Abstract
The guinea pig model of tuberculosis is used extensively in assessing novel vaccines, since Mycobacterium bovis BCG vaccination effectively prolongs survival after low-dose aerosol infection with virulent M. tuberculosis. To better understand how BCG extends time to death after pulmonary infection with M. tuberculosis, we examined cytokine responses postvaccination and recruitment of activated T cells and cytokine response postinfection. At 10 weeks postvaccination, splenic gamma interferon (IFN-γ) mRNA was significantly elevated compared to the levels at 5 weeks in ex vivo stimulation assays. At 15, 40, 60, and 120 days postinfection, T-cell activation (CD4+ CD62Llow and CD8+ CD62Llow) and mRNA expression of IFN-γ, tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-10, IL-12, and eomesodermin were assessed. Our data show that at day 40, BCG-vaccinated guinea pigs had significantly increased levels of IFN-γ mRNA expression but decreased TNF-α mRNA expression in their lungs compared to the levels in nonvaccinated animals. At day 120, a time when nonvaccinated guinea pigs succumbed to infection, low levels of IFN-γ mRNA were observed even though there were increasing levels of IL-1, IL-12, and IL-10, and the numbers of activated T cells did not differ from those in BCG-vaccinated animals. BCG vaccination conferred the advantage of recruiting greater numbers of CD4+ CD62Llow T cells at day 40, although the numbers of CD8+ CD62Llow T cells were not elevated compared to the numbers in nonvaccinated animals. Our data suggest that day 40 postinfection may be a pivotal time point in determining vaccine efficacy and prolonged survival and that BCG promotes the capacity of T cells in the lungs to respond to infection.
Vaccination with Mycobacterium bovis BCG (bacillus Calmette-Gúerin) has been used extensively to combat tuberculosis since its introduction in the early part of the 20th century. Many meta-analyses of clinical studies have since been conducted to estimate the efficacy of BCG vaccination for prevention of adult pulmonary disease, which varies between 0 and 80%, a statistic attributed to multiple factors (10). One explanation includes waning immunity after childhood vaccination, resulting in renewed susceptibility to disease later in life (41). Due to acceptable efficacy of BCG for the prevention of childhood complications of tuberculosis, BCG is still recommended for use as a neonatal vaccine in countries where tuberculosis is endemic (42). There is now a robust effort to develop novel vaccines that will boost neonatal BCG vaccination (28). However, this strategy requires a more thorough understanding of the immunological responses elicited by BCG and of how these immune responses correlate with the prevention or amelioration of pathologies, and most importantly, immunological functions have to be improved upon to create a more efficacious vaccination strategy against adult pulmonary tuberculosis.
In the current endeavors to develop a new antituberculosis vaccine, the guinea pig has been used extensively as a model for testing novel vaccine candidates prior to clinical trials (14). M. tuberculosis infection in guinea pigs has been well characterized and is known to produce several disease characteristics that are similar to those seen in humans, namely, weight loss, intra- and extrapulmonary dissemination of the infectious organism, and subsequent death (32). The pulmonary pathology resembles that seen in humans, with progressive lung involvement and the production of “primary” and “secondary” granulomas characterized on the basis of their size and extent of necrosis (6, 18, 35, 39). Despite its usefulness for replicating certain tuberculosis pathologies for humans, the guinea pig was not tractable to detailed immune studies until recently. An important area of study using the guinea pig model is the correlation of disease pathology with immune responses and the comparison of these to what is known in humans to confirm which experimental observations and immunological endpoints in the guinea pig model have the greatest biological relevance for human disease. The expression of immune responses to M. tuberculosis in humans includes priming of macrophages and the activation of CD4+ and CD8+ T cells, with subsequent production of key cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (11, 12). Thus, it is of great interest to know if these cytokines are produced in the guinea pig model of tuberculosis and if they play a role in the animal's immune response to infection.
Although the guinea pig has been instrumental in early estimation of the efficacy of vaccine candidates and has been used to select clinical candidates, very little is known about the immune response generated as a result of infection and after vaccination, and therefore, correlation of immune responses to pathologies and disease progression was not possible. As a result, studies of tuberculosis immunology are being performed in mice, while pathology studies are being done with guinea pigs, without a common denominator to track “efficacy” between different animal species and humans. Extensive studies by Smith and colleagues and, subsequently, by McMurray have helped to elucidate aspects of guinea pig immune responses to experimental tuberculosis, but these efforts have been hindered by a lack of key immune reagents for this animal (1, 4, 7, 16, 17, 21-23, 31). More recently, numerous investigations of tuberculosis in the guinea pig have used more modern approaches to repeat histological studies of the pathology during the early phase of infection in nonvaccinated and vaccinated animals to confirm earlier findings (5, 18). Immune studies have remained limited to the characterization of a small number of cell surface markers and T-cell phenotypes during infection in vaccinated and nonvaccinated guinea pigs (24, 25). Thus, a more extensive study of the immune response in guinea pigs during infection and after vaccination is needed to provide a more integrated view of immunology and pathology in this model.
The current studies were performed to examine the clinical course of disease and the kinetics and magnitude of the immune responses generated by BCG vaccination before and after aerosol challenge with a low dose of virulent M. tuberculosis. Specifically, cellular recruitment and activation and cytokine and transcription factor expression profiles were assessed using guinea pig-specific or cross-reactive reagents, such as monoclonal antibodies (MAbs), to identify CD4 and CD8 T cells and a guinea pig-specific MAb against CD62L (L-selectin) to determine the activation state of T cells during infection (13, 36). L-Selectin is an adhesion molecule used by naïve T cells to traffic into peripheral lymph nodes. Once the T cell encounters its cognate antigen on the dendritic cell, CD62L is shed as the T cell is activated. Activated cells then use other adhesion molecules to migrate into inflammatory foci in infected tissue (19, 38, 40). In addition to characterizing T-cell phenotypes, we also delineated the kinetics of the immune response to vaccination and infection by measuring cytokine mRNA levels. Specifically, primers for guinea pig IFN-γ, TNF-α, interleukin-1 (IL-1), IL-12, and IL-10 were used to determine cytokine expression profiles during the initial 120-day period of infection. We also chose to measure eomesodermin (EOMES), a member of the T-box family of transcription factors that has been shown to be involved in the differentiation of CD8+ T cells into effector and memory cells (3, 26). It has been hypothesized that if CD8+ T cells are an essential component of the immune response during infection with M. tuberculosis, then upregulation of EOMES expression will be observed after vaccination or during infection.
MATERIALS AND METHODS
Animals.
Out-bred female Hartley guinea pigs weighing 450 to 500 grams were purchased from Charles River Laboratories (Wilmington, MA). Guinea pigs were maintained under animal biosafety level 3 barrier conditions in isolator cages (Thoren, Hazleton, PA) for the entire period of each experiment, had access to chow and water ad libitum, and received daily environmental enrichment. All experimental procedures were approved by the Colorado State University Institutional Animal Care and Use Committee.
