Abstract
The nanATEK-yhcH, yjhATS, and yjhBC operons in Escherichia coli are coregulated by environmental N-acetylneuraminic acid, the most prevalent sialic acid in nature. Here we show that YjhS (NanS) is a probable 9-O-acetyl N-acetylneuraminic acid esterase required for E. coli to grow on this alternative sialic acid, which is commonly found in mammalian host mucosal sites.
The coregulated nanATEK-yhcH, yjhATS, and yjhBC operons involved in sialic acid catabolism in Escherichia coli are thought to be induced by the most common sialic acid, N-acetylneuraminic acid (Neu5Ac), through reversible inactivation of the NanR repressor encoded by nanR mapping immediately upstream of nanA (15, 27, 28; http://vetmed.illinois.edu/path/sialobiology/). Sialic acids are a family of over 40 naturally occurring 9-carbon keto sugar acids found mainly in metazoans of the deuterostome (starfish to human) developmental lineage and in some, mostly pathogenic, bacteria, where sialic acids expressed at the microbial cell surface inhibit host innate immunity (27). By contrast, most bacterial commensals and pathogens catabolize sialic acids as sole carbon and nitrogen sources, indicating exploitation of the sialic acid-rich host mucosal environment by a wide range of species (2, 27, 28). Interestingly, in vivo experimental evidence further indicates that sialic acid catabolism functions directly (nutrition) or indirectly (surface decoration and cell signaling) in host-microbe commensal and pathogenic interactions in organisms such as E. coli, Haemophilus influenzae, Pasteurella multocida, Salmonella enterica serovar Typhi, Streptococcus pneumoniae, Vibrio vulnificus, and Vibrio cholerae (1, 3, 5, 6, 10, 14, 23, 24, 26, 29). The animal species used for these studies include rodent models and natural hosts such as cattle and turkeys. The structural diversity of sialic acids at the terminal positions on glycoconjugates (glycoproteins and glycolipids) of mucosal surfaces of these hosts requires sialidases, acetyl esterases, and probably other enzymes that convert alternative or at least minor sialic acids to the more digestible Neu5Ac form (8, 9). We have previously demonstrated that E. coli has an epicurean propensity for metabolizing alternative sialic acids (30, 31). In the current communication, we show that YjhS is required for growth of E. coli on 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2).
Because most sialic acids are bound to other sugars, including other sialic acids, as part of the oligosaccharide chains on glycoconjugates, either microbial or endogenous (host) sialidases (NanH, or N-acylneuraminate hydrolases) are needed to release free sugar, which is then transported by NanT in E. coli (15, 16, 26, 31). Once internalized, sialic acid is cleaved by an nanA-encoded aldolase or lyase to yield the 6-carbon hexosamine, N-acetylmannosamine (ManNAc), and pyruvate, with the latter entering the tricarboxylic acid cycle or gluconeogenesis. ManNAc is converted to its 6-phosphate derivative by a specific kinase encoded by nanK and epimerized by NanE to yield N-acetylglucosamine 6-phosphate, which is converted to fructose 6-phosphate by products of the nag operon (15, 17, 31, 32). The functions of the coregulated yjhS, yjhB, yjhC, and yhcH gene products are unknown but are not required for growth on Neu5Ac (15). However, YjhA (NanC) is an outer membrane porin required for diffusion of Neu5Ac in the absence of the major porins (7), while YjhT (NanM) is a mutarotase that catalyzes the conversion of the alpha sialic acid isomer to the more thermodynamically stable beta form (21). Neither nanC nor nanM is required for growth on Neu5Ac (15), suggesting that yjhS, yjhBC, and yhcH are involved in reactions that convert alternative sialic acids to Neu5Ac (22, 23). YhcH was crystallized and has been suggested to be an isomerase or epimerase involved in processing N-glycolylneuraminic acid (Neu5Gc) (25), but deletion of yhcH did not affect growth on this sialic acid as a sole carbon source (16).
