Abstract
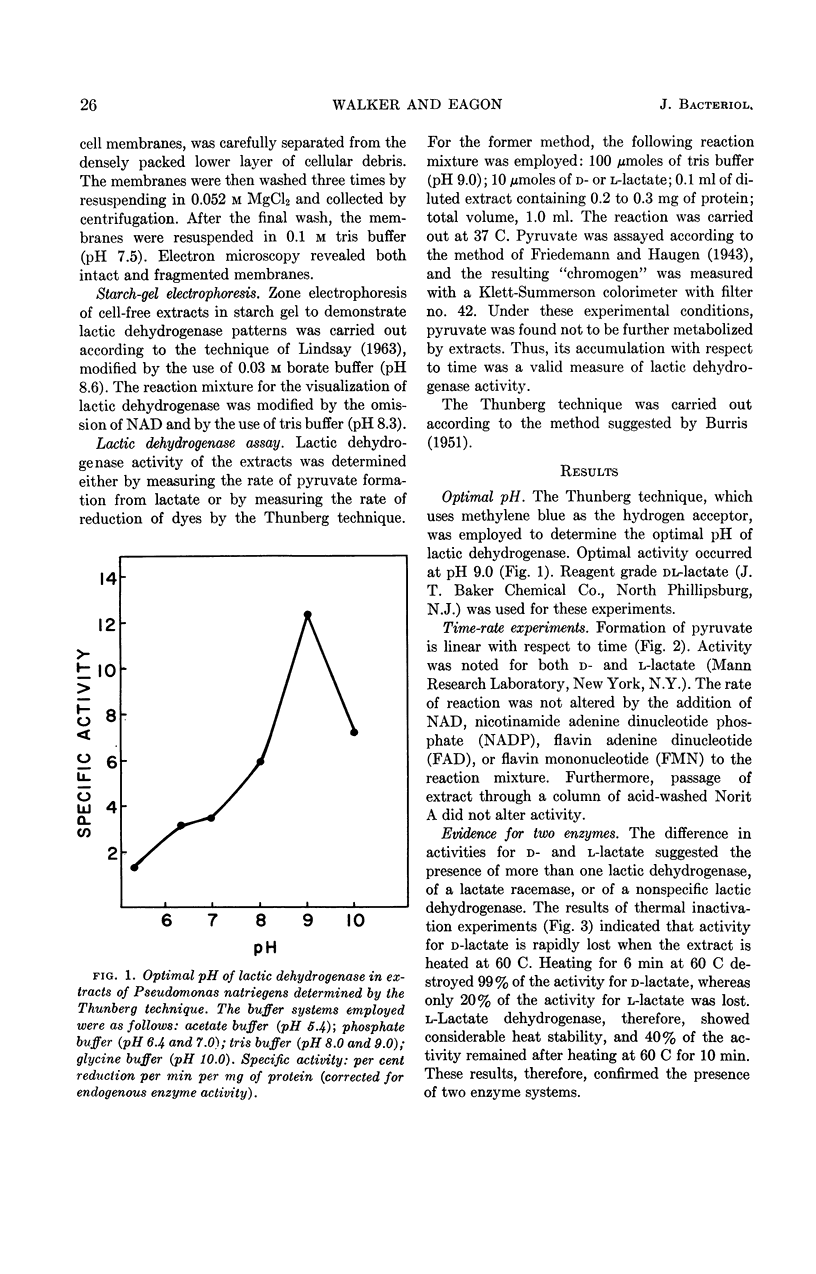
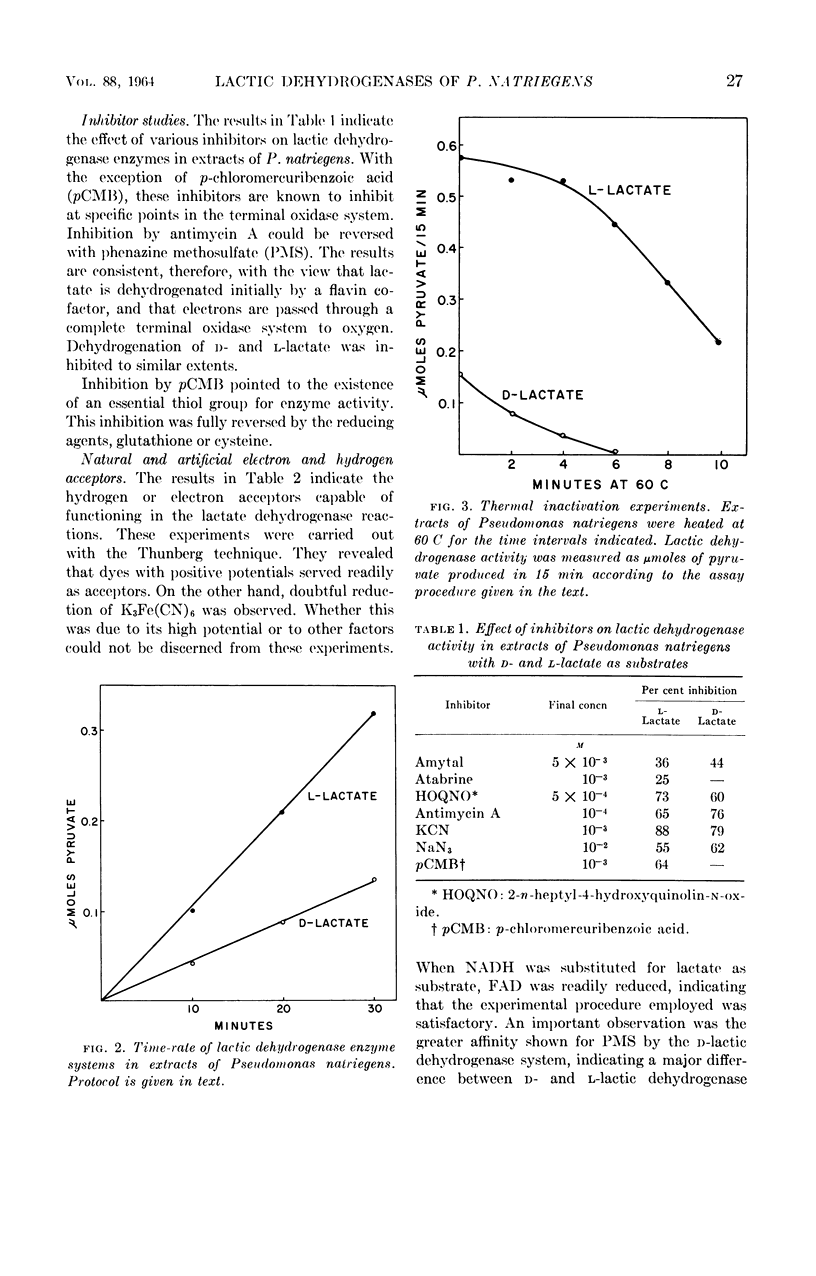
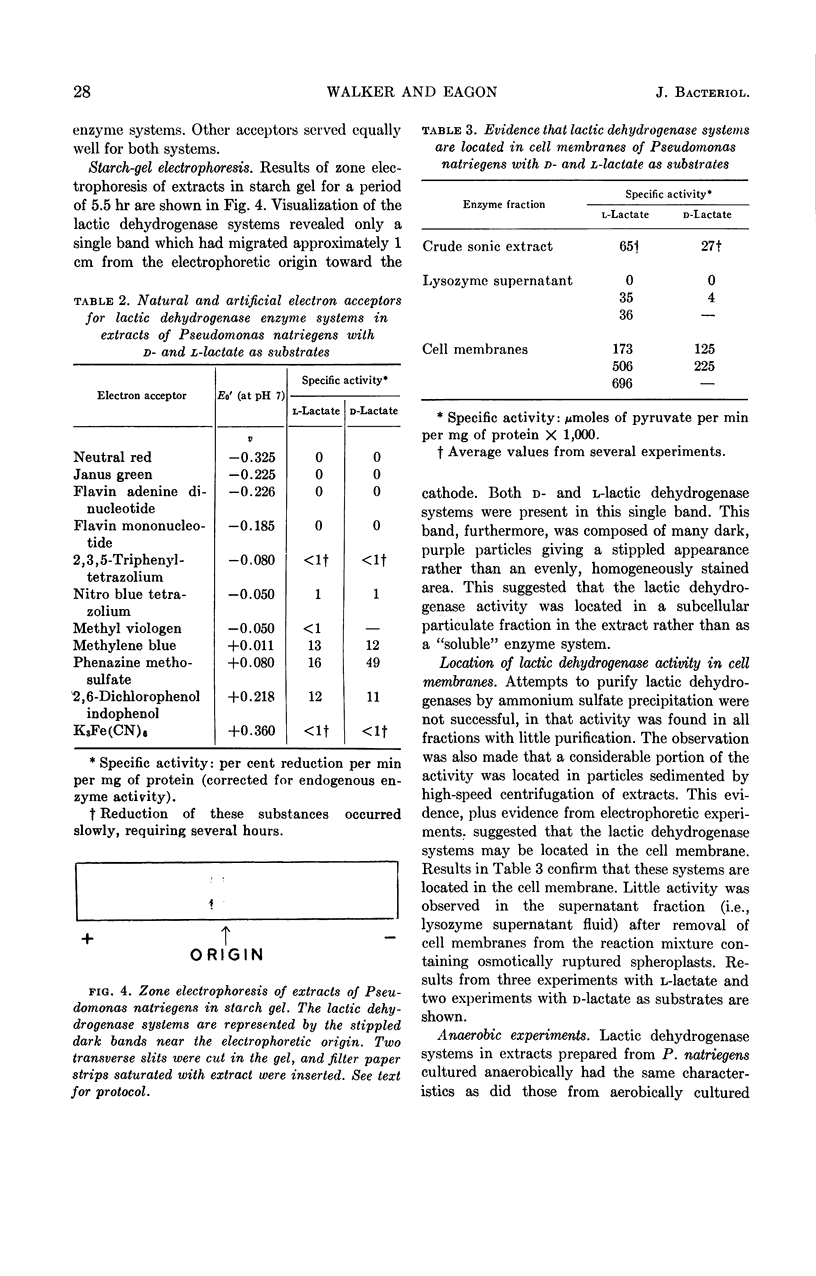
Walker, Hazel (University of Georgia, Athens), and R. G. Eagon. Lactic dehydrogenases of Pseudomonas natriegens. J. Bacteriol. 88:25–30. 1964.—Lactic dehydrogenases specific for d- and l-lactate were demonstrated in Pseudomonas natriegens. The l-lactic dehydrogenase showed considerable heat stability, and 40% of the activity remained in extracts after heating at 60 C for 10 min. An essential thiol group for enzyme activity was noted. The results of these experiments were consistent with the view that lactate was dehydrogenated initially by a flavin cofactor and that electrons were transported through a complete terminal oxidase system to oxygen. The intracellular site of these lactic dehydrogenases was shown to be the cell membrane. It was suggested that the main physiological role of these lactic dehydrogenases is that of lactate utilization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE LEY J., SCHEL J. Studies on