Mycobacterium species.
Mycobacterium bovis BCG Pasteur (TMCC 1011) strain was grown in Proskaur and Beck (P&B) medium with 0.1% Tween 80 to mid-log phase. Aliquots were stored at −80°C and thawed before use. M. tuberculosis H37Rv (TMCC 102) was initially grown for three passages as a pellicle on P&B medium to produce seed stocks. Working stocks with a maximum of six passages were expanded from the seed stocks in P&B medium with 0.1% Tween 80. Working stocks were prepared at the mid-log phase, and aliquots were stored at −80°C.
Infection and survival studies.
Guinea pigs were exposed via the respiratory route to 10 to 20 CFU of virulent M. tuberculosis H37Rv using a Madison aerosol chamber (Madison, WI). Working stocks of M. tuberculosis H37Rv were diluted to 106 CFU/ml in sterile distilled water and placed in the nebulizer jar, and animals were exposed to the aerosol for 5 min. The time course experiments were performed twice with 8 guinea pigs per group per time point, and animals were sacrificed at 15, 40, 60, and 120 days postinfection. The right cranial lobe of the lung and half of the spleen were sampled to assess CFU numbers. Bacterial load was determined by plating organ homogenates onto nutrient 7H11 agar supplemented with oleic acid-albumin-dextrose-catalase. Colonies were enumerated after 21 days of incubation at 37°C. For survival studies, guinea pigs were monitored daily and weighed once each week. Guinea pigs were euthanized when they reached set criteria established by the animal care and use committee, such as being moribund or exceeding acceptable weight loss and/or being affected in their respiratory rate (labored/heavy breathing). Time to euthanasia is used as time to death. Survival experiments were performed with between 8 and 10 guinea pigs per group. Body temperature was measured to track clinical progression of disease. For this, guinea pigs received a subcutaneous microchip implant (IPT-300 Bio Medic Data Systems [BMDS], Inc., Seaford, DE) that allowed measurement of temperature and also carried information about experiment number and animal number. The body temperatures of individual guinea pigs were assessed each day at approximately the same time in the afternoon using a DAS-6006/7 scanner transponder (BMDS). Previous studies had shown that the body temperature of guinea pigs did not vary significantly during the day and that deviations from normal temperature curves were indicative of disease progression. Temperature experiments have been performed with 10 to 15 guinea pigs per group.
Vaccination and immunogenicity studies of BCG-vaccinated guinea pigs.
Guinea pigs were vaccinated with 103 CFU of BCG Pasteur via the intradermal route on the belly, using a tuberculin syringe attached to a 26-gauge, 1/2-in. needle. At 5 and 10 weeks postvaccination, animals were sacrificed and their spleens were aseptically removed. Spleen cells were isolated, and antigen-specific responses for IFN-γ and TNF-α were measured indirectly through mRNA expression via real-time PCR. Briefly, spleens were excised, cut into small pieces, and then passed through a sterile cell strainer (BD Biosciences, Mountain View, CA) to obtain single-cell suspensions. The cells were washed with complete RPMI medium (c-RPMI medium; RPMI 1640 plus 10% heat-inactivated fetal bovine serum [Atlas Biologicals, Fort Collins, CO], and Gey's solution (0.15 M NH4Cl and 10 mM KHCO3) was used to lyse red blood cells. The cells were washed twice with c-RPMI medium containing 200 mM l-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin [Sigma-Aldrich]), and viable cell numbers determined by counting using trypan blue exclusion in a Neubauer chamber (IMV International, Minneapolis, MN). The cell concentration for each PCR experiment was adjusted to 2 × 106 cells/ml with c-RPMI medium, and cells were cultured in duplicate and stimulated with 1 μg/ml of culture filtrate protein (obtained through the TB Vaccine Testing and Research Materials contract at CSU through NIH grant no. HHSN266200400091c) for 24 h at 37°C in 5% CO2. At the time of harvest, the cells and supernatants were removed by aspirating the well contents and transferring them to a microcentrifuge tube. The well was then checked using an inverted microscope to ensure that all the cells had been removed. The tubes were centrifuged at 135 × g, and the supernatant removed. One milliliter of TRIZol (Invitrogen, Carlsbad, CA) was then added to each tube. RNA was extracted according to the manufacturer's protocol and stored at −80°C in sterile, nuclease-free water (Invitrogen). cDNA was transcribed from mRNA made using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and real-time PCR was performed using iQ Syber green Supermix (Bio-Rad). Primers for IFN-γ and TNF-α were derived from previously published sequences (2).
Cell preparation for flow cytometric analysis.
Guinea pig accessory lung lobes were perfused with phosphate-buffered saline containing 50 U/ml of heparin (Sigma-Aldrich, St. Louis, MO) and placed into a small tissue culture dish containing incomplete RPMI 1640 medium (Invitrogen). Each lung lobe was cut into small pieces, using a sterile razor blade, and incubated in collagenase D (0.7 mg/ml; Roche, Nutley, NJ) and DNase (30 μg/ml; Sigma-Aldrich) for 30 min at 37°C. Half of the spleen from each guinea pig was placed into a small tissue culture dish and cut into small pieces. Mediastinal lymph nodes were processed similarly. To obtain single-cell suspensions from all organs collected, the predigested lung pieces, the spleen pieces, and the mediastinal lymph node pieces were passed through cell strainers (BD Biosciences) and then rinsed with c-RPMI medium containing 5% heat-inactivated fetal bovine serum (Atlas Biologicals). After the cells were washed in c-RPMI Gey's solution was used to lyse red blood cells, and the cells were washed with c-RPMI medium. Finally the cells were resuspended in c-RPMI medium and counted in a Neubauer chamber (IMV International), using a 2% trypan blue solution.
Flow cytometric analysis of cell surface markers.
Single-cell suspensions from organs prepared from each individual guinea pig were stained with fluorescence-labeled MAbs against CD4 (clone FITC CT7; Serotec, Inc., Raleigh, NC) (37), CD8 (clone FITC CT6; Serotec, Inc.) (37), pan-T cells (clone APC CT5; Serotec, Inc.) (37), and CD62L (clone PE lam1-116; Santa Cruz Biotechnology, Santa Cruz, CA) (13) at 4°C for 30 min in the dark after the cells were washed with phosphate-buffered saline containing 0.1% sodium azide (Sigma-Aldrich). Antibodies were used at 0.2 μg/106 cells. Cells were gated on lymphocytes by forward and side scatter according to their characteristic scatter profile and further gated based upon on pan-T-cell and CD4 or CD8 expression. All analyses were performed with an acquisition of at least 100,000 events on a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed using CellQuest software (BD Biosciences, San Jose, CA).
Analysis of mRNA by quantitative real-time PCR.