Computer-assisted analysis indicated that YjhB is a permease similar to NanT (16) whereas YjhC is a likely oxidoreductase or dehydrogenase. Orthologs of yhcH, nanC, nanM, and yjhBC are found in most bacterial species with intact Neu5Ac utilization systems, while yjhS is confined to E. coli and shigellae, either as part of the chromosomes in these strains or integrated with phages or phage remnants. However, a significant match (E value = 0.0007) was found between YjhS and AxeA in Rhodopirellula baltica, where AxeA is an acetyl xylan esterase (11), suggesting YjhS might be a sialate esterase. We propose that YjhS should be designated NanS to indicate its direct participation in utilization of an alternative sialic acid.
Phenotypic characterization of nanS mutants.
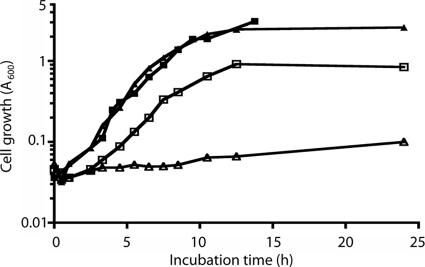
Neu5,9Ac2 (lot no. 160309-75) and Neu4,5Ac2 (lot no. 58-5#9) were purchased from Applied Biotechnology, Austria. Fluorometric analysis of Neu5,9Ac2 by detection of 1,2-diamino-4,5-methylenedioxybenzene (DMB)-labeled sialyl derivatives after separation by reverse-phase chromatography (22) indicated contamination with 7% Neu5,8Ac2 and 3% Neu5Ac, while Neu4,5Ac2 contained 34% unknown contaminants compared to the 10% reported by the manufacturer. The O-acetylated sialic acids were used as supplied for growth experiments by dissolving them to achieve a 0.1% final concentration in minimal salts medium followed by filter sterilization before immediate inoculation with the indicated bacterial strains. All chromatographic peak assignments were made on the basis of the specifications provided by the manufacturer and previous analyses of modified sialic acids as previously described (22). To determine whether nanS is required for E. coli K-12 growth on O-acetylated sialic acids, we constructed a deletion of nanS in strain BW30270 (E. coli Genetic Stock Collection) and tested its ability to utilize Neu5,9Ac2 as a sole carbon source. It and the other nanS mutants described below were constructed with forward (5′-AATACCATTCATGATAATAAAAAAGGAAAATGCCGCACTCATTCCGGGGATCCGTCGACC-3′) and reverse (5′-ATATGGCGTAATATCGGGCGTCATATGGTATTGTAGAACTGTAGGCTG GAGCTGCTTCG-3′) primers designed to amplify the kanamycin resistance cassette in pKD13 (pKD13 homologous regions are underlined) to yield in-frame deletions as previously described for construction of the Keio collection (4). All constructions were verified by diagnostic PCR analyses. Overnight cultures of the wild type and its isogenic mutant were prepared in M63 minimal medium with 0.4% glycerol as the carbon source. Unless indicated otherwise, cultures were grown at 37°C with vigorous aeration. Samples of each overnight culture were diluted 1:50 in M63 medium containing glycerol or 0.1% Neu5,9Ac2 as the carbon source, with growth monitored by changes in A600 values by the use of a Beckman DU-640 spectrophotometer. As shown in Fig. 1, the wild type and the mutant utilized glycerol at similar rates and to similar extents whereas the mutant did not appear to metabolize the acetylated sialic acid. We presume that the lower growth yield of the wild type on Neu5,9Ac2 relative to glycerol was due to the higher starting glycerol concentration. Similar growth rates of the mutant and the wild type on glycerol indicated that disruption of nanS has no effect on general cell physiology. By contrast, neither the mutant nor the wild type grew on Neu4,5Ac2 as the sole carbon source (data not shown), though when cultures containing this acetylated sialic acid were supplemented with glycerol, both the mutant and the wild type grew normally, indicating an inability to remove the acetyl group at carbon position 4. This conclusion follows from our previous demonstration that NanA-initiated metabolism of intracellular diacetylated sialic acids containing acetyl groups at carbon positions 7 to 9 requires deacetylation by the NeuA-Star (NeuA*) esterase encoded by neuA in E. coli K1 strains (22). Those previous results indicate that catabolism of exogenous diacetylated sialic acid by E. coli K-12 requires prior deacetylation, thus clearly implicating NanS as a probable esterase, because this strain lacks NeuA* (22). Additionally, we previously identified the presence of nonspecific esterase activity in E. coli (22) that is evidently insufficient for the growth of the wild type observed on Neu5,9Ac2, because the mutant A600 value barely doubled during 24 h of incubation (Fig. 1).