the metabolism of Acetobacter peroxydans. II. The enzymic mechanism of lactate metabolism. Biochim Biophys Acta. 1959 Sep;35:154–165. doi: 10.1016/0006-3002(59)90344-0. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., WANG C. H. Dissimilation of glucose and gluconic acid by Pseudomonas natriegens. J Bacteriol. 1962 Apr;83:879–886. doi: 10.1128/jb.83.4.879-886.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHEL H. J., REM L. T. Respiratory enzyme studies in Tetrahymena pyriformis. V. Some properties of an L-lactic oxidase. J Biol Chem. 1962 Mar;237:940–945. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., FOWLER L. R., GRIFFITHS D. E. Studies on the electron transfer system. XLII. Reconstitution of the electron transfer system. J Biol Chem. 1962 Aug;237:2661–2669. [PubMed] [Google Scholar]
- HAUGAARD N. D- and L-lactic acid oxidases of Escherichia coli. Biochim Biophys Acta. 1959 Jan;31(1):66–72. doi: 10.1016/0006-3002(59)90439-1. [DOI] [PubMed] [Google Scholar]
- LINDSAY D. T. Isozymic patterns and properties of lactate dehydrogenase from developing tissues of the chicken. J Exp Zool. 1963 Feb;152:75–89. doi: 10.1002/jez.1401520108. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORTON R. K. Structure and properties of the flavohaemoprotein, cytochrome b2 (L-lactate dehydrogenase of baker's yeast) and of haemoprotein, flavoprotein and apoprotein derivatives. Nature. 1961 Nov 25;192:727–731. doi: 10.1038/192727a0. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P. Various forms of D- and L-lactate dehydrogenases in yeast. Ann N Y Acad Sci. 1961 Nov 2;94:774–779. doi: 10.1111/j.1749-6632.1961.tb35572.x. [DOI] [PubMed] [Google Scholar]
- PACKER L. Respiratory carriers involved in the oxidation of hydrogen and lactate in a facultative autotroph. Arch Biochem Biophys. 1958 Nov;78(1):54–65. doi: 10.1016/0003-9861(58)90314-x. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J., EAGON R. G., WILLIAMS A. K. Some observations on the physiology of Pseudomonas natriegens nov. spec. Antonie Van Leeuwenhoek. 1961;27:121–128. doi: 10.1007/BF02538432. [DOI] [PubMed] [Google Scholar]