At the designated time points, guinea pig left caudal lung lobes were placed directly into 4 ml of TRIzol (Invitrogen) and immediately homogenized, and RNA extracted according to the manufacturer's protocol. cDNA was produced by using an iScript cDNA synthesis kit (Bio-Rad), and quantitative real-time PCR was performed using iQ Syber green Supermix (Bio-Rad). Primers to amplify IFN-γ, TNF-α, IL-12, IL-1β, and β-actin for real-time PCR were obtained from previously published sequences (2, 7, 29, 43). The primer sequences for IL-10 were forward, TTCTTCCAAACACAGGATCAGC, and reverse, TCATTTCCGATAGGGCTTGG. The primer sequences for EOMES were forward, ATAAACGGACTCAATCCCACCGCCCA, and reverse, ATTATTGTCGGCTTTGCCGCAGGTCAC. The primer sequences for IL-10 and EOMES were derived from the guinea pig genome database available at www.sanger.ac.uk. All primers were validated prior to use in preliminary experiments by amplifying guinea pig cDNA and sequencing the amplicon. The extent of mRNA induction for each cytokine was calculated by initially determining its mRNA expression level in naïve guinea pigs (n = 8) and then calculating the increases in mRNA induction postvaccination and postinfection with reference to the level of expression in naïve guinea pigs.
Histology.
The right caudal lobe of the guinea pig lung was utilized to analyze pathological lesions. The excised lobe was inflated with formalin and placed in total into formalin. For processing, the lobe was embedded in paraffin and sections cut and stained with hematoxylin and eosin (H&E; IHC Tech, Aurora, CO). Photomicrographs were taken using an Olympus BX41 microscope attached to a Dell Precision computer with DP2-BSW software for image capture.
Statistical analyses.
Guinea pig survival was plotted using the Kaplan-Meier method, and differences between curves were analyzed using the log-rank test. The Mann-Whitney test was used to characterize changes in cell surface markers and cytokine mRNA expression between groups of guinea pigs for each time point.
RESULTS
Survival.
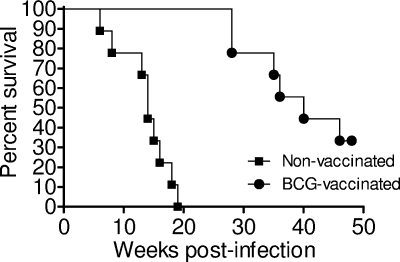
Guinea pigs were vaccinated via the intradermal route with 103 CFU BCG Pasteur or injected via the same route with sterile nonpyrogenic saline and rested for 10 weeks. All guinea pigs were infected with a low-dose aerosol of virulent M. tuberculosis H37Rv after week 10 and then monitored for weight loss, respiration rate, and failure to thrive and were euthanized as outlined in Materials and Methods. Figure 1 shows the Kaplan-Meier guinea pig survival plot for M. tuberculosis-infected animals that had received either BCG or saline placebo (nonvaccinated). Nonvaccinated guinea pigs succumbed to disease within 20 weeks, while vaccinated guinea pigs had a significantly prolonged survival time of up to 50 weeks (P < 0.001), suggesting that BCG induced an immune response that was sufficient to alter the course of disease. Interestingly, when the lungs and spleens from necropsied animals were plated for CFU counts, there was no indication of increased mycobacterial numbers in either group (data not shown).
FIG. 1.
Kaplan-Meier plot of the survival of nonvaccinated and BCG-vaccinated guinea pigs after low-dose aerosol infection with M. tuberculosis H37Rv. Guinea pigs were vaccinated with 103 CFU BCG Pasteur or with an equal volume of pyrogen-free sterile saline. Guinea pigs were monitored daily and weighed weekly to assess their health status. n = 10 guinea pigs per group.
Reduction of mycobacterial burden in BCG-vaccinated, M. tuberculosis-infected guinea pigs.
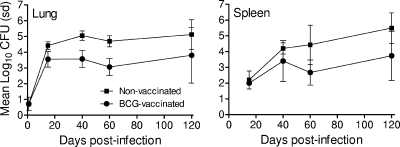
Given the significantly prolonged survival of BCG-vaccinated guinea pigs, we next assessed whether survival is correlated with a reduction in mycobacterial burden in the lungs and spleens. Nonvaccinated and BCG-vaccinated guinea pigs were infected with a low-dose aerosol of virulent M. tuberculosis; animals sacrificed at days 15, 40, 60, and 120 postinfection; and the lung and spleen homogenates plated to enumerate M. tuberculosis organisms. Figure 2 shows that BCG-vaccinated guinea pigs had significantly lower numbers of M. tuberculosis organisms in their lungs at days 15 (P = 0.002), 40 (P < 0.001), and 60 (P < 0.001) postinfection and significantly lower numbers in the spleens at day 60 (P = 0.003) than nonvaccinated animals. Therefore, BCG vaccination provided guinea pigs with an increased capacity to reduce mycobacterial burden.
FIG. 2.
Growth of M. tuberculosis in the lungs and spleens of nonvaccinated and BCG-vaccinated guinea pigs during the first 120 days after low-dose aerosol infection as described in the Fig. 1 legend. n = 8 guinea pigs per group per time point. Error bars show standard deviations (sd).
Induction of IFN-γ and TNF-α in BCG-vaccinated guinea pigs.
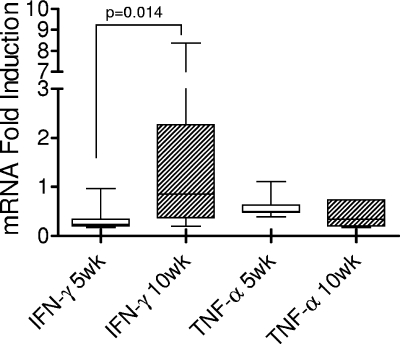
Since detailed immune studies of guinea pigs had not been feasible until now, we analyzed whether basic immune responses generated by BCG vaccination could be detected. Guinea pigs were vaccinated but not infected with M. tuberculosis, their spleen cells were stimulated, and resultant levels of IFN-γ and TNF-α mRNA expression were examined at 5 and 10 weeks postvaccination. Spleen cells from vaccinated guinea pigs were stimulated ex vivo with culture filtrate protein for 24 h, and then mRNA expression levels measured via real-time PCR. Figure 3 shows that stimulated spleen cells from animals sacrificed at 10 weeks produced higher levels of IFN-γ mRNA than those from animals sacrificed at 5 weeks postvaccination (P = 0.014). Interestingly, not all BCG-vaccinated guinea pigs responded to the ex vivo culture filtrate protein stimulation even at 10 weeks postvaccination. This may reflect the variation in immune responses to BCG in this out-bred animal. TNF-α mRNA expression did not differ between the two time points tested and was scarcely above the background level.
FIG. 3.
