− | 65 |
| Plasmids | ||
| pHT304 | Apr Emr low-copy-number cloning vector | 2 |
| pHT315 | Apr Emr mid-copy-number cloning vector | 2 |
| pHT370 | Apr Emr high-copy-number cloning vector | 2 |
| pHT315-dltBc | pHT315 carrying dltXABCD of ATCC 14579 | This study |
| pHT304-dltTT01 | pHT304 carrying dltDBAC of TT01 | This study |
| pHT315-dltTT01 | pHT315 carrying dltDBAC of TT01 | This study |
| pHT370-dltTT01 | pHT370 carrying dltDBAC of TT01 | This study |
| pHT304-Km | aphAIII with its own promoter cloned in SalI and PstI sites of pHT304 | 6 |
| pMAD | Apr Emr thermosensitive plasmid containing the lacZ gene | 3 |
| pMAD-updlt-aphA3Km-downdlt | 2.3-kb BamHI-EcoRI fragment containing the 845-bp dlt upstream region, aphA3 Km, and the 835-bp dltD downstream region, inserted into pMAD/BamHI-EcoRI | This study |
Construction of the B. cereus dlt mutant.
The thermosensitive pMAD vector (3) was used to delete the dltXABCD operon (BC1373, BC1372, BC1371, BC1370, and BC1369) from B. cereus strain ATCC 14579 (GenBank accession no. AE016877) by allelic exchange. A Km resistance (Kmr) cassette was purified from pHT304-Km (6), which was obtained by cloning the aphA3 gene (Kmr) of pDG783-aphA3 (38) as a 1.5-kb XbaI-PstI fragment carrying the aphA3 gene. An 846-bp XbaI-EcoRI fragment (extending from position −865 to −27 with respect to the dltX translation initiation site) and an 838-bp PstI-BamHI fragment (extending from position +9 to +855 with respect to the dltD stop codon) were generated by PCR, using the following pairs of oligonucleotides: dlt1 (5′-GCTCTAGAGCTCTTTGTAGGTATCATGT-3′) and dlt2 (5′-CGGAATTCTACGTCTGGAGCTGCCATC-3′), containing the sites for XbaI and EcoRI, respectively (underlined), and dlt3 (5′-AAAGTGCAGCGCTTGAATTACTCAAGTGGTC-3′) and dlt4 (5′-CGGGATCCGAGAAGGTAGATGAGGCGA-3′), containing the sites for PstI and BamHI, respectively (underlined). These two amplified DNA fragments were inserted into E. coli TG1, on each side of the 1.5-kb XbaI-PstI fragment carrying the aphA3 gene, between the EcoRI and the BamHI sites of the thermosensitive plasmid pMAD, conferring Em resistance to B. cereus and Ap resistance to E. coli. The resulting pMAD-updlt-aphA3Km-downdlt plasmid was verified by restriction mapping and introduced, by electroporation, into E. coli ET 12567. The demethylated plasmid was then prepared from E. coli ET 12567 and introduced, by electroporation, into B. cereus ATCC 14579 (64). Transformants were obtained after growth for 2 days at 30°C on LB plates containing 120 μg·ml−1 X-Gal, 5 μg·ml−1 Em, and 200 μg·ml−1 Km. Gene deletion was achieved by recombination, as previously described (63), but with minor modifications (3).
A pool of individual blue clones was used to inoculate an overnight culture of LB medium supplemented with 10 μg·ml−1 Em, which was incubated at 30°C until stationary phase was reached. The culture was diluted in fresh medium and incubated for 2 hours at 42°C. Two more cycles of dilution in fresh medium and growth under the same conditions were then carried out. Finally, single white and blue colonies were isolated by plating dilutions of the culture onto LB agar plates containing X-Gal and incubating at 40°C. Several white colonies were isolated and checked for resistance to Km and susceptibility to Em. Strains resistant to Km and susceptible to Em arose through a double-crossover event in which the chromosomal wild-type copies of the dltXABCD genes were deleted and replaced with the Kmr cassette. The chromosomal allele exchange was checked by PCR, using appropriate primer pairs hybridizing outside the cloned DNA fragments in the B. cereus genome (UpdltX3 [5′-GCTCCGATAGCAATAGGATCTA-3′] and DowndltD3 [5′-GGTATGGCAGGCGCGATTACGA-3′]) and within the Kmr cassette (Km3 [5′-ACTCTGATGTTTTATATCTTTTCTA-3′] and Km-R [5′-CTTTGGAACAGGCAGCTTTC-3′]). The resulting recombinant clone was named ΔdltBc.
Construction of vectors for complementation tests.
For the complemented strains, the entire dltXABCD open reading frame (ORF) of B. cereus without its promoter region was amplified by PCR with Taq DNA polymerase (Extensor High-Fidelity; Thermo Scientific), using B. cereus ATCC 14579 genomic DNA as the template and using the forward primer SacI-dltD (5′-GCGCGAGCTCGAGCGAGTTATGAAAGAAGC-3′) and the reverse primer SmaI-dltX (5′-AGGCCCGGGGAGAGGGAAAGACATGGAA-3′), which include the restriction sites for SacI and XmaI, respectively (underlined). The resulting 4.447-kb fragment was hydrolyzed with SacI and XmaI, gel purified, and ligated to the pHT315 shuttle vector previously hydrolyzed with the same enzymes. An aliquot of ligation mixture (∼50 ng DNA) was used to transform E. coli TG1 by electroporation. The resulting construct, pHT315-dltBc, was verified by restriction mapping and was then directly introduced into ΔdltBc by electroporation. The dltCDBA operon of P. luminescens was amplified by PCR with Taq DNA polymerase (Invitrogen), using TT01 genomic DNA as the template and the primers dltA bis (5′-GCGCGAATTCGGGCTAAAAATACTGGGCTGA-3′) and Down dltC (5′-GCGCCTGCAGCGGGTATAGAGAACTATGTGA-3′), which include the restriction sites for EcoRI and PstI, respectively (underlined). The resulting 4.4-kb fragment was hydrolyzed by EcoRI and PstI; gel purified; ligated to pHT304, pHT315, and pHT370; and used to electroporate E. coli XL1-Blue. The resulting constructs, pHT304-dltTT01, pHT315-dltTT01, and pHT370-dltTT01, were verified by restriction mapping and sequencing (Macrogen, Seoul, South Korea) and introduced into ΔdltBc as described above. In these plasmids, the dlt gene transcription is under the control of the lacZ promoter, which is constitutively active in LB medium (25).
Autolysis test.
B. cereus wild-type and ΔdltBc strains were grown in LB medium at 37°C, with shaking. Cells were harvested in exponential growth phase, washed twice with cold phosphate-buffered saline (PBS; Gibco), and resuspended in the same buffer supplemented with 0.05% Triton X-100 (Roche). Bacterial cells were incubated at 37°C without shaking, and autolysis was monitored by measuring the decrease in absorbance at 600 nm (A600) at 30-min intervals on a microplate reader system (Tecan Infinite), recording changes in A600 over time. The initial A600 value was fixed at 100%, and the results shown are the means for three experiments.
Susceptibility to cationic AMPs.