FIG. 1.
nanS is required for E. coli to grow on Neu5,9Ac2 as the sole carbon source. Overnight cultures of BW30270 and its isogenic nanS mutant derivative grown on glycerol as the carbon source were diluted 1:50 into fresh medium containing either glycerol or Neu5,9Ac2 as follows: BW30270 with glycerol (closed squares); nanS mutant with glycerol (closed triangles); BW30270 with Neu5,9Ac2 (open squares); nanS mutant with Neu5,9Ac2 (open triangles).
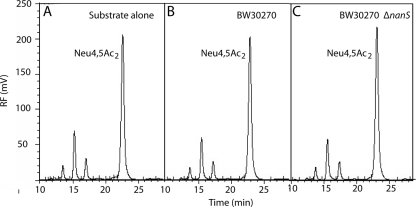
To demonstrate that the inability to metabolize Neu4,5Ac2 as a carbon source was caused by a failure to deacetylate this sialic acid, we analyzed the spent culture supernatants of strains cocultured with glycerol and Neu4,5Ac2 by chromatography of DMB derivatives. As shown in Fig. 2, the peak representing Neu4,5Ac2 plus contaminants in medium alone was indistinguishable from that seen with the spent media of either the wild type or the mutant, indicating that Neu4,5Ac2 is neither transported nor deacetylated by E. coli K-12. We conclude that nanS is required for growth on Neu5,9Ac2, with the growth process most likely involving deacetylation prior to transport by NanT, and that failure to utilize Neu4,5Ac2 is caused by an inability to remove the carbon-4 acetyl group of this sialic acid. The idea of a requirement for NanS as a probable exocyclic sialyl deacetylase is supported by the ability of a pGEM-T Easy clone (Promega, Madison, WI) harboring a full-length nanS PCR amplicon to restore growth of the nanS mutant on Neu5,9Ac2 as a sole carbon source.
FIG. 2.
Chemical analysis of Neu4,5Ac2 in spent culture medium. (A) Neu4,5Ac2 at a 0.1% final concentration was incubated in minimal glycerol medium without cells for 7 h and then subjected to DMB analysis as described in the text. (B) Neu4,5Ac2 in the spent culture supernatant of wild type was grown for 7 h in the presence of glycerol. (C) Neu4,5Ac2 in the spent culture supernatant of the nanS mutant was grown in glycerol medium for 7 h. Note that the same profiles were obtained after comparable chemical analyses of 24-h cultures, indicating the stability of Neu4,5Ac2 and its resistance to metabolism by E. coli K-12. RF, relative fluorescence.