Induction of IFN-γ and TNF-α mRNA in spleen cells from BCG-vaccinated guinea pigs at 5 and 10 weeks postvaccination. Single-cell suspensions were prepared and cultured at 2 × 106 cells/ml for 24 h with culture filtrate protein derived from M. tuberculosis. Cells were then treated for RNA isolation. Data are expressed as the level of induction of cytokine mRNA in relation to the expression of cytokine mRNA in naïve guinea pigs. Each box shows the median and 25th and 75th percentiles. The “whiskers” show the minimum and maximum values. n = 5 guinea pigs per time point.
Cellular analysis of CD4 and CD8 T cells.
We next determined whether guinea pigs vaccinated with BCG were better able to initiate cellular immunity and recruit T cells into infected organs after challenge with virulent M. tuberculosis H37Rv than nonvaccinated animals. The numbers of activated CD4+ (CD4+ CD62Llow) and CD8+ (CD8+ CD62Llow) T cells were examined at days 15, 40, 60, and 120 postinfection in the lungs, spleens, and mediastinal lymph nodes (Table 1). In the lungs, a significantly greater number of total T cells were recruited at day 15 postinfection in BCG-vaccinated animals (P < 0.05). Beyond this time point, no difference was observed between groups in the total number of T cells recruited into the lungs. The kinetics of recruitment of activated CD4+ T cells into the lungs was in general similar between vaccinated and nonvaccinated groups, although a significantly greater number of CD4+ CD62Llow T cells were recruited at day 15 of infection (P < 0.001) in BCG-vaccinated guinea pigs, while a small but not significant increase in the number of CD4+ CD62Llow T cells in the lungs of nonvaccinated guinea pigs occurred at day 40 postinfection (P = 0.05). As infection progressed beyond day 15, there was a trend for pulmonary CD4+ CD62Llow T cells to increase in both groups, even though BCG-vaccinated animals had significantly fewer mycobacteria in their lungs (Fig. 2). Recruitment of activated CD8+ T cells into infected lungs was significantly increased in nonvaccinated animals at day 40 (P < 0.01) postinfection, beyond which increases were observed for both groups as infection persisted. In the spleen, there was no significant difference between groups in the total numbers of recruited cells at each of the time points. The numbers of CD4+ CD62Llow T cells in the spleen were similar in both groups at day 15 postinfection; however, it was significantly elevated at day 40 postinfection in the BCG-vaccinated group (P < 0.05). No significant difference in numbers of spleen CD8+ CD62Llow T cells was observed between groups at all time points examined. In the lymph nodes, the total numbers of cells were significantly increased at day 15 postexposure in the BCG-vaccinated animals (P < 0.05), although the numbers of CD4+ CD62Llow T cells were not significantly elevated in this group in the lymph nodes until days 40, 60, and 120 postexposure (P < 0.05). However, despite an initial increase in CD8+ CD62Llow T cells at day 15, there was no difference in the numbers of these cells in the lymph nodes.
TABLE 1.
Mean numbers of T cells in the lung, spleen, and mediastinal lymph nodes of vaccinated or mock-vaccinated guinea pigs
| Tissue assayed and no. of days postinfection | Mean no. (±SEM) of cells (105) in indicated vaccination groupa |
|||||
|---|---|---|---|---|---|---|
| Total |
CD4+ CD62Llow |
CD8+ CD62Llow |
||||
| Saline | BCG | Saline | BCG | Saline | BCG | |
| Lung | ||||||
| 15 | 33.6 (4.79) | 50.6 (5.98)* | 0.08 (0.01) | 0.24 (0.03)*** | 0.13 (0.03) | 0.38 (0.13) |
| 40 | 55.8 (7.90) | 42.2 (63.5) | 4.07 (1.12) | 1.56 (0.35) | 2.73 (1.12) | 0.37 (0.07)* |
| 60 | 56.6 (14.0) | 49.8 (8.72) | 1.73 (0.63) | 0.87 (0.19) | 1.33 (0.56) | 0.52 (0.14) |
| 120 | 97.4 (20.6) | 111.0 (58.7) | 5.96 (1.52) | 4.37 (2.17) | 4.77 (2.05) | 4.94 (2.99) |
| Spleen | ||||||
| 15 | 301 (37.0) | 368 (49.4) | 15.7 (1.18) | 16.5 (1.84) | 20.1 (2.68) | 16.5 (1.91) |
| 40 | 509 (45.1) | 473 (48.1) | 27.3 (4.36) | 36.7 (2.16)* | 24.3 (4.43) | 17.9 (1.71) |
| 60 | 459 (52.2) | 398 (34.0) | 17.2 (3.20) | 18.2 (1.53) | 16.4 (2.48) | 12.7 (1.30) |
| 120 | 401 (23.0) | 438 (55.0) | 13.0 (1.56) | 16.6 (3.19) | 10.3 (1.89) | 14.9 (3.57) |
| Lymph node | ||||||
| 15 | 7.53 (4.83) | 26.9 (6.96)* | 2.37 (0.95) | 4.45 (1.01) | 1.69 (0.55) | 3.07 (0.43) |
| 40 | 14.9 (2.66) | 12.5 (2.58) | 0.57 (0.17) | 2.33 (0.55)* | 0.32 (0.43) | 0.81 (0.27) |
| 60 | 12.7 (6.08) | 9.36 (1.82) | 0.45 (0.14) | 1.14 (0.29)* | 0.30 (0.01) | 0.55 (0.13) |
| 120 | 2.78 (1.55) | 27.7 (21.2) | 0.57 (0.37) | 4.42 (2.72)* | 0.29 (0.17) | 0.29 (0.12) |
Guinea pigs were vaccinated via the intradermal route with 103 CFU BCG Pasteur or saline 10 weeks prior to infection with a low-dose aerosol of M. tuberculosis H37Rv. Cells from the accessory lobe of the lung, half of the spleen, and all visible mediastinal lymph nodes were stained with monoclonal antibodies that recognize guinea pig pan-T-cell marker, CD4, CD8, and CD62L at the different time points postinfection. n = 8 guinea pigs per group per time point; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
These data suggest that BCG vaccination provided guinea pigs with the capacity to recruit activated CD4+ T cells into the lungs after pulmonary infection sooner than nonvaccinated animals and that BCG vaccination promotes seeding of peripheral lymphoid organs with activated T cells. In contrast, BCG vaccination had little effect on the CD8+ T-cell response during infection, as this was no different and was at times lower than the CD8+ T-cell response in nonvaccinated guinea pigs.
Formation of early pulmonary granulomas.
