Abstract
Thioredoxin-like proteins of the TlpA/ResE/CcmG subfamily are known to face the periplasm in gram-negative bacteria. Using the tlpA gene of Bradyrhizobium japonicum as a query, we identified a locus (NGO1923) in Neisseria gonorrhoeae that encodes a thioredoxin-like protein (NG_TlpA). Bioinformatics analysis indicated that the predicted NG_TlpA protein contained a cleavable signal peptide at the N terminus, and secondary structure analysis identified a thioredoxin fold with a helical insertion (∼25 residues), similar to that found in B. japonicum TlpA but absent in cytoplasmic thioredoxins. Biochemical characterization of a recombinant form of NG_TlpA revealed a standard redox potential (E0′) of −206 mV. This property and the observation that the oxidized form of the protein exhibited greater thermal stability than the reduced species indicated that NG_TlpA is a reducing thioredoxin and not an oxidizing thiol-disulfide oxidoreductase like DsbA. The thioredoxin activity of NG_TlpA was confirmed in an insulin disulfide reduction assay. A tlpA mutant of N. gonorrhoeae strain 1291 was found to be highly sensitive to oxidative killing by paraquat and hydrogen peroxide, indicating an antioxidant role for the NG_TlpA in this bacterium. The tlpA mutant also exhibited reduced intracellular survival in human primary cervical epithelial cells.
Thioredoxins are small proteins with a conserved secondary structure and a CXXC active site motif (11, 17). Using NADPH-dependent thioredoxin reductase as an electron donor, thioredoxins can catalyze reduction of cysteine disulfides in proteins. Thus, in the cytoplasm thioredoxins have a role in the catalytic cycle of redox enzymes, such as ribonucleotide reductase, and they also have a role in preventing protein disulfide bridge formation in the cytoplasm (27). The extracytoplasmic compartments of the gram-negative bacterial cell, including the periplasm, represent a more oxidizing environment than the cytoplasm. Over the last decade a sophisticated system for disulfide bond formation and protein folding in the periplasm of gram-negative gammaproteobacteria has been described using Escherichia coli as a model: the Dsb system involves thioredoxin-related proteins that are involved in oxidation-reduction of thiols/disulfides. Oxidation of protein thiols is catalyzed by DsbA, and recycling of this protein involves its reoxidation by membrane-bound DsbB, which is connected to the respiratory chain (9, 13). Reductive reactions are also an integral part of protein folding processes in the periplasm, and in this case the protein disulfide isomerase DsbC catalyzes the removal of nonnative disulfide bonds from proteins and enables them to refold in the correct conformation. Reducing power for DsbC is provided by the transmembrane protein DsbD, which uses cytoplasmic thioredoxin as an electron donor (34).
In addition to its role as an electron donor for the action of DsbC, DsbD can also provide reducing power for the membrane-bound, thioredoxin-like protein CcmG (also known as DsbE), which faces the periplasm and is required for the reduction of cysteine residues in apo-cytochromes c, which form thioether linkages to heme groups in a reaction catalyzed by CcmF and CcmH (15, 26, 34). Thus, a concept is emerging in which DsbD plays as role as a “redox hub” for the translocation of reducing power to periplasmic “thioredoxin-like” proteins. In addition to CcmG, in gram-negative bacteria a number of thioredoxin-like proteins have been identified that are periplasmic or are anchored to the cytoplasmic membrane but face the periplasm. An example is the thioredoxin-like protein A (TlpA) of the nitrogen-fixing soil bacterium Bradyrhizobium japonicum. Mutational analysis indicates that this protein is essential for the biogenesis and maturation of cytochrome oxidase of the aa3 type (18). Although bioinformatics analysis of bacterial genomes identifies proteins related to CcmG and TlpA (the TlpA/ResE/CcmG family), the function of these thioredoxin-like proteins has not been fully explored.
Neisseria gonorrhoeae is a gram-negative betaproteobacterium that is predicted from bioinformatics analysis to possess a Dsb system for protein disulfide bond formation. Genes encoding homologues to DsbB, DsbC, and DsbD can be identified within the genome, and two DsbA proteins are predicted by genomic analysis (31). The presence of a CcmG homologue is also predicted by the presence of several species of c-type cytochrome (36). N. gonorrhoeae is a mucosal pathogen that is adapted to its human host. One of these interactions in females is the invasion and survival of this bacterium within cervical epithelial cells (5). Indeed, analysis of mutants of N. gonorrhoeae has demonstrated that defense against oxidative and nitrosative stress is important for its intracellular survival (29). In this study, we characterized a novel periplasmic thioredoxin-like protein (TlpA) of N. gonorrhoeae. This reveals a new role for a periplasmic thioredoxin-like protein in defense against oxidative stress and for survival of N. gonorrhoeae within cervical epithelial cells.
MATERIALS AND METHODS
Materials.
Except when stated otherwise, all chemicals were purchased from Sigma (Castle Hill, NSW, Australia). Restriction enzymes were from New England BioLabs (Arundel, QLD, Australia).