In vitro susceptibility to polymyxin B sulfate (Sigma), colistin methanesulfate (Sigma), protamine sulfate (Sigma), lysozyme from chicken egg white (Sigma), and nisin (Fluka) was evaluated by determining MICs for B. cereus ATCC 14579 and its isogenic derivatives. Stock solutions of colistin, polymyxin B, protamine, and lysozyme were prepared in sterile water to obtain concentrations of 20 mg·ml−1, 50 mg·ml−1, 10 mg·ml−1, and 500 mg·ml−1, respectively. Nisin was dissolved in 20 mM HCl to give a final concentration of 250 μg·ml−1. Bacterial strains were cultured to late exponential growth phase (optical density at 600 nm [OD600], ∼1.2) in LB broth (Difco) at 37°C and diluted 1:100 into individual wells containing various concentrations of cationic AMPs (series of twofold dilutions). Growth was scored after 24 h of incubation at 37°C, and microtiter plates were read visually. The MIC was considered to be the lowest concentration of compound that severely inhibited cell growth. In vitro susceptibility to Spodoptera frugiperda cecropin B (Cec B) was also evaluated by MIC determination using 25, 50, 75, and 150 μg/ml of S. frugiperda Cec B. S. frugiperda Cec B was synthesized using tert-butyldicarbonate chemistry (Polypeptide Group, Strasbourg, France). The growth kinetics of the wild-type B. cereus strain and its isogenic ΔdltBc mutant in LB broth (Difco) supplemented with the above-cited S. frugiperda Cec B concentrations at 37°C with shaking (Tecan Infinite) were monitored for 15 h. Growth data were recorded in triplicate for strains incubated with the antimicrobial compound, and A600 measurements recorded after 15 h of incubation were averaged for the plotting of growth curves.
In vivo pathogenicity assays.
The common cutworm, S. littoralis, was reared on an artificial diet at 23°C, with a photoperiod of 12 h. Greater wax moth (G. mellonella) eggs were hatched at 25°C, and larvae were reared on beeswax and pollen (Naturalim). The surfaces of insect fifth-instar larvae were sterilized with 70% (vol/vol) ethanol. A Hamilton syringe was then used to inject appropriate dilutions of bacteria into insect larvae. S. littoralis and G. mellonella were injected with 20 μl and 10 μl, respectively, of bacterial suspension, using 0.45 × 13-mm needles (Terumo) and a 1-ml syringe with a microinjector (Kd Scientific). Groups of 20 larvae were injected, at the base of the last proleg (G. mellonella) or the second proleg (S. littoralis), with vegetative bacterial suspension (grown in LB medium at 30°C to an OD600 of 1 to 2) or PBS buffer as a control. We tested doses of 5 × 102 to 2 × 104 CFU and recorded mortality for up to 168 h of incubation at 30°C for G. mellonella. For S. littoralis, doses of 3 × 103 to 1 × 104 CFU were used and mortality was recorded for up to 80 h of incubation at 23°C. Ingestion assays were performed on groups of 20 G. mellonella larvae force fed (using 0.5 × 25-mm needles) with a mixture of 4 × 105 to 5 × 106 vegetative bacteria and 3 μg of purified and activated CryIC toxin (10 μl/larva) or with 10 μl of toxin or PBS alone as a control (5). Mortality was recorded for between 18 and 48 h after ingestion. We recorded the time of insect death to establish the 50% lethal time (LT50; the time by which 50% of the insects had died), and various doses were used to investigate dose-dependent mortality. Bacterial concentrations (doses) were determined by counting the number of CFU formed after the plating of dilutions on LB agar. At least three independent experiments were performed for each strain. The in vivo plasmid stability test was performed as follows. Dilutions of ΔdltBc/pHT315-dltBc and ΔdltBc/pHT315 supplemented or not supplemented with 10 μg·ml−1 Em were injected to insects. Bacterial dilutions prepared from infected or dead larvae were plated onto LB agar plates containing or not containing 10 μg/ml of Em (for positive pHT315 plasmid selection and for negative selection of coccus insect microflora) and incubated for 24 h at 37°C. Plasmid stability rate was calculated by dividing the number of CFU counted on plates supplemented with antibiotic by the number of CFU counted on plates without antibiotic.
RESULTS
Identification of the Bacillus cereus dlt operon.
Analysis of the B. cereus ATCC 14579 genome sequence (49) revealed the presence of a dlt locus containing five ORFs, dltX, dltA, dltB, dltC, and dltD (BC1373 to BC1369), which were 100%, 99%, 95%, 100%, and 98% identical to those of B. thuringiensis, respectively. Moreover, all the dlt ORFs of B. anthracis have levels of identity similar to those of B. cereus, with the exception of the dltB gene, which is 90% identical to that of B. cereus (Fig. 1A). The dltX gene encodes a putative 48-amino-acid (aa) peptide 40% identical to the DltX protein of Bacillus subtilis. The putative DltX protein is of unknown function. The dltA gene encodes a 504-aa protein 56% identical to the d-alanine-d-alanyl carrier protein ligase (Dcl or DltA) of B. subtilis. The dltC gene encodes a 79-aa protein 55% identical to the B. subtilis Dcp protein (or DltC). The dltB gene encodes a 391-aa transmembrane protein 59% identical to the DltB protein of B. subtilis, and dltD encodes a 391-aa protein 48% identical to the DltD protein of B. subtilis. The five ORFs of the dlt locus are potentially transcribed in the same direction. The presence of short intergenic sequences, an absence of putative terminators between the genes, and the presence of a putative terminator downstream from dltD strongly suggest that the dlt locus of B. cereus is an operon.
FIG. 1.
The dlt operon involved in the d-alanylation of TAs is part of the core genome of gram-positive bacteria, whereas it is located in plastic regions in gram-negative bacterial genomes. Shown are the organization and genomic environment of the dlt operon in several gram-positive bacterial species (Bc, Bacillus cereus ATCC 14579 [GenBank accession no. AE016877]; Bt, Bacillus thuringiensis subsp. konkukian strain 97-27 [AE017355]; Ba, Bacillus anthracis strain Sterne [AE017225]; Bl, Bacillus licheniformis ATCC 14580 [AE017333]; Bs, Bacillus subtilis 168 [AL009126]; Sp, Streptococcus pneumoniae R6 [AE007317]; Sag, Streptococcus agalactiae 2603V/R [AE009948]; Saur, Staphylococcus aureus JH1 [CP000736]; Lc, Lactobacillus casei BL23 [FM177140]; Ef, Enterococcus faecalis V583 [AE016830]; and Lmo, Listeria monocytogenes EGD-e [AL591824]) (A) and in several gram-negative bacterial species (Eca, Erwinia carotovora subsp. atroseptica SCRI1043 [GenBank accession no. BX950851]; Pl, Photorhabdus luminescens TT01 [BX470248]; Bp, Bordetella pertussis Tohama I [BX470248]; Bpp, Bordetella parapertussis 12822 [BX470249]; and Bbr, Bordetella bronchiseptica RB50 [BX470250]) (B). Black blocks and arrows indicate the core genome, gray blocks indicate regions of genomic plasticity, horizontal arrows indicate the different dlt genes, and white vertical arrowheads indicate core genome genes or operons flanking the dlt region in gram-negative bacteria. kb, kilobases.