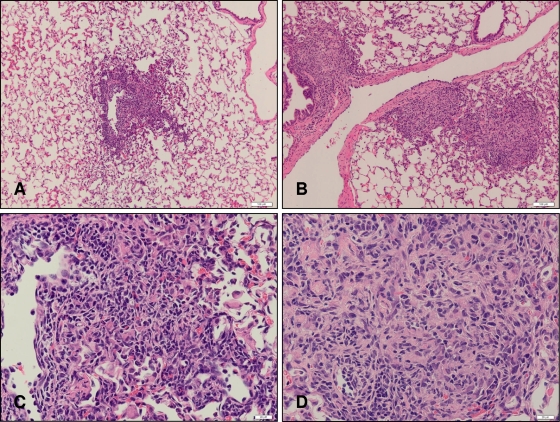
To correlate disease pathology with bacterial burden and fundamental immune responses, we followed granuloma formation during the early phase of infection in hematoxylin-and-eosin-stained sections of the right caudal lung lobe of guinea pigs. It was of particular interest to determine whether BCG-vaccinated guinea pigs were able to form granulomatous lesions at day 15 postinfection, since CFU determinations showed that at this time, animals had already begun to arrest the growth of M. tuberculosis. Based on previous data, we had anticipated that M. tuberculosis was multiplying specifically in the lungs and that there would be limited extrapulmonary dissemination (unpublished results). Furthermore, since bacteria can be recovered from the right caudal lobe, we expected that foci of growing mycobacteria would stimulate the formation of granulomatous lesions (data not shown). Nonvaccinated guinea pigs had discrete foci of cellular accumulations that were scattered throughout the lung, as well as defined lesions in perivascular regions (Fig. 4A). These lesions had a poorly organized appearance and consisted predominantly of mononuclear cells (Fig. 4C). Granulomatous lesions in BCG-vaccinated guinea pigs were also predominantly perivascular; there were more of them (Fig. 4B); and they had greater demarcation and were better organized, with areas of centralized macrophages surrounded by a ring of lymphocytes (Fig. 4D).
FIG. 4.
Representative photomicrographs of lungs from nonvaccinated (A and C) and BCG-vaccinated (B and D) guinea pigs at day 15 postinfection. Lung lobes from guinea pigs at day 15 postinfection were processed and stained with hematoxylin and eosin. Bar = 50 μm.
Analysis of cytokine and EOMES mRNA after pulmonary infection.
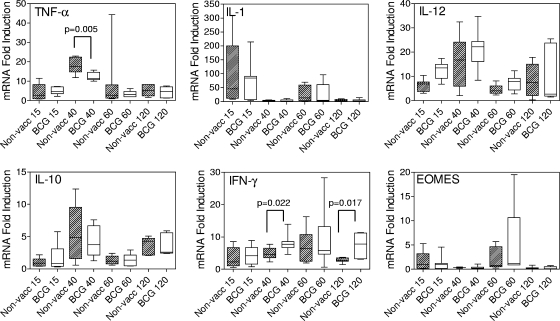
Lung and spleens from infected guinea pigs with and without BCG vaccination were taken at days 15, 40, 60, and 120 postinfection, and the relative expression levels of TNF-α, IL-1, IL-12, IL-10, IFN-γ, and EOMES mRNA determined by real time PCR. The overall expression of each cytokine mRNA response to infection at certain time points seemed broader than at others and was usually due to one or two overresponding guinea pigs in each group. This observation may be attributed to Hartley guinea pigs being an out-bred strain of animals. Nevertheless, the pattern of expression for each cytokine mRNA was quite discernible over the time course of observation and between groups. At day 15 postinfection, there was no significant difference in the expression of cytokines and EOMES in the lungs of animals from the two groups (Fig. 5). The immediate response to infection in both groups was manifested in elevated levels of IL-1 mRNA, which subsided by day 40, while the mRNA expression levels for TNF-α, IL-12, and IL-10 had increased by day 40. Overall, there was a significantly greater increase in TNF-α mRNA expression in the nonvaccinated animals than in the vaccinated group (P = 0.005), indicating a stronger inflammatory response. At day 60 postinfection, IL-1 mRNA expression had increased yet further, while TNF-α, IL-12, and IL-10 mRNA levels had subsided in both groups. Then, similar to day 40 postinfection, at day 120, the levels of IL-1 mRNA decreased, while those of TNF-α, IL-12, and IL-10 increased. Thus, immediately after infection, there was an initial burst of IL-1 mRNA expression in the lungs which then subsided and was replaced by increased TNF-α, IL-12, and IL-10 mRNA levels, and this pattern was repeated at days 60 and 120, respectively. The repeat pattern suggests that macrophages continue to be recruited into sites of infectious foci although the overall growth of M. tuberculosis has reached a plateau, indicating that while the total number of mycobacterial cells that can be recovered from the lung at these time points remains stable, dynamic growth and continued spread of infection occurs (Fig. 2).
FIG. 5.
TNF-α, IL-1β, IL-12, IL-10, IFN-γ, and EOMES mRNA expression profiles in the lungs of guinea pigs at days 15, 40, 60, and 120 postinfection. A lung lobe from each guinea pig was treated for isolation of RNA and subjected to quantitative real-time PCR analysis as described in Materials and Methods. Data are expressed as the level of induction of cytokine mRNA in relation to the expression of cytokine mRNA in naïve guinea pigs. Each box shows the median and 25th and 75th percentiles. The “whiskers” show the minimum and maximum values. n = 8 guinea pigs per group. BCG, BCG vaccinated; non-vacc, nonvaccinated.
For IFN-γ and EOMES, the levels of mRNA expression followed a different course than for IL-1, IL-12, and IL-10. BCG-vaccinated guinea pigs had recruited greater numbers of CD4+ CD62Llow T cells into their lungs at day 15 (Table 1) than nonvaccinated animals, but the expression levels of IFN-γ mRNA did not differ between groups, despite the fact that there were fewer M. tuberculosis bacteria in the lungs of the vaccinated group. The pattern of expression for IFN-γ mRNA differed between groups at days 40 and 120 postinfection. mRNA levels were significantly elevated in the BCG-vaccinated group at day 40 postinfection compared to the levels in nonvaccinated animals (P = 0.022). IFN-γ mRNA levels increased in the nonvaccinated group around day 60 and then declined by day 120, while levels in the BCG-vaccinated group remained significantly elevated (P = 0.017) throughout this time period. This seems to indicate that although by day 120 postinfection, nonvaccinated guinea pigs had the capacity to produce proinflammatory cytokines and recruited T cells into the lungs, these cells were limited in their ability to respond to infection. BCG vaccination had provided an immunological advantage by stimulating T cells that could maintain their effector function. Finally, the mRNA for transcriptional regulator EOMES, used to assess the differentiation state of CD8+ T cells, was elevated in the lungs of both groups at days 15 and 60 postinfection. EOMES mRNA expression followed a different pattern from that of IFN-γ and may reflect the transient expression of this intracellular protein in that once the protein has been produced and used by the cell, it is immediately degraded.