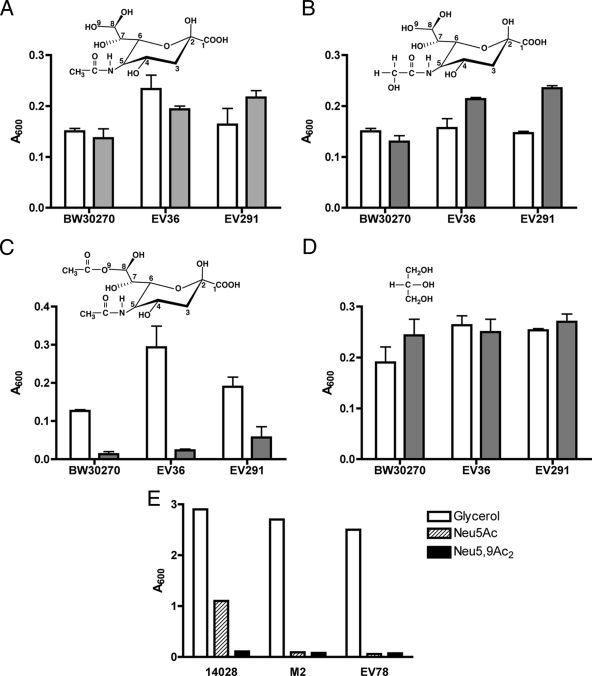
To demonstrate that the phenotype of the nanS mutant was not strain dependent and that the general Neu5Ac catabolic pathway was not affected by disruption of nanS, we determined the growth of the BW30270-derived nanS mutant compared to that of two independent nanS deletion mutants constructed from the E. coli K-12/K1 EV36 hybrid strain (31) and strain EV291, derived from clinical isolate RS218 (12); both strains produce NeuA* (22). As shown in Fig. 3A and B, all three wild-type strains and mutant derivatives grew similarly in the presence of the common sialic acids Neu5Ac and N-glycolylneuraminic acid (Neu5Gc), demonstrating that the central sialocatabolic system encoded by nanATEK genes is not affected by loss of NanS. As expected from the results shown in Fig. 1, growth on glycerol was unaffected by the nanS mutations (Fig. 3D). By contrast, the mutants did not grow or grew poorly when Neu5,9Ac2 was the carbon source (Fig. 3C), thus confirming the results represented in Fig. 1. We conclude that the growth defect of nanS mutants on Neu5,9Ac2 results specifically from loss of NanS.
FIG. 3.
Growth of the wild type and the yjhS and nanA mutants on different sialic acids. In panels A to D, the indicated wild-type strains (open boxes) and their isogenic yjhS deletion derivatives (shaded boxes) were diluted 50-fold from stationary-phase cells grown in M63 medium plus glycerol (0.4%) into 0.1 ml of M63 medium containing 0.1% of the indicated sialic acids or glycerol in 96-well plates. (A) Growth on Neu5Ac. (B) Growth on Neu5Gc. (C) Growth on Neu5,9Ac2. (D) Growth on glycerol. The plate was incubated without shaking at 37°C for 16 h, and A600 values were recorded with a microplate reader. Data represent the means of three independent experiments ± standard deviations. (E) S. enterica serovar Typhimurium wild-type strain 14028, nanA strain LT2 derivative M2, and E. coli nanA mutant EV78 were diluted 60-fold into 0.5 ml of M63 medium containing 0.4% glycerol, 0.1% Neu5Ac, or 0.1% Neu5,9Ac2. Cultures were grown at 37°C with vigorous aeration; the A600 values were recorded after 7 h.
Because S. enterica serovar Typhimurium has an intact nanATEK system and was previously shown to grow on Neu5Ac (13) but lacks an ortholog of NanS, we tested whether the 14028 wild-type strain purchased from the ATCC could utilize Neu5,9Ac2 and found that it could not (Fig. 1E), indicating that without NanS, the presence of nonspecific esterase is insufficient to support growth of this strain on acetylated sialic acid as a sole carbon source. Failure of the S. enterica serovar Typhimurium strain LT2-derived nanA M2 mutant to grow on Neu5Ac and Neu5,9Ac2 indicates that, as discussed above, utilization of acetylated sialic acid requires NanA and prior deacetylation of Neu5,9Ac2 (Fig. 1E). This conclusion was supported by a similar result obtained with the E. coli K-12 nanA EV78 mutant (31) (Fig. 1E). These results further suggest that NanS is a probable sialyl esterase that catalyzes the removal of the 9-O-acetyl group, most likely in the periplasm, and that NanT transports the resulting Neu5Ac for subsequent dissimilation by NanATEK. We have not yet determined whether a plasmid harboring a wild-type copy of E. coli nanS is able to confer the ability to grow on Neu5,9Ac2 to S. enterica.