The dlt operon is widespread in gram-positive bacteria, and the four genes (dltA, dltB, dltC, and dltD) are highly conserved and organized similarly in other gram-positive bacterial species, such as B. anthracis, B. thuringiensis, B. subtilis, Bacillus licheniformis, Lactobacillus casei, L. monocytogenes, Streptococcus pneumoniae, Streptococcus agalactiae, S. aureus, and Enterococcus faecalis (Fig. 1A). Although flanking regions differ between gram-positive bacteria, remarkable similarities in genomic organization are observed between phylogenetically closely related bacterial species, such as the various Bacillus spp. Furthermore, no features indicative of mobility (tRNA, insertion sequences, and integrase genes) were identified in any of the studied dlt environments in gram-positive bacteria. Thus, the dlt operon of gram-positive bacteria probably belongs to the core genome (Fig. 1A).
Identification of the dlt locus in gram-negative bacterial genomes.
Genome database searches led to the identification of dlt operons in the genomes of three gram-negative genera: Erwinia (the phytopathogenic bacterium Erwinia carotovora subsp. atroseptica [4]), Bordetella (the three species B. pertussis, B. parapertussis, and B. bronchiseptica [73]), and Photorhabdus (the entomopathogenic bacterium P. luminescens TT01 [28]) (Fig. 1B). These dlt operons contained four ORFs corresponding to dltA, dltB, dltC, and dltD and encoding proteins 34%, 29%, 29%, and 21% identical to those of B. subtilis, respectively. Their order differs from that described for gram-positive bacteria. The dlt operons of P. luminescens and Bordetella spp. were identical and were found to contain an additional ORF between the dltD and dltB genes, predicted to encode a 41-aa protein 32% identical to a hypothetical protein of Arcobacter butzleri RM4018 (GenBank accession no. CP000361).
The dlt loci of the three gram-negative bacterial genera were probably acquired by horizontal genetic transfer and therefore belong to the flexible gene pool. Indeed, the dlt locus of E. carotovora is part of a 60-kb putative genomic island flanked by a tRNAArg gene. The dlt locus of P. luminescens has a G+C content very different from that of the rest of the chromosome (30% versus 43% for the genome as a whole) and is located in a putative region of genomic plasticity absent from the genomes of most enterobacteria. Finally, the dlt locus of Bordetella spp. is itself a putative region of genomic plasticity, as it is present in some Bordetella spp. (B. pertussis, B. parapertussis, and B. bronchiseptica) but absent from Bordetella petrii DSM 12804 (GenBank accession no. AM902716).
The regions of genomic plasticity containing the dlt locus in both P. luminescens and E. carotovora are flanked by core genome genes also involved in bacterial cell surface modifications reducing the overall negative charge of the cell wall. In P. luminescens, these genes include lysC and aceK (mprF homolog), which are involved in the pathways of l-lysine synthesis and incorporation into cell membrane phospholipids, respectively (76). In Erwinia, homologs of the pbgPE operon (yfbEFGHIWJ or pmrHFIJKLM) and the ugd gene (pmrE) were identified. The pbgPE and ugd genes encode enzymes mediating aminoarabinose synthesis and the transfer to lipid A of several bacterial lipopolysaccharides (LPSs) conferring resistance to cationic AMPs in gram-negative bacteria (24, 39, 40).
Construction and phenotypic characterization of the isogenic B. cereus dlt mutant.
We investigated the role of the dlt operon in B. cereus ATCC 14579 by deleting the entire operon by allelic exchange. The phenotypic traits of the resulting ΔdltBc mutant and the wild-type strain were compared. ΔdltBc colonies were smaller than wild-type colonies after growth on LB agar plates for 15 h at 37°C (Fig. 2A). Furthermore, light microscopy showed that mutant cells had an aberrant shape (pleomorphism) and were smaller (5 μm) than the wild type (5 to 30 μm) (Fig. 2A). Similar differences were observed during the exponential (Fig. 2A) and stationary (not shown) growth phases. ΔdltBc also formed shorter chains than the wild type during the exponential and stationary phases. In LB medium, the wild-type and mutant B. cereus strains grew similarly, but with a slightly longer lag phase for the dlt mutant (30 min) (Fig. 2B). However, both strains reached the same OD600 after 4 h of growth in LB medium at 37°C. The ΔdltBc strain was able to form spores on HCT (hydrolysate casein-tryptone) agar sporulation-specific medium, with the same efficiency as the wild-type strain.
FIG. 2.
Phenotypes of the wild-type B. cereus and ΔdltBc strains. (A) Photograph of B. cereus ATCC 14579 wild-type (Bc WT) colonies and B. cereus dlt mutant (ΔdltBc) colonies grown on LB agar for 15 h at 37°C. The corresponding phase-contrast transmission micrographs of Bc WT and ΔdltBc in the exponential growth phase are shown. Note the smaller sizes of ΔdltBc mutant colonies and bacteria than of the wild type. (B) Growth curves for the wild-type (black diamond) and the dlt knockout mutant (ΔdltBc) (white square) B. cereus strains, grown in LB medium at 37°C with shaking. Absorbances (A600) were scored with a real-time microplate reader system (Tecan Infinite). Means and standard errors of the means (bars) of results from triplicate experiments are shown. (C) Autolysis rates of the wild-type (black diamond) and the dlt knockout mutant (ΔdltBc) (white square) B. cereus strains in the presence of Triton X-100. Autolysis rates were determined by a microplate reader system (Tecan Infinite) monitoring decreases in OD600 over a period of 2 h (120 min) in PBS at 37°C, without shaking. Means and standard errors of the means (bars) of results from triplicate experiments are shown.
The mutation of dlt genes in other species of gram-positive bacteria increases autolysis (56, 58, 72, 84, 90). Autolysis rates were similar for the ΔdltBc mutant and wild-type strains during growth in LB medium (data not shown). In contrast, autolysis tests with Triton X-100 showed that autolysis was more rapid in ΔdltBc than in the wild-type strain (Fig. 2C). The B. cereus wild-type and ΔdltBc strains displayed similar levels of autolysis after 6 hours of incubation (not shown).
The B. cereus dlt operon is required for in vitro resistance to structurally different cationic AMPs.
We assessed the role of the dltXABCD operon in the resistance of B. cereus to cationic AMPs by determining MICs for the wild-type and ΔdltBc strains (Table 2). When challenged with bacterial polymyxin B, colistin, and nisin and salmon sperm protamine, the MICs obtained were lower for ΔdltBc than for the wild-type strain by factors of 2 to 64. Full (nisin) or partial (polymyxin B, colistin, and protamine) complementation was obtained by introducing the dltXABCD operon under the control of the lacZ constitutive promoter from the pHT315 plasmid (pHT315-dltBc; 15 copies/bacterium) into the ΔdltBc mutant, demonstrating that dlt deletion was responsible for the resistance defect. The dlt operon of B. cereus is therefore an important component of the intrinsic resistance of B. cereus to cationic AMPs.
TABLE 2.