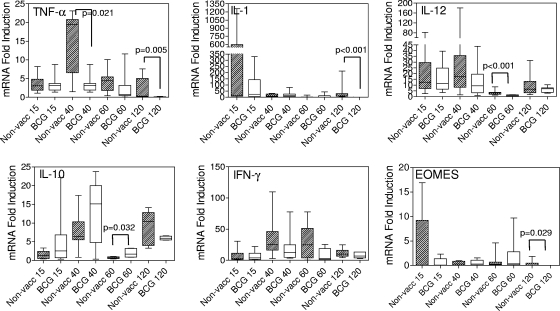
In the spleens of infected guinea pigs, the expression of IFN-γ mRNA in both groups followed a similar trend, and no significant differences were observed at any time point during the study (Fig. 6). EOMES mRNA expression levels were similar between groups at each time point except at day 120, when the nonvaccinated animals showed higher expression levels (P = 0.029). At day 15, two guinea pigs in the nonvaccinated group had relatively high levels of EOMES mRNA. These were the same animals that also showed elevated IL-1 mRNA at this time point. BCG vaccination did not seem to provide guinea pigs with an early immune advantage in the spleen, as was seen in the lung, as the levels of EOMES mRNA remained similar to those of nonvaccinated animals and the bacterial load in the spleen at day 15 was not decreased (Fig. 2). Differences in TNF-α mRNA expression were observed at days 40 (P = 0.021) and 120 (P = 0.005) postinfection, with nonvaccinated guinea pigs producing significantly greater levels. This may be the result of reduced dissemination of M. tuberculosis to the spleens of BCG-vaccinated guinea pigs at the earlier time point. Similarly, IL-1 mRNA expression levels were significantly elevated at day 120 postinfection in the spleens of nonvaccinated guinea pigs (P < 0.001). IL-10 and IL-12 mRNA expression in the spleen followed trends similar to those seen in the lungs, but in the spleen there was significantly greater expression of IL-10 mRNA at day 60 in the BCG-vaccinated group (P = 0.032) and significantly greater expression of IL-12 mRNA in the nonvaccinated group.
FIG. 6.
TNF-α, IL-1β, IL-12, IL-10, IFN-γ, and EOMES mRNA profiles in the spleens of guinea pigs at days 15, 40, 60, and 120 days postinfection. A fragment of the spleen from each guinea pig was treated for isolation of RNA and subjected to quantitative real-time PCR analysis as described in Materials and Methods. Data are expressed as the level of induction of cytokine mRNA in relation to the expression of cytokine mRNA in naïve guinea pigs. Each box shows the median and 25th and 75th percentiles. The “whiskers” show the minimum and maximum values. n = 8 guinea pigs per group. BCG, BCG vaccinated; non-vacc, nonvaccinated.
Taken together, these data suggest that day 40 may be a critical time point at which the progression of disease may be affected. It is suggested that increased expression of IFN-γ relative to TNF-α mRNA prolongs the survival of guinea pigs, while the opposite will cause them to succumb at an earlier time point. Succumbing to infection early may also be due to the limited capacity of T cells from nonvaccinated infected guinea pigs to stimulate increases in IFN-γ mRNA at day 120.
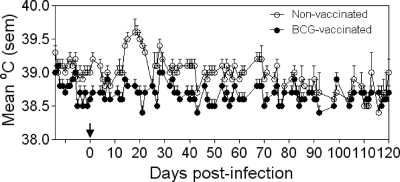
Time course of body temperature postinfection.
Changes in body temperature have long been considered a reliable indicator of the course of progressive M. tuberculosis infection in humans. To assess whether changes in body temperature correlate with immune response, we examined the daily body temperature of guinea pigs throughout the study (Fig. 7). At approximately days 25 to 35 after infection, only nonvaccinated guinea pigs exhibited elevation in body temperature, which mirrored the expression of the inflammatory cytokines IL-1 and TNF-α in the spleens and lungs.
FIG. 7.
Body temperature measurement of guinea pigs after low-dose aerosol infection with virulent M. tuberculosis via implanted temperature-measuring microchips. Animals were monitored daily at approximately the same time of day. n = 16 guinea pigs per group. Arrow indicates day of low-dose aerosol infection (14 days after the initiation of temperature monitoring). Error bars show standard errors of the means (sem).
DISCUSSION
The data presented here show that BCG-vaccinated guinea pigs infected by the pulmonary route with a low dose of M. tuberculosis displayed favorable immune responses within the first 40 days after infection that appeared to prolong survival. By day 15 postinfection, BCG had affected the growth of M. tuberculosis in the lungs by significantly reducing the numbers of recoverable bacterial colonies. By day 15, vaccinated animals were also able to recruit a larger number of activated CD4+ CD62Llow T cells. The elevated levels of IFN-γ and decreased levels of TNF-α mRNA expression at day 40 in vaccinated animals may also indicate a key set point for determining whether infection can be controlled sufficiently to allow prolonged survival. Conversely, at day 120 postinfection, T cells in the lungs of nonvaccinated guinea pigs had reduced capacity to produce IFN-γ mRNA, despite the fact that mRNA levels of immune-enhancing cytokines, such as TNF-α and IL-12, were increasing at this stage.
Overall, our data suggest that BCG affects the growth of M. tuberculosis as early as 15 days postinfection. Our data are supported by those of Smith et al., who examined even earlier time points and showed that during the first 2 weeks of infection with M. tuberculosis, bacteria multiplied at a lower rate in the lungs of vaccinated guinea pigs (30, 33, 34). In our hands, histological analysis indicated that BCG-induced immunity was evidenced as early as 15 days postinfection by the appearance of the first stages of granulomas (areas of loose cell accumulations), as well as more defined and better organized granuloma-like structures in perivascular regions, while nonvaccinated animals had only loose aggregates of cells scattered throughout their lungs (Fig. 4). In association with the organized granulomatous structures, we also observed greater numbers of CD4+ CD62Llow T cells in vaccinated animals, suggesting that adaptive immunity had been generated, which was correlated with a significant decrease in the mycobacterial burden. These data also suggest that vaccination against tuberculosis will result in lung granulomas and that in vaccinated humans, granulomas may need to be formed to limit infection. Thus, in clinical trials for new vaccines, it may be difficult to determine the efficacy of a vaccine based solely on the presence of a granulomatous lesion on a chest X ray.
Despite the early differences in the numbers of pulmonary CD4+ T cells, we did not observe notable differences in the levels of IFN-γ and TNF-α mRNA until day 40, a time at which the numbers of activated CD4+ T cells continued to increase in vaccinated and nonvaccinated animals. Our data differ from those of previous studies by others (15), in which cells from infected guinea pig lungs and spleens were stimulated ex vivo with purified protein derivative. In those studies, there were significant increases in IFN-γ and TNF-α mRNA in nonvaccinated compared to BCG-vaccinated guinea pigs at day 35 postinfection. The difference may be due to the fact that we did not culture cells from infected animals. Conversely, activated CD8+ T cells remained at stable levels only in the vaccinated group. In nonvaccinated guinea pigs, IFN-γ mRNA expression increased up to day 60 and then declined to day 120 postinfection, while mRNA levels in the BCG-vaccinated group were maintained at an elevated level between day 60 and 120, suggesting that BCG vaccination led to the induction of T cells that could maintain effector function. In comparison, the mRNA levels for the predominantly macrophage-derived cytokines IL-1, IL-12, and IL-10 were increased by day 120 postinfection in both groups. These data suggest that guinea pig macrophages were capable of responding to the ongoing M. tuberculosis infection, but T cells were unable to respond efficiently. This suggests that, due to an apparent disconnect between two key cellular immune components thought to be important for productive immune responses to M. tuberculosis infection, nonvaccinated guinea pigs tend to succumb to infection earlier than vaccinated animals.