Bacterial strains and culture conditions.
N. gonorrhoeae strain 1291 (supplied by Michael Apicella, University of Iowa) was grown on brain heart infusion agar (BHI; Acumedia) supplemented with 10% Levithal's base and 1% IsoVitaleX (Difco) at 37°C in 5% CO2. Growth in liquid culture was conducted in supplemented BHI broth (Oxoid) at 37°C under aerobic conditions at 170 rpm. Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C; for growth on solid medium LB broth was supplemented with 1.5% agar. Unless stated otherwise, ampicillin, kanamycin, and tetracycline were used at final concentrations of 100 μg·ml−1, 50 μg·ml−1, and 5 μg·ml−1, respectively.
Cloning of tlpA.
The tlpA coding sequence was amplified by PCR from N. gonorrhoeae strain 1291, using the primers TlpAFN (5′-TAACCATGGACGAACTGGCCGGATGGAAA-3′) and TlpAR_IN (5′-TAACCATGGACGAACTGGCCGGATGGAAA-3′). These primers were designed to omit the region encoding the N-terminal signal sequence to the periplasm and contained restrictions sites for NcoI (underlined in the forward primer) or PstI (underlined in the reverse primer). The resulting PCR product was digested with NcoI and PstI restriction enzymes and cloned into the NcoI and PstI sites of the pPROEX HTa expression vector (Invitrogen), which contains an N-terminal His6 tag used for purification.
Expression and purification of TlpA.
For expression of NG_TlpA, E. coli BL21 cells harboring pPROEX::tlpA were grown in LB medium supplemented with ampicillin. When cells reached an A600 of 0.3, the temperature was lowered from 37°C to 30°C, 0.6 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added, and the induction phase was conducted for 6 h. The cells were harvested by centrifugation at 6,000 × g for 15 min at 4°C. Pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mg ml−1 lysosyme) and incubated for 15 min on ice before sonication. Lysed cells were then centrifuged at 18,000 × g for 50 min at 4°C. The cleared lysate was filtered and loaded onto a Ni-nitrilotriacetic acid column (HisTrap HP 5 ml; GE Healthcare) equilibrated with buffer A (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 20 mM imidazole). After an exhaustive washing with buffer A, the retained proteins were eluted with a gradient going from 0% to 100% of buffer B (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 500 mM imidazole) in 3 column volumes. The fractions corresponding to purified NG_TlpA were pooled, and the preparation was dialyzed overnight against 50 mM HEPES (pH 7.0)-100 mM NaCl. NG_TlpA was more than 99% pure as determined by Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The concentration of purified NG_TlpA (molecular weight of 18,097.5 g·mol−1) was determined by measuring A280 values and using a calculated molar absorption coefficient (ɛ280 nm = 38,180 M−1·cm−1), which was calculated from the amino acid composition of the recombinant NG_TlpA using the method of Gill and von Hippel (7).
Thermal unfolding of TlpA.
The temperature-induced unfolding curves of the reduced and oxidized forms of NG_TlpA were monitored by the change in the UV circular dichroism signal. The complete oxidation of NG_TlpA was ensured by incubation of the purified protein with copper phenanthroline (1.7 mM) for 1 h at 4°C. After removal of the oxidant using a PD10 column (GE Healthcare) equilibrated with 100 mM phosphoric acid-NaOH and 1 mM EDTA, pH 7.0, half of the preparation was used to prepare the reduced NG_TlpA sample by addition of 0.75 mM dithiothreitol (DTT).
UV circular dichroism spectra (from 250 to 190 nm) of 10 μM protein solutions were recorded at 25 and 95°C on a Jasco J-715 spectropolarimeter. The wavelength with the largest change in signal was determined from the differential spectrum at 25 and 95°C. Thermal unfolding was then monitored at 220 nm from 25 to 90°C with a heating rate of 1°C·min−1. All measurements were carried out in 100 mM phosphoric acid-NaOH with 1 mM EDTA, pH 7.0 (in the presence of 0.75 mM DTT for reduced NG_TlpA), using a 1-mm quartz cuvette. Transitions were normalized by assuming a linear dependence of the spectroscopic signal of the native and unfolded states on temperature.
Determination of the redox properties of TlpA.
The redox equilibrium of NG_TlpA with glutathione (GSH) was determined using the method described by Wunderlich and Glockshuber (37). Briefly, 0.95 to 1.5 μM protein was incubated for 16 h at 25°C in 100 mM phosphoric acid-NaOH with 0.1 mM EDTA, pH 7.0, containing 1 mM oxidized GSH (GSH disulfide [GSSG]) and increasing concentrations of reduced GSH. The redox equilibrium of DsbA, a periplasmic disulfide isomerase from E. coli, was also determined as a positive control. The redox state of NG_TlpA and DsbA was followed by fluorescence emission at 333 nm and 322 nm, respectively (excitation at 280 nm). Measurements were performed in triplicate, and experiments were repeated with three independent enzyme preparations. The equilibrium constants (Keq) were determined by fitting the original fluorescence data according to equation 1:
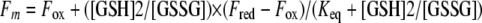 |
(1) |
where Fm is the measured fluorescence, and Fox and Fred are the fluorescence intensities of the oxidized and reduced proteins, respectively (21).