MICsa of various cationic AMPs for wild-type B. cereus ATCC 14579, the dlt knockout mutant (ΔdltBc) strain, and complemented dlt mutants
| B. cereus strain or mutation | MIC (μg/ml) of cationic AMP |
||||
|---|---|---|---|---|---|
| Polymyxin B | Colistin | Nisin | Protamine | Lysozyme | |
| ATCC 14579 | 781 | >5,000 | 62 | 2,500 | >125,000 |
| ΔdltBc | 24 | 78 | 31 | 39 | 7,812 |
| ΔdltBc/pHT315 | 24 | 78 | 31 | 39 | 7,812 |
| ΔdltBc/pHT315-dltBc | 97 | 625 | 62 | 156 | 62,500 |
| ΔdltBc/pHT315-dltTT01 | 24 | 78 | 31 | 39 | 7,812 |
| ΔdltBc/pHT304 | 24 | 78 | 31 | 39 | 7,812 |
| ΔdltBc/pHT304-dltTT01 | 24 | 78 | 31 | 39 | 7,812 |
| ΔdltBc/pHT370 | 24 | 78 | 31 | 39 | 7,812 |
| ΔdltBc/pHT370-dltTT01 | 24 | 78 | 31 | 39 | 7,812 |
As determined by the broth dilution method (three replicates). MICs were scored after 24 h of incubation at 37°C.
TAs have been found only in gram-positive bacteria (69). Nevertheless, we identified dlt operons in some gram-negative bacterial species. We investigated the possible role of these genes in cationic AMP resistance by functional complementation of the ΔdltBc mutant with the dltDBAC operon of another entomopathogenic bacterium, P. luminescens TT01. We inserted the TT01 dlt operon downstream from the constitutive lacZ promoter in plasmids for which there were 4, 15, or 70 copies per bacterial cell (pHT304-dltTT01, pHT315-dltTT01, or pHT370-dltTT01, respectively) (Table 1) and introduced them into the ΔdltBc strain. The MICs of polymyxin B, colistin, nisin, and protamine for ΔdltBc harboring the TT01 dlt operon were similar to those for the ΔdltBc mutant (Table 2), despite the confirmation by reverse transcription-PCR that the TT01 dlt operon was expressed (data not shown). This finding suggests that the dlt operon of P. luminescens has functions other than d-alanylation. However, it should be noted that the production of functional enzymes and their appropriate addressing in the bacterial cell have not been demonstrated.
Contribution of the dlt operon to insect humoral immune resistance.
We investigated the requirement of the B. cereus dlt operon for resistance to the molecules of the insect humoral immune system. We first assessed resistance to lysozyme from chicken egg white. Lysozymes are major constitutive enzymes of the insect humoral immune response that were isolated from the lepidopteran insects Bombyx mori, G. mellonella, and S. littoralis and were structurally classified among the C (chicken) type lysozymes (52). We found that the MIC of lysozyme for ΔdltBc was lower than that for the wild type by a factor of at least 16 (Table 2). We also assessed resistance to S. frugiperda Cec B, a natural inducible cationic AMP isolated from the moth Spodoptera frugiperda. We showed that wild-type B. cereus is highly resistant to S. frugiperda Cec B, with a MIC greater than 125 μg/ml. This high MIC of S. frugiperda Cec B made MIC experiments too expensive. We therefore restricted MIC determination test to four concentrations of S. frugiperda Cec B (25, 50, 75, and 150 μg/ml). The isogenic ΔdltBc mutant could not grow in LB medium to which 150 μg/ml of S. frugiperda Cec B had been added, whereas the parental strain grew well and was highly resistant (Fig. 3). Thus, the dlt operon mediates resistance to insect humoral immune mediators in B. cereus.
FIG. 3.
MICs of S. frugiperda (Sf) Cec B for the wild-type (black diamond) and dlt knockout mutant (ΔdltBc) (white square) B. cereus strains. All strains were diluted (1:100) in LB medium containing the indicated concentration of S. frugiperda Cec B. Real-time changes in A600 were monitored with a microplate reader system (Tecan Infinite) at 37°C, with shaking. Data represent A600 values scored at 15 h of incubation, and results are means for three different experiments. Standard errors of the means (bars) are shown.
The B. cereus dlt operon mutation abolishes or greatly attenuates virulence in lepidopteran insects.
The finding that the dlt operon is essential for B. cereus resistance to insect cationic AMPs led us to investigate the effects of mutating this operon on bacterial virulence in insects. We injected similar doses (3 × 103 to 1 × 104 CFU) of cells of the parental strain (B. cereus ATCC 14579), the dlt knockout mutant harboring pHT315 (ΔdltBc/pHT315), and the complemented dlt mutant (ΔdltBc/pHT315-dltBc) directly into the hemocoels of S. littoralis larvae and monitored insect mortality after injection (Fig. 4A). Remarkably, only between 0 and 5% mortality was observed with ΔdltBc/pHT315. The introduction of pHT315-dltBc into the dlt mutant partially restored virulence after a slight time lag. The LT50 values for a dose of 1 × 104 CFU/larva were 15 h for the wild type and 32 h for the complemented strain. We confirmed the maintenance of pHT315-dltBc in the bacterial population in vivo more than 24 h after injection, even in dead larvae (100% plasmid stability rate). Injection experiments were also carried out with G. mellonella, previously shown to be susceptible to the wild-type strain studied here. In this insect, the lower level of virulence of the ΔdltBc mutant than of the parental strain was less marked than in S. littoralis. However, clear differences were observed in lethal time analysis (Fig. 4B). Following the injection of 1 × 104 CFU/larva, 90% mortality was observed for the wild-type strain, 50% mortality was observed for the ΔdltBc mutant, and 70% mortality was observed for the complemented strain 168 h after injection. The LT50 of G. mellonella was 10 h for the wild type, 168 h for the ΔdltBc mutant, and 40 h for the complemented strain (Fig. 4B).
FIG. 4.
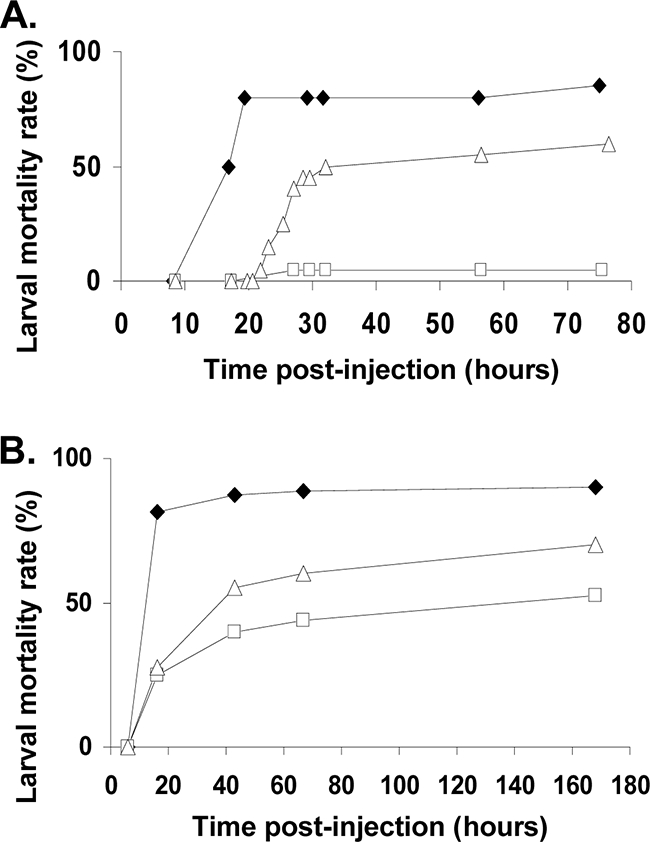
Mortality of insect larvae injected with the wild-type, ΔdltBc/pHT315 mutant, and complemented ΔdltBc/pHT315-dltBc mutant B. cereus strains. Bacteria were collected at the end of the exponential phase and injected into 20 fifth-instar larvae. All experiments were repeated at least three times, and data represent one representative experiment. (A) Mortality kinetics of S. littoralis after injection with 3 × 103 to 1 × 104 CFU of the wild-type (black diamond), ΔdltBc/pHT315 mutant (white square), and complemented ΔdltBc/pHT315-dltBc mutant (white triangle) B. cereus strains. The mortality values presented correspond to the result obtained over a period of 3 days after injections. (B) Mortality kinetics of G. mellonella after injection with 1 × 104 CFU of the wild-type (black diamond), ΔdltBc/pHT315 mutant (white square), and complemented ΔdltBc/pHT315-dltBc mutant (white triangle) B. cereus strains. The mortality values presented correspond to the result obtained over a 6-day period after injection.