Chemical analysis of Neu5,9Ac2 in spent culture media.
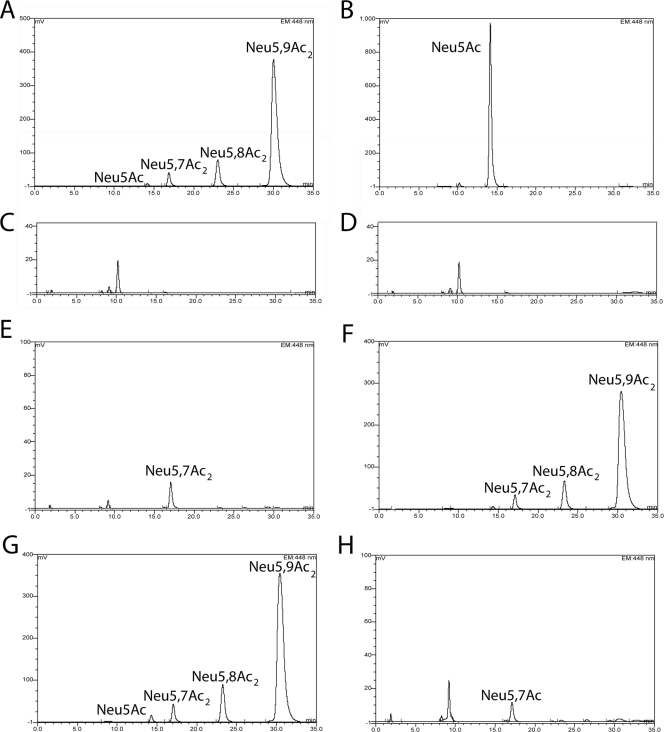
To directly demonstrate utilization of Neu5,9Ac2 under in vivo growth conditions, we carried out a chromatographic analysis of DMB-labeled spent culture medium of BW30270 and its isogenic nanS deletion derivative grown in M63 minimal medium containing glycerol (0.4%) and either Neu5Ac or Neu5,9Ac2 at a 0.1% final concentration. Figure 4A and B show the expected profiles for Neu5,9Ac2 and Neu5Ac, respectively, after incubation without cells in M63 glycerol medium for 7 h at 37°C with vigorous aeration. DMB-labeled peaks in media alone or in spent culture supernatants represent the relative amounts of sialic acids in 3 μl of medium. Medium alone yielded a flat signal, indicating the absence of any DMB-modifiable keto acids. Although the percentages of Neu5,8Ac2 and Neu5Ac contaminants did not change substantially after 7 h (Fig. 4A) or 17 h (Fig. 4G), detection of Neu5,7Ac2 that was not present in the original sample lot indicates some migration of the 9-O-acetyl group to the carbon-7 and carbon-8 positions under the conditions of temperature and pH used in the experiments. As expected, the wild-type strain depleted Neu5Ac and Neu5,9Ac2 (Fig. 4C and E, respectively) but not Neu5,7Ac2, while the mutant depleted Neu5Ac (Fig. 4D) but not the majority of the O-acetylated forms (Fig. 4F). The results shown in Fig. 4E indicate that Neu5,8Ac2 is a NanS substrate. Additionally, we observed poor growth of the mutant exposed to Neu5,9Ac2 and glycerol at 7 h compared to the mutant grown on glycerol (Fig. 1), suggesting inhibition by an unknown mechanism that did not seem to involve transport of Neu5,9Ac2, because the culture medium after 7 h of incubation contained approximately the input amount of sialic acids (Fig. 4F). Further research is needed to determine whether NanT transports acetylated sialic acids. However, when the mutant was incubated with Neu5,9Ac2 and glycerol for 17 h, we observed a profile similar to that of the wild type (compare Fig. 4E with H), suggesting that nonspecific esterase(s) eventually removes the inhibitor by converting acetylated sialic acids to Neu5Ac. Whether relief is caused by periplasmic esterase(s) or intracellular enzymes will require further analysis. If nonspecific esterase(s) is involved in catabolism of Neu5,9Ac2, it is evidently insufficient to allow growth on diacetylated sialic acid as a sole carbon source (Fig. 1 and 3).