The standard redox potentials of NG_TlpA and DsbA were calculated from the Nernst equation using the GSH standard potential (E0′ GSH/GSSG = −240 mV): E0′ (pH of 7) = E0′ GSH/GSSG − (RT/nF) ln(Keq), where n is the number of electrons transferred in the reaction, F is Faraday's constant, and R is the gas constant. The equilibrium constant Keq was calculated by fitting the data according to equation 2:
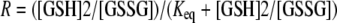 |
(2) |
where R is the fraction of reduced protein at equilibrium measured using the specific NG_TlpA or DsbA fluorescence (8).
Thioredoxin activity assay.
A thioredoxin activity assay was carried out essentially as described by Holmgren (10). Briefly, enzymatic reactions were conducted in 20 mM potassium phosphate, pH 8, at 25°C and were followed by measuring the reduction of disulfide bonds in insulin, as indicated by precipitation of this protein with the attendant increase in optical density at 650 nm. Reactions were started by the addition of 2 μg of purified NG_TlpA, previously reduced with 100 mM DTT, to 1 mg of insulin from bovine pancreas (Sigma) in 1 ml of 20 mM potassium phosphate buffer, pH 8.0, and reactions were monitored for 1 h. Thioredoxin from E. coli (Sigma) was treated as NG_TlpA and used as a reference. Experiments were performed in triplicate and repeated using independent enzyme preparations.
Chromosomal inactivation of tlpA and marker rescue.
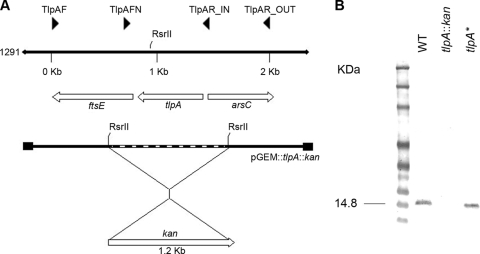
The tlpA gene was amplified by PCR from N. gonorrhoeae strain 1291, using the primers TlpAF (5′-TCATCCGTTTGGGCGGTTTATCGGA-3′) and TlpAR_OUT (5′-ATGATGCTCATGCGAGTCGTCCTT-3′), and the resulting PCR product was cloned into pGem-T Easy (Promega) according to the manufacturer's instructions. The tlpA knockout was constructed by digesting pGEM::tlpA with RsrII, which cut the plasmid once in the coding region of tlpA (see Fig. 4A). The restriction enzyme was then heat inactivated for 20 min at 65°C, and the ends of the linearized plasmids were filled with T4 DNA polymerase. pUC4Kan (Pharmacia), a plasmid containing a kanamycin resistance cassette, was digested with HincII, and the fragment containing the kanamycin resistance gene was isolated and ligated to the linearized plasmid. The plasmid, designated pGEM::tlpA::kan, for the tlpA::kan mutant, was linearized with NotI prior to transformation. N. gonorrhoeae strain 1291 was transformed as described previously (12), and recombinant strains were selected by growth on BHI agar containing kanamycin (50 μg·ml−1 and 100 μg·ml−1). The presence of the inactive allele was confirmed by Southern hybridization analysis as previously described by Tseng and coworkers (35) using BglI restriction endonuclease-digested genomic DNA of N. gonorrhoeae 1291 and digoxigenin-labeled tlpA probes (data not shown). The tlpA+ strain (tlpA*) was constructed by marker exchange: the pGEM::tlpA plasmid was linearized with NotI and transformed into N. gonorrhoeae 1291 tlpA::kan mutant strain as described previously. Recombinant strains were screened by the absence of growth on BHI agar containing kanamycin. The N. gonorrhoeae 1291 tlpA* strain was confirmed by PCR amplification with TlpAF and TlpAR_OUT (see Fig. 4A).
FIG. 4.
(A) Organization of N. gonorrhoeae 1291 chromosomal region containing the tlpA gene and strategy for insertional inactivation of tlpA using a kanamycin cassette. (B) Western blot of soluble extracts from N. gonorrhoeae 1291 (WT), 1291 tlpA::kan, and 1291 tlpA* strains probed using anti-TlpA antibody.
Western blotting.
Antibodies were raised against NG_TlpA by immunization of rabbits essentially as described by Power and coworkers (24). Western blotting was performed essentially as previously described (6) and developed using anti-TlpA serum (1:100,000) and anti-rabbit immunoglobulin G conjugated to alkaline phosphatase secondary antibody (1:5,000; Sigma-Aldrich) with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium) as chromogenic substrates.
Survival assays.