In contrast, oral infection of G. mellonella with vegetative bacteria associated with Cry1C toxin (82) resulted in similar mortality rates for the wild-type, ΔdltBc mutant, and complemented strains. Indeed, 50% mortality at a dose of 5 × 105 CFU and 70% at a dose of 5 × 106 CFU were recorded 48 h after ingestion (data not shown) for all strains. Thus, the dlt operon is involved in B. cereus virulence when injected directly into insects but plays only a minor role in oral infection.
DISCUSSION
The widespread distribution of the dlt operon among gram-positive pathogens and commensal species suggests that d-alanyl-TAs are biologically important in various microbial niches. In a number of medically important gram-positive pathogens, including S. aureus and Staphylococcus xylosus (77), S. pyogenes (58), L. monocytogenes (1), B. anthracis (34), and S. pneumoniae (56), d-alanylation of cell wall-bound LTA decreases the overall negative charge of the cell wall, decreasing the binding of cationic AMPs and autolysins to the bacterial cell surface. Studies of the role of dlt in rodent infection models showed that this operon was required for host-pathogen interactions involving L. monocytogenes, S. aureus, B. anthracis, and S. agalactiae (1, 13, 34, 78). We show here, for the first time, that the dlt locus of the human opportunistic and entomopathogenic bacterium B. cereus ATCC 14579 is important for protection against insect humoral immune mediators, including the cationic AMPs produced in hemolymph following bacterial infection. Such protection may be critical for the establishment of lethal septicemia by B. cereus.
AMPs constitute families of bactericidal peptides widely produced by microbes to inhibit the growth of competing strains (14, 81) and by higher organisms as part of the humoral immune response to bacterial infections (42, 95). Many AMPs are thought to act by accumulating in the cytoplasmic membrane to a critical concentration allowing the assembly of structures that permeabilize the cell (41, 42). As the B. cereus dlt mutant was much less susceptible than the wild type to the highly cationic peptides polymyxin B, colistin, protamine, Cec B from insects, and C-type lysozyme, we hypothesize that the greater susceptibility of the B. cereus dlt mutant and its more rapid autolysis were due to changes in the electrostatic interactions of peptides and autolysins with the mutant cells. These findings also suggest that this bacterium may use dlt-mediated d-alanylation of TAs as a strategy for adaptation to insect hemolymph, into which humoral mediators, such as cecropins and lysozymes, are secreted.
We showed in vivo that the B. cereus dlt mutant was almost completely nonvirulent after injection into S. littoralis but not after injection into G. mellonella, in which the virulence of the dlt mutant was only attenuated. Furthermore, preliminary data indicate that septicemia in S. littoralis hemolymph was observed 8 to 10 h after the injection of wild-type bacteria but that the ΔdltBc mutant was rapidly eliminated from hemolymph (data not shown). This efficient clearance of the B. cereus dlt mutant in the S. littoralis hemolymph probably results from its greater susceptibility to S. littoralis circulating humoral mediators. In contrast, the B. cereus dlt mutant is probably able to persist in the hemocoel of G. mellonella, since this insect shows only a 50% decrease in mortality. One could ask if G. mellonella AMPs are different from those produced by S. littoralis. AMPs are classified into three major groups on the basis of amino acid sequence and structural characteristics (10). Biochemical approaches conducted on G. mellonella led to the purification of about 10 AMPs belonging to those three groups, that is to say, (i) linear α-helical peptides (5 cecropins and moricins), (ii) cysteine-stabilized peptides (3 defensin-like AMPs), and (iii) peptides rich in certain amino acids (2 prolin-rich AMPs) (7, 15, 53, 60, 66). A similar pattern of AMPs in Spodoptera spp. was characterized (68, 71, 88). Consequently, the differences in dlt mutant virulence observed between G. mellonella and S. littoralis could not be assigned to a defect in one of the major groups of AMPs. Nevertheless, it is noteworthy that there are no data available concerning the concentration of the different AMPs in the hemolymph of the two insects. We hypothesized that the difference in dlt mutant virulence observed between G. mellonella and S. littoralis is due to a difference in the concentration and/or synergistic effect of the AMPs in the bloodstream.
With the exceptions of Xenorhabdus and Photorhabdus strains, the ingestion of entomopathogenic microorganisms is probably the main route of insect infection under natural conditions (87). This is clearly the case for B. thuringiensis, because Cry toxins are activated only after oral ingestion. The ingested bacteria may persist in the gut, where they may trigger local AMP gene expression and cross the intestinal barrier to reach the hemocoel (61). In this study, we used the lepidopteran model G. mellonella for oral infection, because this model has already been used to identify various virulence genes in the B. cereus group (5, 30, 32, 82). However, little is known about AMP production in the gut of this insect. Ingestion bioassays with G. mellonella showed the B. cereus dlt mutant and wild-type strains to be similarly virulent and efficient, indicating that the role of the dlt operon in virulence in this insect depends on the route of inoculation. Nevertheless, the insect gut lumen is an environment hostile to microbial colonization, due to its physical and physiological properties and the lysozyme secretion (16, 48). Therefore, although the dlt operon increases the resistance of B. cereus to both lysozyme and Cec B, it is not involved in the colonization of the G. mellonella gut. This suggests that the AMPs secreted locally in the gut differ quantitatively or qualitatively from those of the systemic response in hemolymph. Alternatively, bacteria may be resistant to the AMPs in the insect gut through strategies other than cell surface modifications. For instance, effectors, such as proteases, may specifically degrade the AMPs produced in the gut or by AMP-producing cells. Previous studies consistent with this notion showed that the entomopathogenic bacterium B. thuringiensis secretes metalloproteases also present in B. cereus: InhA1, which specifically hydrolyzes cecropins in the immune hemolymph of Hyalophora cecropia in vitro (17, 29), and InhA2, which has been shown to be essential for virulence after oral ingestion in G. mellonella larvae (32). Nevertheless, InhA1 and InhA2 were not necessary for virulence by injection in B. mori larvae and were not able to ensure bacterial survival in immunized hemolymph (32). Hence, we can speculate that a massive production of InhA1 and InhA2 proteases during stationary phase allows multiplication of the bacteria in the insect gut (31, 37) whereas in hemolymph, the cationic AMP resistance of the vegetative cells is primarily dependent on Dlt alanylating proteins. The difference in dlt-mediated virulence after administration by injection and ingestion may also be related to the cellular immune response. Following injection into insect hemolymph, one of the earliest immune responses against bacterial infection in lepidopteran hemolymph is hemocyte-mediated phagocytosis (80). In contrast, hemocytes are unlikely to be encountered in the gut lumen. Cellular reactions may therefore lead to the clearance of the B. cereus dlt mutant after injection. Indeed, several studies indicate important roles for TA d-alanyl substitutions in bacterial adhesion to and infection of host cells (92). These roles have been studied thoroughly for L. monocytogenes, S. pyogenes, and S. aureus, whose respective dlt mutants were impaired in their ability to adhere to epithelial or endothelial cells (1, 58, 91, 93), thereby decreasing the intracellular survival of these bacterial species confronted with antimicrobial activities during phagocytosis by mammalian immunocompetent cells (1, 13, 34). Moreover, LTAs of S. aureus, S. pneumoniae, and many other gram-positive bacterial species are thought to have immunostimulatory activity (67) by eliciting the proinflammatory responses through the transmembrane protein Toll-like receptor 2 (21). These responses are efficiently amplified only if LTAs are modified with d-alanine (20).