FIG. 4.
Utilization of Neu5Ac or Neu5,9Ac2 by BW30270 and its yjhS deletion derivative growing simultaneously in the presence of glycerol. BW30270 or its yjhS derivative was diluted 60-fold into 0.5 ml of M63 medium plus glycerol containing either 0.1% Neu5Ac or Neu5,9Ac2 and incubated at 37°C with vigorous aeration for 7 h unless indicated otherwise. (A) Neu5,9Ac2 incubated without cells. (B) Neu5Ac incubated without cells. (C) Wild type grown with Neu5Ac. (D) Mutant grown with Neu5Ac. Note that the minor peak eluting at about 10 min as shown in these panels and in the succeeding panel is likely to represent a trace amount of 2-deoxy-d-manno-octulosonic acid derived from sloughed lipopolysaccharide during the incubation that was not removed by the centrifugation step used to produce the spent culture media for the analyses (22). (E) Wild type grown with Neu5,9Ac2. (F) Mutant grown with Neu5,9Ac2. (G) Neu5,9Ac2 incubated without cells for 17 h. (H) Mutant grown with Neu5,9Ac2 for 17 h.
Conclusions.
Our results indicate that NanS is a probable sialate esterase in E. coli and that this protein is absent in the close relative S. enterica. It may be that enhanced utilization of alternative sialic acids could account for the success of E. coli as the predominant intestinal facultative anaerobe in the lower intestinal environment. If this tentative conclusion is true, it might be possible to determine the niche preferences of the majority of the intestinal microbiome and that of microorganisms in other mucosal sites by analysis of their relative sialometabolic activities. This idea could be supported by quantitative measurements of mucosal sialic acids; in fact, two recent studies performed with human tissues demonstrated an increasing gradient of sialic acids from the ileum to the rectum together with changes in the relative amounts of 10 different sialic acids (19, 20). These studies revealed that Neu5,9Ac2 was the second or third most prevalent sialic acid after Neu5Ac in the human intestine. Similar region specificity was observed in the mouse intestine, along with wide variations in sialic acid amounts and their various alternate chemical forms in the liver and central nervous system compared to the intestinal tract (18). Those authors also found substantial amounts of intestinal Neu4,5Ac2, which might not have been observed in the human studies due to methodological differences (18). Our results indicate that if Neu4,5Ac2 is present in substantial amounts in any tissue, an esterase other than NanS must exist to convert Neu4,5Ac2 to Neu5Ac. However, in addition to the potentially large amounts of Neu4,5Ac2 reported in studies of the mouse intestine, the level of Neu5,9Ac2 in this tissue was reportedly low compared to levels in the human intestine (18). If these differences are real instead of just methodological artifacts, our current results indicate that the mouse would not be a relevant model for investigating enteric colonization of the human intestine in terms of sialometabolism. Despite these potential discrepancies, the metabolism of host-derived sialic acids is likely to involve a complex competitive as well as synergistic interplay between different bacterial species and the host, suggesting that a simplified model of mucinolysis is urgently needed for progress in this research area. Because E. coli lacks nanH, our results further imply that this species and other sialidase-negative bacteria efficiently scavenge free sialic acids released by host sialidases or sialidase-positive bacteria. We conclude that further analysis of sialometabolism in E. coli and other bacteria should lead to a more complete understanding of host-microbe colonization and disease potential and might suggest new therapeutic targets, as we have already posited (30). Future studies will focus on determining the relative contributions of different sialic acids to colonization and diseases caused by mucosal pathogens and on understanding the functions of yhcH and yjhBC.