Oxidative killing assays using paraquat and hydrogen peroxide were carried out as previously described (35). Briefly, approximately 106 N. gonorrhoeae cells (wild type, tlpA::kan mutant, or tlpA*) were added to a solution of BHI broth to a final volume of 100 μl. Experiments were initiated by the addition of paraquat at a final concentration of 1 mM or of hydrogen peroxide at a final concentration of 40 mM. The initial number of cells and the number of viable cells remaining after 15, 30, 45, or 60 min of treatment were determined by direct colony counts.
Cervical cell invasion and survival assays.
Assays of the survival of N. gonorrhoeae in primary human cervical epithelial (pex) cells were performed as described in a previous publication (23). Briefly, pex cells were challenged with gonococci at a multiplicity of infection of 100 for 90 min. Gentamicin was then omitted from (association assays) or added to (invasion and intracellular survival assays) infected pex cell monolayers to kill extracellular cell-associated bacteria. The pex cell monolayers were subsequently lysed, or they were subjected to a second incubation in antibiotic-free medium before cell lysis (intracellular survival assays). For all assays, serial dilutions of the cell lysates were plated to enumerate viable CFU.
RESULTS
Identification and in silico analysis of a periplasmic thioredoxin in N. gonorrhoeae.
Using B. japonicum TlpA (GenBank accession number NP_009593) as a query in BLASTP searches, locus NGO1923 was identified in the N. gonorrhoeae strain FA1090 as a gene encoding a thioredoxin-like protein (NG_TlpA) with 28% amino acid sequence identity to B. japonicum TlpA. The NGO1923 open reading frame is 614 nucleotides in length, and the predicted NG_TlpA protein has a calculated relative molecular mass of 17,595 Da. The TlpA protein of B. japonicum has been shown to consist of an N-terminal membrane-spanning helix that anchors a periplasmic-facing water-soluble domain to the cytoplasmic membrane (3). In contrast, NG_TlpA contains a signal peptidase I cleavage site typical of gram-negative bacteria, with a cleavage site between amino acids 22 and 23 in the TlpA sequence, as predicted using SignalP, version 3.0 (1). This is consistent with a periplasmic location for NG_TlpA.
Protein folding analysis using Phyre (16) shows that NG_TlpA incorporates a thioredoxin fold with a helical insertion (∼25 residues), similar to that found in B. japonicum TlpA but absent in thioredoxin (3, 14). NG_TlpA also contains the Cys-X-X-Cys motif characteristic of thiol-disulfide oxidoreductases of the thioredoxin superfamily (20). There is a correlation between the dipeptide sequence between the cysteine residues and the redox potential of thioredoxin-like proteins (21, 25). In NG_TlpA this is Gly-Pro, similar to that of a thioredoxin and suggesting that the E0′ of the thiol/disulfide couple is probably around −200 mV. According to the analysis of Wunderlich and Glockshuber (37), this would be consistent with the idea that NG_TlpA acts as an electron donor.
Biochemical characterization of neisserial TlpA.
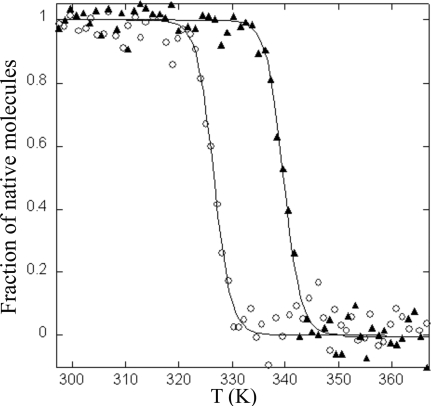
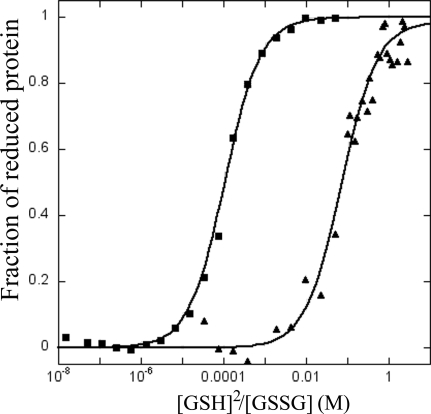
N. gonorrhoeae TlpA was overexpressed in E. coli as a recombinant protein in which the 22 amino acid residues at the N terminus were deleted and replaced with a six-histidine tag. The recombinant NG_TlpA was purified as described in the Materials and Methods section. Thioredoxins operate as disulfide reductases, and as a consequence the oxidized form of this protein is more stable toward thermal denaturation. Figure 1 shows the thermal denaturation curves for reduced and oxidized forms of NG_TlpA. The oxidized form of NG_TlpA was denatured at 339 K while the reduced species was denatured at 326 K, consistent with NG_TlpA's being a reducing thioredoxin. Sequence analysis predicted that the E0′ of the thiol/disulfide couple of NG_TlpA would be around −200 mV. We investigated this prediction using redox potentiometry. Figure 2 shows the change in the redox state of NG_TlpA, as indicated by Trp fluorescence, as a function of ambient redox potential defined by various ratios of GSH and GSSG. The equilibrium constant (Keq) for the thiol-disulfide exchange reaction with GSH (Fig. 2) for NG_TlpA is 0.75 ± 0.1 × 10−1 M, which converts into a standard redox potential (E0′) of −206 mV. Figure 2 also shows a redox titration using E. coli DsbA. The E0′ measured for this oxidizing thioredoxin-like protein is in good agreement with the previously published value (−122.8 mV versus −119 mV) (38) and confirms that neisserial TlpA is, indeed, a thioredoxin that is likely to have a role as a disulfide reductase. This was confirmed using the insulin reduction assay in which purified NG_TlpA protein exhibited disulfide reductase activity, which is typical of a thioredoxin (Fig. 3).