Finally, we cannot exclude the possibility of another, as-yet-unidentified function of the dlt operon to account for the difference in dlt-mediated virulence after administration by injection and ingestion. Indeed, TAs were described only for gram-positive bacteria, but we showed that the dlt operon had been acquired through horizontal genetic transfer by five gram-negative bacterial pathogen species. Since the dlt operon of the entomopathogenic P. luminescens TT01 strain could not restore the cationic AMP resistance of the B. cereus dlt mutant, the gram-negative dlt genes may therefore be involved in the d-alanylation of other substrates on bacterial cell surfaces. In gram-negative bacteria, LPS is the major cell surface glycopolymer. As TAs, LPS is highly anionic and can be modified by addition of polar groups in many pathogenic bacterial species, such as Salmonella and Photorhabdus species (24, 39, 47). Consequently, reducing the overall cell surface negative charge confers resistance to cationic AMP in these bacterial species. To the best of our knowledge, it has never been shown that the LPS of gram-negative bacteria could be alanylated. Indeed, studies of Bordetella and Erwinia LPS structures are consistent with this notion (35, 79). The structure of Photorhabdus LPS has never been elucidated. Finally, d-alanylation may occur on other cell surface components. Indeed, d-alanine is a structural component of peptidoglycan in both gram-positive and gram-negative bacteria (43). Moreover, d-alanyl esters in B. subtilis are linked not only to TAs but also to two membrane proteins of unknown function (12).
In conclusion, we show here, for the first time, that the Dlt resistance system of an entomopathogenic bacterium is effective against the humoral immune system of insects, contributing to the virulence and persistence of these bacteria. However, the impact of this system on cellular responses to B. cereus infection in insects is currently being studied, and the results of these studies may increase our understanding of the cross talk between insects and bacteria.
Acknowledgments
This work was supported by the CEDRE program (project no. 07EF21/L12 [Résistance aux peptides antimicrobiens chez les bactéries pathogènes d'insectes Bacillus thuringiensis and Photorhabdus luminescens]), the Agence Universitaire de la Francophonie (project no. 63 13PS808 [Etude de la résistance de bactéries pathogènes aux peptides antimicrobiens et recherche de nouveaux peptides d'origine végétale]), and the research council of Université Saint-Joseph, Beirut (project FS17). Z.A.K. was funded by grant P6-411/3089/166 from the Agence Universitaire de la Francophonie.
Footnotes
Published ahead of print on 18 September 2009.
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115-119. [DOI] [PubMed] [Google Scholar]
- 3.Arnaud, M., A. Chastanet, and M. Debarbouille. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 70:6887-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, K. S., M. Sebaihia, L. Pritchard, M. T. Holden, L. J. Hyman, M. C. Holeva, N. R. Thomson, S. D. Bentley, L. J. Churcher, K. Mungall, R. Atkin, N. Bason, K. Brooks, T. Chillingworth, K. Clark, J. Doggett, A. Fraser, Z. Hance, H. Hauser, K. Jagels, S. Moule, H. Norbertczak, D. Ormond, C. Price, M. A. Quail, M. Sanders, D. Walker, S. Whitehead, G. P. Salmond, P. R. Birch, J. Parkhill, and I. K. Toth. 2004. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. USA 101:11105-11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillaut, L., N. Ramarao, C. Buisson, N. Gilois, M. Gohar, D. Lereclus, and C. Nielsen-Leroux. 2005. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 71:8903-8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brillard, J., K. Susanna, C. Michaud, C. Dargaignaratz, M. Gohar, C. Nielsen-Leroux, N. Ramarao, A. B. Kolsto, C. Nguyen-the, D. Lereclus, and V. Broussolle. 2008. The YvfTU two-component system is involved in plcR expression in Bacillus cereus. BMC Microbiol. 8:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, S. E., A. Howard, A. B. Kasprzak, K. H. Gordon, and P. D. East. 2008. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 38:201-212. [DOI] [PubMed] [Google Scholar]
- 8.Bucher, G. E. 1960. Potential bacterial pathogens of insects and their characteristics. J. Insect Pathol. 2:172-195. [Google Scholar]
- 9.Bulet, P., C. Hetru, J. L. Dimarcq, and D. Hoffmann. 1999. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 23:329-344. [DOI] [PubMed] [Google Scholar]
- 10.Bulet, P., R. Stocklin, and L. Menin. 2004. Anti-microbial peptides: from invertebrates to vertebrates. Immunol. Rev. 198:169-184. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury, B., C. Leoff, E. Saile, P. Wilkins, C. P. Quinn, E. L. Kannenberg, and R. W. Carlson. 2006. The structure of the major cell wall polysaccharide of Bacillus anthracis is species-specific. J. Biol. Chem. 281:27932-27941. [DOI] [PubMed] [Google Scholar]
- 12.Clark, V. L., and F. E. Young. 1978. d-Alanine incorporation into macromolecules and effects of d-alanine deprivation on active transport in Bacillus subtilis. J. Bacteriol. 133:1339-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. Van Kessel, J. A. Van Strijp, F. Gotz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186:214-219. [DOI] [PubMed] [Google Scholar]
- 14.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 15.Cytrynska, M., P. Mak, A. Zdybicka-Barabas, P. Suder, and T. Jakubowicz. 2007. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 28:533-546. [DOI] [PubMed] [Google Scholar]
- 16.Daffre, S., P. Kylsten, C. Samakovlis, and D. Hultmark. 1994. The lysozyme locus in Drosophila melanogaster: an expanded gene family adapted for expression in the digestive tract. Mol. Gen. Genet. 242:152-162. [DOI] [PubMed] [Google Scholar]
- 17.Dalhammar, G., and H. Steiner. 1984. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insects. Eur. J. Biochem. 139:247-252. [DOI] [PubMed] [Google Scholar]
- 18.Debabov, D. V., M. P. Heaton, Q. Zhang, K. D. Stewart, R. H. Lambalot, and F. C. Neuhaus. 1996. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J. Bacteriol. 178:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debabov, D. V., M. Y. Kiriukhin, and F. C. Neuhaus. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J. Bacteriol. 182:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deininger, S., I. Figueroa-Perez, S. Sigel, A. Stadelmaier, R. R. Schmidt, T. Hartung, and S. von Aulock. 2007. Use of synthetic derivatives to determine the minimal active structure of cytokine-inducing lipoteichoic acid. Clin. Vaccine Immunol. 14:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deininger, S., A. Stadelmaier, S. von Aulock, S. Morath, R. R. Schmidt, and T. Hartung. 2003. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J. Immunol. 170:4134-4138. [DOI] [PubMed] [Google Scholar]