Studies by Ly et al. examining primary and secondary lung lesions in BCG-vaccinated and nonvaccinated guinea pigs demonstrated that mRNA for transforming growth factor-β and some IFN-γ and IL-12p40 could be detected in primary tubercles from BCG-vaccinated guinea pigs only, while primary lesions from nonvaccinated animals contained predominantly TNF-α mRNA (20). Although our studies were of a broader nature, examining immune responses in the whole lung and spleen, our data concur with those described by Ly et al. in that we documented that nonvaccinated guinea pigs tended to produce higher levels of proinflammatory cytokine mRNAs, while BCG vaccination resulted in increased levels of IFN-γ mRNA during the early stage of infection. Our studies differ from those of Ly et al. in that we examined later time points postinfection and revealed that during the period in which infected guinea pigs tend to succumb to infection, despite the fact that macrophages are primed during this time, T cells seem to be unable to induce IFN-γ mRNA, a key cytokine required for activation of macrophages and killing of M. tuberculosis (8, 11). This reduced ability to induce IFN-γ mRNA was not due to an inability to recruit T cells into the lung, as our data showed that total numbers of CD4+ and CD8+ T cells at day 120 were similar to those observed in BCG-vaccinated animals. To characterize activated CD4+ and CD8+ T-cell subpopulations in the lungs, spleens, and mediastinal lymph nodes, we employed a cross-reactive (human, mouse, and guinea pig) MAb recognizing CD62L (2, 13). CD62L is a key adhesion molecule for T-cell trafficking into lymph nodes. Once T cells encounter their cognate antigen on dendritic cells and become activated, they shed CD62L (19, 38, 40). BCG vaccination resulted in significantly greater recruitment of pulmonary CD4+ CD62Llow T cells soon after infection, corresponding to previous reports showing recruitment of CD4+ T cells expressing the CD45 molecule into infected lungs after vaccination (24). In contrast, the levels of CD8+ CD62Llow T cells were not significantly elevated in vaccinated guinea pigs and may reflect the inability of BCG to induce large numbers of these cells. A similar finding was reported by Jeevan et al. (15), who showed a lower percentage of CD8 T cells in BCG-vaccinated guinea pigs at 5 weeks postinfection. Our observation contrasts with reports by others (24), who showed increases in “activated” CD8 T cells using the CD45 (leukocyte common antigen) marker, and may reflect the relative times at which these two molecules are either upregulated (in the case of CD45) or shed (in the case of CD62L).
IL-1β and TNF-α are known to be pyrogenic cytokines that are produced in response to infection, injury, or immunologic challenge and, at low concentrations, cause fever and hypotension (9). mRNA for these cytokines was detected early during the infection process, with peak IL-1β mRNA expression preceding that of TNF-α and occurring at the same time that we observed an increase in body temperature, between 25 and 35 days postinfection, in nonvaccinated animals. In other studies using the guinea pig as an animal model, others have demonstrated the dependence of the fever response on such cytokines such as IL-1β, TNF-α, and IL-6 (27), and thus, it appears plausible that these cytokines mediate an increase in body temperature during infection with M. tuberculosis.
Taken together, our data provide evidence to suggest that in the guinea pig model, BCG vaccination provides a distinct immunological advantage for the response to M. tuberculosis infection by stimulating early recruitment of activated T cells that facilitate both early and also late-stage responses to infection, resulting in increased survival. Based on these data, to improve the protective immune response to M. tuberculosis infection and disease, novel vaccines would be expected to demonstrate a capacity for this stimulation that is similar to or better than that of BCG. Furthermore, our studies offer a set of immune responses that are easily measured and should be employed when assessing the effects of novel vaccines in the guinea pig model. We conclusively show that no one immune, histological, or microbiological marker is sufficient or universally correlated with survival and that the analysis of a composite of clinical, immunological, and histological data will provide opportunities for more in-depth comparisons of vaccine candidates.
Acknowledgments
Funding for this research was provided by NIH, NIAID (grant no. NO1-AI-40091).
We thank C. Sizemore for critical review of the manuscript.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 8 September 2009.
REFERENCES
- 1.Allen, S. S., L. Cassone, T. M. Lasco, and D. N. McMurray. 2004. Effect of neutralizing transforming growth factor β1 on the immune response against Mycobacterium tuberculosis in guinea pigs. Infect. Immun. 72:1358-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, S. S., and D. N. McMurray. 2003. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect. Immun. 71:4271-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., R. R. Beerli, P. Agnellini, P. Wolint, K. Schwarz, and A. Oxenius. 2006. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur. J. Immunol. 36:842-854. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian, V., E. H. Wiegeshaus, and D. W. Smith. 1994. Mycobacterial infection in guinea pigs. Immunobiology 191:395-401. [DOI] [PubMed] [Google Scholar]
- 5.Basaraba, R. J. 2008. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis (Edinburgh) 88(Suppl. 1):S35-S47. [DOI] [PubMed] [Google Scholar]
- 6.Basaraba, R. J., D. D. Dailey, C. T. McFarland, C. A. Shanley, E. E. Smith, D. N. McMurray, and I. M. Orme. 2006. Lymphadenitis as a major element of disease in the guinea pig model of tuberculosis. Tuberculosis (Edinburgh) 86:386-394. [DOI] [PubMed] [Google Scholar]
- 7.Cho, H., T. M. Lasco, S. S. Allen, T. Yoshimura, and D. N. McMurray. 2005. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect. Immun. 73:1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello, C. A. 2004. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J. Endotoxin Res. 10:201-222. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P. E., and E. Vynnycky. 1998. The effect of heterologous immunity upon the apparent efficacy of (e.g., BCG) vaccines. Vaccine 16:1923-1928. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 13.Fryer, A. D., R. W. Costello, B. L. Yost, R. R. Lobb, T. F. Tedder, D. A. Steeber, and B. S. Bochner. 1997. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J. Clin. Investig. 99:2036-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzo, A., L. Brandt, T. Lasco, A. P. Kipnis, and I. Orme. 2005. NIH pre-clinical screening program: overview and current status. Tuberculosis (Edinburgh) 85:25-28. [DOI] [PubMed] [Google Scholar]