FIG. 1.
Thermal unfolding of NG_TlpA measured in 100 mM phosphoric acid-NaOH-1 mM EDTA, pH 7.0. Reduced NG_TlpA was measured in the presence of 0.75 mM DTT. ▴, oxidized NG_TlpA; ○, reduced NG_TlpA; T, temperature.
FIG. 2.
Redox equilibrium of NG_TlpA (▴) and DsbA (▪) with GSH. The relative amount of reduced protein at equilibrium (R) was determined by the redox-state-dependent fluorescence of NG_TlpA and DsbA at 333 nm and 322 nm, respectively (excitation at 280 nm), as described in Materials and Methods. The solid lines correspond to fits of the normalized data according to the overall TlpA/DsbA/GSH equilibrium.
FIG. 3.
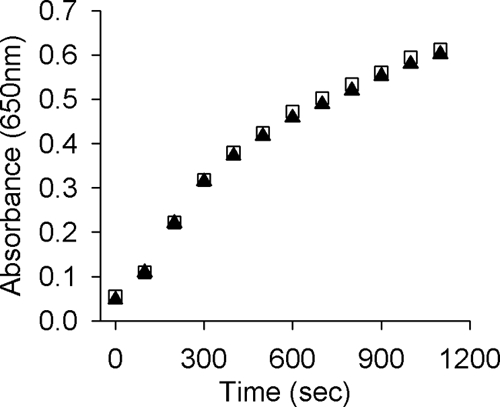
Insulin reduction assay using purified recombinant NG_TlpA protein of N. gonorrhoeae 1291 (▴) and recombinant thioredoxin of E. coli (□). Insulin reduction was indicated by the increase in optical density at 650 nm.
Characterization of a tlpA mutant of N. gonorrhoeae strain 1291.
In view of the importance of proteins exhibiting thiol-disulfide oxidoreductase activity in the periplasm, we investigated the potential function of NG_TlpA by mutational analysis. A tlpA mutant was constructed in N. gonorrhoeae strain 1291 by disruption of the tlpA gene with a promoterless kanamycin antibiotic resistance cassette (Fig. 4A). In addition, a tlpA* strain was constructed to act as a control for second-site suppressor mutations. In this strain the tlpA::kan allele was replaced by wild-type tlpA by homologous recombination. The phenotypes of the tlpA::kan mutant and tlpA* strains were confirmed by probing the gonococcal cells with TlpA antibody, generated using recombinant NG_TlpA protein (Fig. 4B). Investigations in B. japonicum have revealed that TlpA was required for the assembly of the CuA center of the aa3-type cytochrome oxidase (18). Since pathogenic Neisseria cells contain a single oxidase (28), cytochrome cbb3, which lacks a CuA center, it seemed unlikely that NG_TlpA would have a role in the biogenesis of the neisserial cytochrome oxidase. As expected, both wild-type and mutant strains stained positive for the cytochrome oxidase test (data not shown).
Thioredoxins and related proteins with thiol-disulfide oxidoreductase activity have a number of important roles within bacterial cells including maintenance of a reducing environment of the cytoplasm and the protection of cells against peroxide stress (4, 11, 33). As a consequence we tested whether NG_TlpA might have a role in protection against oxidative stress in N. gonorrhoeae as indicated by sensitivity to paraquat, a generator of superoxide ions, or hydrogen peroxide. Figure 5 shows that the tlpA::kan mutant was sensitive to killing by paraquat while the wild-type and tlpA* strains were more resistant, and a significant fraction of the population survived (about 20% of the population) even at 45 min of incubation with paraquat. Figure 6 shows that the tlpA::kan mutant was highly sensitive to killing by hydrogen peroxide while the wild-type and tlpA* strains were resistant. The data obtained from these oxidative killing assays suggest that NG_TlpA contributes to defense against oxidative stress in N. gonorrhoeae.
FIG. 5.