- 22.de Maagd, R. A., A. Bravo, C. Berry, N. Crickmore, and H. E. Schnepf. 2003. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 37:409-433. [DOI] [PubMed] [Google Scholar]
- 23.de Maagd, R. A., M. Weemen-Hendriks, J. W. Molthoff, and S. Naimov. 2003. Activity of wild-type and hybrid Bacillus thuringiensis delta-endotoxins against Agrotis ipsilon. Arch. Microbiol. 179:363-367. [DOI] [PubMed] [Google Scholar]
- 24.Derzelle, S., E. Turlin, E. Duchaud, S. Pages, F. Kunst, A. Givaudan, and A. Danchin. 2004. The PhoP-PhoQ two-component regulatory system of Photorhabdus luminescens is essential for virulence in insects. J. Bacteriol. 186:1270-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Souza, M. T., M. M. Lecadet, and D. Lereclus. 1993. Full expression of the cryIIIA toxin gene of Bacillus thuringiensis requires a distant upstream DNA sequence affecting transcription. J. Bacteriol. 175:2952-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimarcq, J. L., P. Bulet, C. Hetru, and J. Hoffmann. 1998. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers 47:465-477. [DOI] [PubMed] [Google Scholar]
- 27.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307-1313. [DOI] [PubMed] [Google Scholar]
- 29.Edlund, T., I. Siden, and H. G. Boman. 1976. Evidence for two immune inhibitors from Bacillus thuringiensis interfering with the humoral defense system of saturniid pupae. Infect. Immun. 14:934-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedhila, S., N. Daou, D. Lereclus, and C. Nielsen-LeRoux. 2006. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol. Microbiol. 62:339-355. [DOI] [PubMed] [Google Scholar]
- 31.Fedhila, S., M. Gohar, L. Slamti, P. Nel, and D. Lereclus. 2003. The Bacillus thuringiensis PlcR-regulated gene inhA2 is necessary, but not sufficient, for virulence. J. Bacteriol. 185:2820-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedhila, S., P. Nel, and D. Lereclus. 2002. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 184:3296-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer-Le Saux, M., V. Viallard, B. Brunel, P. Normand, and N. E. Boemare. 1999. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov. and P. asymbiotica sp. nov. Int. J. Syst. Bacteriol. 49:1645-1656. [DOI] [PubMed] [Google Scholar]
- 34.Fisher, N., L. Shetron-Rama, A. Herring-Palmer, B. Heffernan, N. Bergman, and P. Hanna. 2006. The dltABCD operon of Bacillus anthracis Sterne is required for virulence and resistance to peptide, enzymatic, and cellular mediators of innate immunity. J. Bacteriol. 188:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuoka, S., Y. A. Knirel, B. Lindner, H. Moll, U. Seydel, and U. Zahringer. 1997. Elucidation of the structure of the core region and the complete structure of the R-type lipopolysaccharide of Erwinia carotovora FERM P-7576. Eur. J. Biochem. 250:55-62. [DOI] [PubMed] [Google Scholar]
- 36.Gibson, T. J. 1984. Ph.D thesis. University of Cambridge, Cambridge, United Kingdom.
- 37.Grandvalet, C., M. Gominet, and D. Lereclus. 2001. Identification of genes involved in the activation of the Bacillus thuringiensis inhA metalloprotease gene at the onset of sporulation. Microbiology 147:1805-1813. [DOI] [PubMed] [Google Scholar]
- 38.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 39.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 40.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hancock, R. E. 1999. Host defence (cationic) peptides: what is their future clinical potential? Drugs 57:469-473. [DOI] [PubMed] [Google Scholar]
- 42.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 43.Heaton, M. P., and F. C. Neuhaus. 1993. The significance of secondary cell wall polymers in gram-positive organisms: Lactobacillus casei as a model system for the study of D-alanyl-lipoteichoic acid biosynthesis and function. Horizon Scientific Press, Wymondham, United Kingdom.
- 44.Heaton, M. P., and F. C. Neuhaus. 1992. Biosynthesis of d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J. Bacteriol. 174:4707-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaton, M. P., and F. C. Neuhaus. 1994. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J. Bacteriol. 176:681-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heimpel, A. M. 1955. Investigations of the mode of action of strains of Bacillus cereus Fr. and Fr. pathogenic for the larch sawfly, Pristiphora erichsonii (Htg). Can. J. Zool. 33:311-326. [DOI] [PubMed] [Google Scholar]
- 47.Helander, I. M., I. Kilpelainen, and M. Vaara. 1994. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol. Microbiol. 11:481-487. [DOI] [PubMed] [Google Scholar]
- 48.Hultmark, D. 1996. Insect lysozymes. EXS 75:87-102. [DOI] [PubMed] [Google Scholar]
- 49.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 50.Iwasaki, H., A. Shimada, and E. Ito. 1986. Comparative studies of lipoteichoic acids from several Bacillus strains. J. Bacteriol. 167:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwasaki, H., A. Shimada, K. Yokoyama, and E. Ito. 1989. Structure and glycosylation of lipoteichoic acids in Bacillus strains. J. Bacteriol. 171:424-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolles, J., F. Schoentgen, G. Croizier, L. Croizier, and P. Jolles. 1979. Insect lysozymes from three species of Lepidoptera: their structural relatedness to the C (chicken) type lysozyme. J. Mol. Evol. 14:267-271. [DOI] [PubMed] [Google Scholar]
- 53.Kim, C. H., J. H. Lee, I. Kim, S. J. Seo, S. M. Son, K. Y. Lee, and I. H. Lee. 2004. Purification and cDNA cloning of a cecropin-like peptide from the great wax moth, Galleria mellonella. Mol. Cells 17:262-266. [PubMed] [Google Scholar]
- 54.Kolsto, A. B., D. Lereclus, and M. Mock. 2002. Genome structure and evolution of the Bacillus cereus group. Curr. Top. Microbiol. Immunol. 264:95-108. [PubMed] [Google Scholar]
- 55.Kolsto, A. B., N. J. Tourasse, and O. A. Okstad. 2009. What sets Bacillus anthracis apart from other Bacillus species? Annu. Rev. Microbiol. 63:451-476. [DOI] [PubMed] [Google Scholar]
- 56.Kovács, M., A. Halfmann, I. Fedtke, M. Heintz, A. Peschel, W. Vollmer, R. Hakenbeck, and R. Brückner. 2006. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 188:5797-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krieg, A. 1981. The genus Bacillus: insect pathogens. Springer, Berlin, Germany.