- 15.Jeevan, A., D. L. Bonilla, and D. N. McMurray. 2009. Expression of interferon-γ and tumour necrosis factor-α messenger RNA does not correlate with protection in guinea pigs challenged with virulent Mycobacterium tuberculosis by the respiratory route. Immunology 128:e296-e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeevan, A., T. Yoshimura, G. Foster, and D. N. McMurray. 2002. Effect of Mycobacterium bovis BCG vaccination on interleukin-1β and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect. Immun. 70:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeevan, A., T. Yoshimura, K. E. Lee, and D. N. McMurray. 2003. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infect. Immun. 71:354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft, S. L., D. Dailey, M. Kovach, K. L. Stasiak, J. Bennett, C. T. McFarland, D. N. McMurray, A. A. Izzo, I. M. Orme, and R. J. Basaraba. 2004. Magnetic resonance imaging of pulmonary lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect. Immun. 72:5963-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley, K., and G. S. Kansas. 2004. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4:325-335. [DOI] [PubMed] [Google Scholar]
- 20.Ly, L. H., M. I. Russell, and D. N. McMurray. 2008. Cytokine profiles in primary and secondary pulmonary granulomas of guinea pigs with tuberculosis. Am. J. Respir. Cell Mol. Biol. 38:455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2004. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 84:283-292. [DOI] [PubMed] [Google Scholar]
- 22.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurray, D. N., S. S. Allen, A. Jeevan, T. Lasco, H. Cho, T. Skwor, T. Yamamoto, C. McFarland, and T. Yoshimura. 2005. Vaccine-induced cytokine responses in a guinea pig model of pulmonary tuberculosis. Tuberculosis (Edinburgh) 85:295-301. [DOI] [PubMed] [Google Scholar]
- 24.Ordway, D., M. Henao-Tamayo, C. Shanley, E. E. Smith, G. Palanisamy, B. Wang, R. J. Basaraba, and I. M. Orme. 2008. Influence of Mycobacterium bovis BCG vaccination on cellular immune response of guinea pigs challenged with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 15:1248-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordway, D., G. Palanisamy, M. Henao-Tamayo, E. E. Smith, C. Shanley, I. M. Orme, and R. J. Basaraba. 2007. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J. Immunol. 179:2532-2541. [DOI] [PubMed] [Google Scholar]
- 26.Pearce, E. L., A. C. Mullen, G. A. Martins, C. M. Krawczyk, A. S. Hutchins, V. P. Zediak, M. Banica, C. B. DiCioccio, D. A. Gross, C. A. Mao, H. Shen, N. Cereb, S. Y. Yang, T. Lindsten, J. Rossant, C. A. Hunter, and S. L. Reiner. 2003. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 302:1041-1043. [DOI] [PubMed] [Google Scholar]
- 27.Roth, J., D. Martin, B. Storr, and E. Zeisberger. 1998. Neutralization of pyrogen-induced tumour necrosis factor by its type 1 soluble receptor in guinea-pigs: effects on fever and interleukin-6 release. J. Physiol. 509(Pt. 1):267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skeiky, Y. A., and J. C. Sadoff. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469-476. [DOI] [PubMed] [Google Scholar]
- 29.Skwor, T. A., H. Cho, C. Cassidy, T. Yoshimura, and D. N. McMurray. 2004. Recombinant guinea pig CCL5 (RANTES) differentially modulates cytokine production in alveolar and peritoneal macrophages. J. Leukoc. Biol. 76:1229-1239. [DOI] [PubMed] [Google Scholar]
- 30.Smith, D. W., J. S. Fok, R. S. Ho, G. E. Harding, E. Wiegeshaus, and P. K. Arora. 1975. Influence of BCG vaccination on the pathogenesis of experimental airborne tuberculosis. J. Hyg. Epidemiol. Microbiol. Immunol. 19:407-417. [PubMed] [Google Scholar]
- 31.Smith, D. W., G. B. Fregnan, L. Delaquerriere, and E. Valdivia. 1964. Induction of acquired resistance in guinea pigs with defatted Mycobacterium Tuberculosis vaccines. J. Bacteriol. 88:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, D. W., and G. E. Harding. 1977. Animal model of human disease. Pulmonary tuberculosis. Animal model: experimental airborne tuberculosis in the guinea pig. Am. J. Pathol. 89:273-276. [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, D. W., D. N. McMurray, E. H. Wiegeshaus, A. A. Grover, and G. E. Harding. 1970. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am. Rev. Respir. Dis. 102:937-949. [DOI] [PubMed] [Google Scholar]
- 34.Smith, D. W., E. Wiegeshaus, R. Navalkar, and A. A. Grover. 1966. Host-parasite relationships in experimental airborne tuberculosis. I. Preliminary studies of BCG-vaccinated and nonvaccinated animals. J. Bacteriol. 91:718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, D. W., and E. H. Wiegeshaus. 1989. What animal models can teach us about the pathogenesis of tuberculosis in humans. Rev. Infect. Dis. 11(Suppl. 2):S385-S393. [DOI] [PubMed] [Google Scholar]
- 36.Steeber, D. A., P. Engel, A. S. Miller, M. P. Sheetz, and T. F. Tedder. 1997. Ligation of L-selectin through conserved regions within the lectin domain activates signal transduction pathways and integrin function in human, mouse, and rat leukocytes. J. Immunol. 159:952-963. [PubMed] [Google Scholar]
- 37.Tan, B. T., F. Ekelaar, J. Luirink, G. Rimmelzwaan, A. J. De Jonge, and R. J. Scheper. 1985. Production of monoclonal antibodies defining guinea pig T-cell surface markers and a strain 13 Ia-like antigen: the value of immunohistological screening. Hybridoma 4:115-124. [DOI] [PubMed] [Google Scholar]
- 38.Tedder, T. F., A. C. Penta, H. B. Levine, and A. S. Freedman. 1990. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J. Immunol. 144:532-540. [PubMed] [Google Scholar]
- 39.Turner, O. C., R. J. Basaraba, and I. M. Orme. 2003. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect. Immun. 71:864-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Andrian, U. H., and C. R. Mackay. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020-1034. [DOI] [PubMed] [Google Scholar]
- 41.Weir, R. E., P. Gorak-Stolinska, S. Floyd, M. K. Lalor, S. Stenson, K. Branson, R. Blitz, A. Ben-Smith, P. E. Fine, and H. M. Dockrell. 2008. Persistence of the immune response induced by BCG vaccination. BMC Infect. Dis. 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. 2004. BCG vaccine. WHO position paper. Wkly. Epidemiol. Rec. 79:27-38. [PubMed] [Google Scholar]
- 43.Yamada, H., T. Udagawa, S. Mizuno, K. Hiramatsu, and I. Sugawara. 2005. Newly designed primer sets available for evaluating various cytokines and iNOS mRNA expression in guinea pig lung tissues by RT-PCR. Exp. Anim. 54:163-172. [DOI] [PubMed] [Google Scholar]