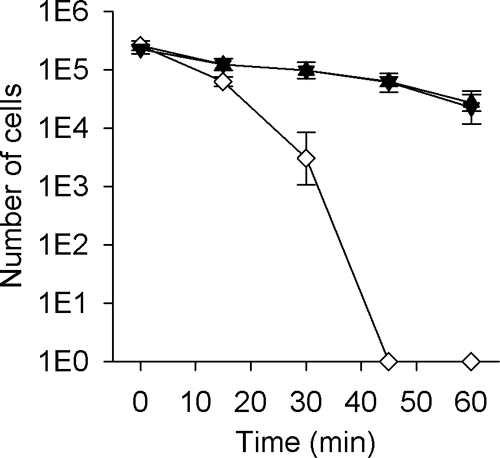
Paraquat killing assay of N. gonorrhoeae 1291 (wild-type; ▴), 1291 tlpA::kan (tlpA mutant; ⋄), and 1291 tlpA* (knock-in mutant; ▾). In this assay, cells were grown on BHI agar and exposed to 1 mM paraquat. Experiments were performed in triplicate and repeated on several occasions. Error bars indicate ± 1 standard deviation of the mean.
FIG. 6.
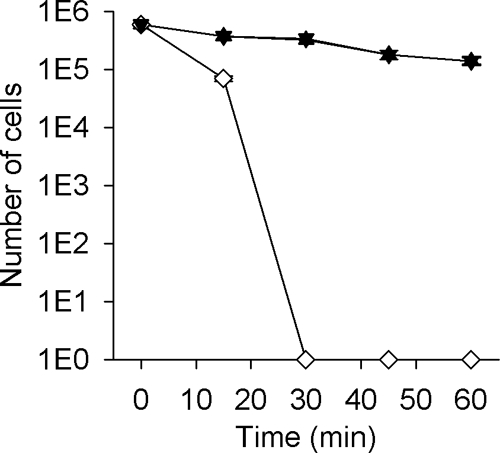
Hydrogen peroxide killing assay of N. gonorrhoeae 1291 (wild-type; ▴), 1291 tlpA::kan (tlpA mutant; ⋄), and 1291 tlpA* (knock-in mutant; ▾). In this assay, cells were grown on BHI agar and exposed to 40 mM hydrogen peroxide. The N. gonorrhoeae 1291 kat::kan mutant strain was used as a control (data not shown) (35). Experiments were performed in triplicate and repeated on several occasions. Error bars indicate ± 1 standard deviation of the mean.
A tlpA mutant exhibits reduced colonization of cervical epithelial cells.
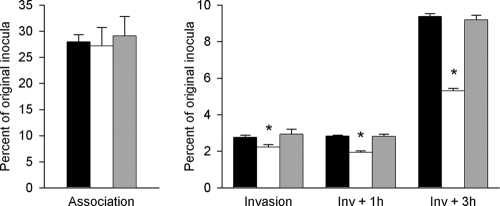
To determine whether TlpA might be physiologically relevant for successful gonococcal infection, the ability of N. gonorrhoeae 1291 and 1291 tlpA::kan to associate with, invade, and survive within pex cells was tested. The pex cells were challenged with either the wild-type or mutant strain, and infection was allowed to progress for up to 5 h. There was no significant difference observed in the ability of the tlpA::kan strain to associate with pex cells in comparison to the wild type (Fig. 7). However, the tlpA::kan strain showed reduced ability to survive and/or replicate within pex cells compared with the wild-type strain (Fig. 7). This deficiency was corrected in the tlpA* strain.
FIG. 7.
TlpA mutant gonococci are impaired in their ability to invade and survive within primary cervical cells: association, invasion (Inv), and survival assays were performed as described in the text, where association corresponds to a 90-min infection and invasion refers to a 90-min infection plus gentamicin treatment. Survival (Inv + 1 h or 3 h) refers to the number of gonococci that survive within pex cells at 1 h or 3 h following gentamicin treatment. Values depicted were determined as a function of the original inoculum and the number of CFU formed with subsequent plating of the pex cell lysates. Data given were obtained from three trials performed in triplicate. P values were determined using a Kruskal-Wallis k-sample analysis of variance calculated for the tlpA::kan mutant (white) or knock-in mutant (gray) gonococci upon comparison to the wild-type bacteria (black), as outlined in the text (*, P < 0.001).
DISCUSSION
The importance of protein disulfide reduction reactions in the periplasm of gram-negative bacteria is now well established with respect to protein disulfide isomerization and protein folding as well as c-type cytochrome maturation. However, it is increasingly clear that there are additional thioredoxin-like proteins which operate in the periplasm that are distinct from DsbC/DsbG and CcmG. NG_TlpA is one example, and our biochemical analysis clearly indicates that the thermodynamic and catalytic properties of this protein are consistent with its operating as a disulfide reductase. As for the DsbC/DsbG and CcmG proteins, the source of electrons for NG_TlpA seems likely to be DsbD, consistent with the latter's role as a redox hub for transfer of thiol reducing power from cytoplasmic thioredoxin to periplasmic-facing thioredoxins (34).