Acknowledgments
The research in this communication was supported by NIH grant AI042015.
Footnotes
Published ahead of print on 11 September 2009.
REFERENCES
- 1.Almagro-Moreno, S., and E. F. Boyd. 29 June 2009, posting date. Sialic acid catabolism confers a competitive advantage to pathogenic Vibrio cholerae in the mouse intestine. Infect. Immun. doi: 10.1128/IAI.00279-09. [DOI] [PMC free article] [PubMed]
- 2.Almagro-Moreno, S., and E. F. Boyd. 2009. Insights into the evolution of sialic acid catabolism among bacteria. BMC Evol. Biol. 9:118-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alteri, C. J., S. N. Smith, and H. L. Mobley. 29 May 2009, posting date. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed]
- 4.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 21 February 2006, posting date. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed]
- 5.Becker, D., M. Selbach, C. Rollenhagen, M. Ballmaier, T. F. Meyer, M. Mann, and D. Bumann. 2006. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature 440:303-307. [DOI] [PubMed] [Google Scholar]
- 6.Chang, D. E., D. J. Smalley, D. L. Tucker, M. P. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 101:7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condemine, G., C. Berrier, J. Plumbridge, and A. Ghazi. 2005. Function and expression of an N-acetylneuraminic acid-inducible outer membrane channel in Escherichia coli. J. Bacteriol. 187:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corfield, A. P., S. A. Wagner, J. R. Clamp, M. S. Kriaris, and L. C. Hoskins. 1992. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, acylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 60:3971-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corfield, A. P., S. A. Wagner, L. J. D. O'Donnell, P. Durdey, R. A. Mountford, and J. R. Clamp. 1993. The roles of enteric bacterial sialidase, sialate O-acetyl esterase and glycosulfatase in the degradation of human colonic mucin. Glycoconj. J. 10:72-81. [DOI] [PubMed] [Google Scholar]
- 10.Fabich, A. J., S. A. Jones, F. Z. Chowdhury, A. Cernosek, A. Anderson, D. Smalley, J. W. McHargue, G. A. Hightowere, J. T. Smith, S. M. Autieri, M. P. Leatham, J. J. Lins, R. L. Allen, D. C. Laux, P. S. Cohen, and T. Conway. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glöckner, F. O., M. Kube, M. Bauer, H. Teeling, T. Lombardot, W. Ludwig, D. Gade, A. Beck, K. Borzym, K. Heitmann, R. Rabus, H. Schlesner, R. Amann, and R. Reinhardt. 2003. Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc. Natl. Acad. Sci. USA 100:8298-8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez, M. D., C. A. Lichtensteiger, and E. R. Vimr. 2001. Adaptation of signature-tagged mutagenesis to Escherichia coli K1 and the infant-rat model of invasive disease. FEMS Microbiol. Lett. 198:125-128. [DOI] [PubMed] [Google Scholar]
- 13.Hoyer, L. L., A. C. Hamilton, S. M. Steenbergen, and E. R. Vimr. 1992. Cloning, sequencing, and distribution of the Salmonella typhimurium LT2 sialidase gene, nanH, provide evidence for interspecies gene transfer. Mol. Microbiol. 6:873-884. [DOI] [PubMed] [Google Scholar]
- 14.Jeong, H. G., M. H. Oh, B. S. Kim, M. Y. Lee, H. J. Han, and S. H. Choi. 2009. The capability of catabolic utilization of N-acetylneuraminic acid, a sialic acid, is essential for Vibrio vulnificus pathogenesis. Infect. Immun. 77:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalivoda, K. A., S. M. Steenbergen, E. R. Vimr, and J. Plumbridge. 2003. Regulation of sialic acid catabolism by the DNA binding protein, NanR, in Escherichia coli. J. Bacteriol. 185:4806-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez, J., S. Steenbergen, and E. Vimr. 1995. Derived structure of the putative sialic acid transporter from Escherichia coli predicts a novel sugar permease domain. J. Bacteriol. 1 77:6005-6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringenberg, M. A., S. M. Steenbergen, and E. R. Vimr. 2003. The first committed step in the biosynthesis of sialic acids by Escherichia coli K1 does not involve a phosphorylated N-acetylmannosamine intermediate. Mol. Microbiol. 50:961-975. [DOI] [PubMed] [Google Scholar]