- 58.Kristian, S. A., V. Datta, C. Weidenmaier, R. Kansal, I. Fedtke, A. Peschel, R. L. Gallo, and V. Nizet. 2005. d-Alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187:6719-6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kristian, S. A., X. Lauth, V. Nizet, F. Goetz, B. Neumeister, A. Peschel, and R. Landmann. 2003. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 188:414-423. [DOI] [PubMed] [Google Scholar]
- 60.Lee, Y. S., E. K. Yun, W. S. Jang, I. Kim, J. H. Lee, S. Y. Park, K. S. Ryu, S. J. Seo, C. H. Kim, and I. H. Lee. 2004. Purification, cDNA cloning and expression of an insect defensin from the great wax moth, Galleria mellonella. Insect Mol. Biol. 13:65-72. [DOI] [PubMed] [Google Scholar]
- 61.Lemaitre, B., and J. Hoffmann. 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25:697-743. [DOI] [PubMed] [Google Scholar]
- 62.Leoff, C., E. Saile, J. Rauvolfova, C. P. Quinn, A. R. Hoffmaster, W. Zhong, A. S. Mehta, G. J. Boons, R. W. Carlson, and E. L. Kannenberg. 2009. Secondary cell wall polysaccharides of Bacillus anthracis are antigens that contain specific epitopes which cross-react with three pathogenic Bacillus cereus strains that caused severe disease, and other epitopes common to all the Bacillus cereus strains tested. Glycobiology 19:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lereclus, D., H. Agaisse, M. Gominet, and J. Chaufaux. 1995. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo0A mutant. Bio/Technology 13:67-71. [DOI] [PubMed] [Google Scholar]
- 64.Lereclus, D., O. Arantes, J. Chaufaux, and M. Lecadet. 1989. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 51:211-217. [DOI] [PubMed] [Google Scholar]
- 65.MacNeil, D. J., K. M. Gewain, C. L. Ruby, G. Dezeny, P. H. Gibbons, and T. MacNeil. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61-68. [DOI] [PubMed] [Google Scholar]
- 66.Mak, P., D. Chmiel, and G. J. Gacek. 2001. Antibacterial peptides of the moth Galleria mellonella. Acta Biochim. Pol. 48:1191-1195. [PubMed] [Google Scholar]
- 67.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Negre, V., T. Hotelier, A. N. Volkoff, S. Gimenez, F. Cousserans, K. Mita, X. Sabau, J. Rocher, M. Lopez-Ferber, E. d'Alencon, P. Audant, C. Sabourault, V. Bidegainberry, F. Hilliou, and P. Fournier. 2006. SPODOBASE: an EST database for the lepidopteran crop pest Spodoptera. BMC Bioinformatics 7:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuhaus, F. C., M. P. Heaton, D. V. Debabov, and Q. Zhang. 1996. The dlt operon in the biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei. Microb. Drug Resist. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 71.Oizumi, Y., H. Hemmi, M. Minami, A. Asaoka, and M. Yamakawa. 2005. Isolation, gene expression and solution structure of a novel moricin analogue, antibacterial peptide from a lepidopteran insect, Spodoptera litura. Biochim. Biophys. Acta 1752:83-92. [DOI] [PubMed] [Google Scholar]
- 72.Palumbo, E., M. Deghorain, P. S. Cocconcelli, M. Kleerebezem, A. Geyer, T. Hartung, S. Morath, and P. Hols. 2006. d-Alanyl ester depletion of teichoic acids in Lactobacillus plantarum results in a major modification of lipoteichoic acid composition and cell wall perforations at the septum mediated by the Acm2 autolysin. J. Bacteriol. 188:3709-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 74.Perego, M., P. Glaser, A. Minutello, M. A. Strauch, K. Leopold, and W. Fischer. 1995. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598-15606. [DOI] [PubMed] [Google Scholar]
- 75.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 76.Peschel, A., and L. V. Collins. 2001. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides 22:1651-1659. [DOI] [PubMed] [Google Scholar]
- 77.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 78.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in D-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 79.Preston, A., B. O. Petersen, J. O. Duus, J. Kubler-Kielb, G. Ben-Menachem, J. Li, and E. Vinogradov. 2006. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J. Biol. Chem. 281:18135-18144. [DOI] [PubMed] [Google Scholar]
- 80.Ribeiro, C., and M. Brehelin. 2006. Insect haemocytes: what type of cell is that? J. Insect Physiol. 52:417-429. [DOI] [PubMed] [Google Scholar]
- 81.Sahl, H. G., R. W. Jack, and G. Bierbaum. 1995. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur. J. Biochem. 230:827-853. [DOI] [PubMed] [Google Scholar]
- 82.Salamitou, S., F. Ramisse, M. Brehelin, D. Bourguet, N. Gilois, M. Gominet, E. Hernandez, and D. Lereclus. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825-2832. [DOI] [PubMed] [Google Scholar]
- 83.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steen, A., E. Palumbo, M. Deghorain, P. S. Cocconcelli, J. Delcour, O. P. Kuipers, J. Kok, G. Buist, and P. Hols. 2005. Autolysis of Lactococcus lactis is increased upon d-alanine depletion of peptidoglycan and lipoteichoic acids. J. Bacteriol. 187:114-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stenfors Arnesen, L. P., A. Fagerlund, and P. E. Granum. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579-606. [DOI] [PubMed] [Google Scholar]
- 86.Stephens, J. M. 1952. Disease in codling moth larvae produced by severa strains of Bacillus cereus. Can. J. Zool. 30:30-40. [Google Scholar]
- 87.Vallet-Gely, I., B. Lemaitre, and F. Boccard. 2008. Bacterial strategies to overcome insect defences. Nat. Rev. Microbiol. 6:302-313. [DOI] [PubMed] [Google Scholar]
- 88.Volkoff, A. N., J. Rocher, E. d'Alencon, M. Bouton, I. Landais, E. Quesada-Moraga, A. Vey, P. Fournier, K. Mita, and G. Devauchelle. 2003. Characterization and transcriptional profiles of three Spodoptera frugiperda genes encoding cysteine-rich peptides. A new class of defensin-like genes from lepidopteran insects? Gene 319:43-53. [DOI] [PubMed] [Google Scholar]
- 89.Walter, J., D. M. Loach, M. Alqumber, C. Rockel, C. Hermann, M. Pfitzenmaier, and G. W. Tannock. 2007. D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100-23 results in impaired colonization of the mouse gastrointestinal tract. Environ. Microbiol. 9:1750-1760. [DOI] [PubMed] [Google Scholar]
- 90.Wecke, J., M. Perego, and W. Fischer. 1996. D-alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 2:123-129. [DOI] [PubMed] [Google Scholar]
- 91.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 92.Weidenmaier, C., and A. Peschel. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6:276-287. [DOI] [PubMed] [Google Scholar]
- 93.Weidenmaier, C., A. Peschel, V. A. Kempf, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2005. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 73:8033-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yeaman, M. R., and N. Y. Yount. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55:27-55. [DOI] [PubMed] [Google Scholar]
- 95.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 96.Zhang, M. Y., A. Lovgren, M. G. Low, and R. Landen. 1993. Characterization of an avirulent pleiotropic mutant of the insect pathogen Bacillus thuringiensis: reduced expression of flagellin and phospholipases. Infect. Immun. 61:4947-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]