Although we can be confident that TlpA operates as a thioredoxin, the nature of the biomolecules to which NG_TlpA donates electrons has not been established. A clue about the potential role for NG_TlpA comes from our observation that the tlpA::kan mutant is sensitive to oxidative killing by hydrogen peroxide and superoxide. One possibility is that NG_TlpA is associated with the maintenance of specific cysteine residues of some periplasmic proteins in a reduced state. Oxidizing agents such as hydrogen peroxide can cause oxidation of cysteine thiols that can react with small-molecule thiols or cysteine thiols in proteins to form mixed disulfides or disulfide bonds between proteins. Such possibilities have been suggested for the periplasmic thioredoxin from Chlamydia pneumoniae, DsbH (19), although it should be noted that DsbH does not appear to be closely related to NG_TlpA or the TlpA/ResE/CcmG family of thioredoxin-like proteins. Bardwell and coworkers have suggested that a generally more reducing periplasm may represent an adaptation to an intracellular environment, as in the case of Chlamydia. Methionine and cysteine are the amino acids that are most sensitive to oxidation by hydrogen peroxide; the former is oxidized to methionine sulfoxide while the latter can form a cysteine sulfenic acid, which in turn can react with a thiol to form a disulfide. N. gonorrhoeae possesses a highly unusual methionine sulfoxide reductase (MsrAB) that is located in the outer membrane rather than the cytoplasm, as in most other bacteria (32). Although there is in vitro biochemical evidence that the thioredoxin domain of MsrAB interacts directly with DsbD (2), another possibility is that periplasmic TlpA mediates electron flow between these inner and outer membrane proteins. In this context it is interesting that a pilB mutant of gonococcus is also sensitive to killing by hydrogen peroxide (32).
N. gonorrhoeae grows extracellularly in the human host, but it can also invade and grow within cervical epithelial cells (5). The potential importance of NG_TlpA to pex cell infection was illustrated by the reduced survival of the tlpA::kan mutant compared to wild-type and tlpA* strains. However, the effect of the tlpA mutation was modest compared to that of other mutations that affect defense against oxidative and nitrosative stress and that have been analyzed using this same (pex cell) model system (22, 30). The host factors (e.g., hydrogen peroxide or nitric oxide production) contributing to the impaired ability of the TlpA strain to survive during the course of pex cell challenge will require further analysis in the future. However, our data may collectively reflect a redundancy in periplasmic oxidative stress defense systems in N. gonorrhoeae.
Acknowledgments
This research is supported by the National Health and Medical Research Council, program grant 284214 to A.G.M. and M.P.J. A.J.H. was the recipient of a University of Queensland Postgraduate Scholarship. J.L.E. received funding from the Research Institute at Nationwide Children's Hospital.
We thank Michael Apicella, Xavier Nassif, and John Tainer for providing strains. We acknowledge the Cooperative Human Tissue Network (Columbus, OH) for providing cervical tissue specimens.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 17 August 2009.
REFERENCES
- 1.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 2.Brot, N., J. F. Collet, L. C. Johnson, T. J. Jonsoon, H. Weissbach, and W. T. Lowther. 2006. The thioredoxin domain of Neisseria gonorrhoeae PiIB can use electrons from DsbD to reduce downstream methionine sulfoxide reductases. J. Biol. Chem. 281:32668-32675. [DOI] [PubMed] [Google Scholar]
- 3.Capitani, G., R. Rossmann, D. F. Sargent, M. G. Grutter, T. J. Richmond, and H. Hennecke. 2001. Structure of the soluble domain of a membrane-anchored thioredoxin-like protein from Bradyrhizobium japonicum reveals unusual properties. J. Mol. Biol. 311:1037-1048. [DOI] [PubMed] [Google Scholar]
- 4.Chae, H. Z., K. Robison, L. B. Poole, G. Church, G. Storz, and S. G. Rhee. 1994. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 91:7017-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards, J. L., and M. A. Apicella. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher, S., S. E. Winston, and J. G. R. Hurrell. 1992. Immunoblotting and immunodetection, p. 10.18.11-10.18.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, NY.