- 18.Rinninger, A., C. Richer, A. Pons, G. Kohla, R. Schauer, H.-C. Bauer, J.-P. Zanetta, and R. Vlasak. 2006. Localisation and distribution of O-acetylated N-acetylneuraminic acids, the endogenous substrates of the hemagglutinin-esterases of murine coronaviruses, in mouse tissue. Glycoconj. J. 23:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbe, C., C. Capon, E. Maes, M. Rousset, A. Zweibaum, J.-P. Zanetta, and J.-C. Michalski. 2003. Evidence of regio-specific glycosylation in human intestinal mucins: presence of an acidic gradient along the intestinal tract. J. Biol. Chem. 278:46337-46348. [DOI] [PubMed] [Google Scholar]
- 20.Robbe-Masselot, C., E. Maes, M. Rousset, J.-C. Michalsjki, and C. Capon. 2009. Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj. J. 26:397-413. [DOI] [PubMed] [Google Scholar]
- 21.Severi, E., A. Müller, J. R. Potts, A. Leech, D. Williamson, K. S. Wilson, and G. H. Thomas. 2008. Sialic acid mutarotation is catalyzed by the Escherichia coli beta-propeller protein YjhT. J. Biol. Chem. 283:4841-4849. [DOI] [PubMed] [Google Scholar]
- 22.Steenbergen, S. M., Y.-C. Lee, W. F. Vann, J. Vionet, L. F. Wright, and E. R. Vimr. 2006. Separate pathways for O-acetylation of polymeric and monomeric sialic acids in Escherichia coli K1. J. Bacteriol. 188:6195-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenbergen, S. M., C. A. Lichtensteiger, R. Caughlan, J. Garfinkle, T. E. Fuller, and E. R. Vimr. 2005. Sialic acid metabolism and systemic pasteurellosis. Infect. Immun. 73:1284-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatum, F. M., L. B. Tabatabai, and R. E. Briggs. 2009. Sialic acid uptake is necessary for virulence of Pasteurella multocida in turkeys. Microb. Pathog. 46:337-344. [DOI] [PubMed] [Google Scholar]
- 25.Teplyakov, A., G. Obmolova, J. Toedt, M. Y. Galperin, and G. L. Gilliland. 2005. Crystal structure of the bacterial YhcH protein indicates a role in sialic acid catabolism. J. Bacteriol. 187:5520-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trappetti, C., A. Kadioglu, M. Carter, J. Hayre, F. Iannelli, G. Pozzi, P. W. Andrew, and M. R. Oggioni. 2009. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J. Infect. Dis. 199:1497-1505. [DOI] [PubMed] [Google Scholar]
- 27.Vimr, E. R., K. A. Kalivoda, E. L. Deszo, and S. M. Steenbergen. 2004. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68:132-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vimr, E., and C. A. Lichtensteiger. 2002. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10:254-257. [DOI] [PubMed] [Google Scholar]
- 29.Vimr, E., C. Lichtensteiger, and S. Steenbergen. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 36:1113-1123. [DOI] [PubMed] [Google Scholar]
- 30.Vimr, E. R., and S. M. Steenbergen. 2006. Targeting microbial sialic acid metabolism for new drug development, p. 125-150. In C. A. Bewley (ed.), Protein-carbohydrate interactions in infectious disease. Royal Society of Chemistry, London, United Kingdom.
- 31.Vimr, E. R., and F. A. Troy. 1985. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J. Bacteriol. 164:845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vimr, E. R., and F. A. Troy. 1985. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J. Bacteriol. 164:854-860. [DOI] [PMC free article] [PubMed] [Google Scholar]