- 7.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins, H. C., M. Denardi, and R. B. Freedman. 1991. Redox properties and cross-linking of the dithiol/disulphide active sites of mammalian protein disulphide-isomerase. Biochem. J. 275:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heras, B., S. R. Shouldice, M. Totsika, M. J. Scanlon, M. A. Schembri, and J. L. Martin. 2009. DSB proteins and bacterial pathogenicity. Nat. Rev. Microbiol. 7:215-225. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren, A. 1979. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J. Biol. Chem. 254:9113-9119. [PubMed] [Google Scholar]
- 11.Holmgren, A. 1985. Thioredoxin. Annu. Rev, Biochem. 54:237-271. [DOI] [PubMed] [Google Scholar]
- 12.Jennings, M. P., D. W. Hood, I. R. A. Peak, M. Virji, and E. R. Moxon. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18:729-740. [DOI] [PubMed] [Google Scholar]
- 13.Kadokura, H., F. Katzen, and J. Beckwith. 2003. Protein disulfide bond formation in procaryotes. Annu. Rev. Biochem. 72:111-135. [DOI] [PubMed] [Google Scholar]
- 14.Katti, S. K., D. M. Lemaster, and H. Eklund. 1990. Crystal-structure of thioredoxin for Escherichia coli at 1.68 Å resolution. J. Mol. Biol. 212:167-184. [DOI] [PubMed] [Google Scholar]
- 15.Katzen, F., and J. Beckwith. 2000. Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell 103:769-779. [DOI] [PubMed] [Google Scholar]
- 16.Kelley, L. A., and M. J. E. Sternberg. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363-371. [DOI] [PubMed] [Google Scholar]
- 17.Lillig, C. H., and A. Holmgren. 2007. Thioredoxin and related molecules—from biology to health and disease. Antioxid. Redox Signal. 9:25-47. [DOI] [PubMed] [Google Scholar]
- 18.Loferer, H., M. Bott, and H. Hennecke. 1993. Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa3 and development of symbiosis. EMBO J. 12:3373-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mac, T. T., A. von Hacht, K. C. Hung, R. J. Dutton, D. Boyd, J. C. A. Bardwell, and T. S. Ulmer. 2008. Insight into disulfide bond catalysis in Chlamydia from the structure and function of DsbH, a novel oxidoreductase. J. Biol. Chem. 283:824-832. [DOI] [PubMed] [Google Scholar]
- 20.Martin, J. L. 1995. Thioredoxin—a fold for all reasons. Structure 3:245-250. [DOI] [PubMed] [Google Scholar]
- 21.Mossner, E., M. Huber-Wunderlich, and R. Glockshuber. 1998. Characterization of Escherichia coli thioredoxin variants mimicking the active-sites of other thiol/disulfide oxidoreductases. Protein Sci. 7:1233-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter, A. J., S. P. Kidd, J. L. Edwards, M. Falsetta, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2009. Esterase D is essential for protection of Neisseria gonorrhoeae against nitrosative stress and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 200:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potter, A. J., S. P. Kidd, J. L. Edwards, M. L. Falsetta, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2009. Thioredoxin reductase is essential for protection of Neisseria gonorrhoeae against killing by nitric oxide and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 199:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power, P. M., L. F. Roddam, M. Dieckelmann, Y. N. Srikhanta, Y. C. Tan, A. W. Berrington, and M. P. Jennings. 2000. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology 146:967-979. [DOI] [PubMed] [Google Scholar]
- 25.Quan, S., I. Schneider, J. Pan, A. Von Hacht, and J. C. A. Bardwell. 2007. The CXXC motif is more than a redox rheostat. J. Biol. Chem. 282:28823-28833. [DOI] [PubMed] [Google Scholar]
- 26.Reid, E., J. Cole, and D. J. Eaves. 2001. The Escherichia coli CcmG protein fulfils a specific role in cytochrome c assembly. Biochem. J. 355:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritz, D., and J. Beckwith. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21-48. [DOI] [PubMed] [Google Scholar]
- 28.Seib, K. L., M. P. Jennings, and A. G. McEwan. 2003. A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett. 546:411-415. [DOI] [PubMed] [Google Scholar]
- 29.Seib, K. L., H. J. Wu, S. P. Kidd, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 70:344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seib, K. L., H. J. Wu, Y. N. Srikhanta, J. L. Edwards, M. L. Falsetta, A. J. Hamilton, T. L. Maguire, S. M. Grimmond, M. A. Apicella, A. G. McEwan, and M. P. Jennings. 2007. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol. Microbiol. 63:54-68. [DOI] [PubMed] [Google Scholar]
- 31.Sinha, S., P. R. Langford, and J. S. Kroll. 2004. Functional diversity of three different DsbA proteins from Neisseria meningitidis. Microbiology 150:2993-3000. [DOI] [PubMed] [Google Scholar]
- 32.Skaar, E. P., D. M. Tobiason, J. Quick, R. C. Judd, H. Weissbach, F. Etienne, N. Brot, and H. S. Seifert. 2002. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc. Natl. Acad. Sci. USA 99:10108-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits, W. K., J. Y. F. Dubois, S. Bron, J. M. van Dijl, and O. P. Kuipers. 2005. Tricksy business: transcriptome analysis reveals the involvement of thioredoxin a in redox homeostasis, oxidative stress, sulfur metabolism, and cellular differentiation in Bacillus subtilis. J. Bacteriol. 187:3921-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stirnimann, C. U., M. G. Grutter, R. Glockshuber, and G. Capitani. 2006. nDsbD: a redox interaction hub in the Escherichia coli periplasm. Cell. Mol. Life Sci. 63:1642-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 36.Turner, S., E. Reid, H. Smith, and J. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a gram-negative bacterium. Biochem. J. 373:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wunderlich, M., and R. Glockshuber. 1993. Redox properties of protein disulfide isomerase (DsbA) from Escherichia coli. Protein Sci. 2:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zapun, A., J. C. A. Bardwell, and T. E. Creighton. 1993. The reactive and destabilizing disulfide bond of DsbA, a protein required for protein disulfide bond formation in vivo. Biochemistry 32:5083-5092. [DOI] [PubMed] [Google Scholar]