Abstract
Infertility in men and women is frequently associated with genital contamination by various commensal or uropathogenic microbes. Since many microorganisms are known to release quorum-sensing signals in substantial amounts, we raised the question whether such molecules can directly affect human spermatozoa. Here we show that farnesol and 3-oxododecanoyl-l-homoserine lactone, employed by the opportunistic pathogenic yeast Candida albicans and the gram-negative bacterium Pseudomonas aeruginosa, respectively, induce multiple damage in spermatozoa. A reduction in the motility of spermatozoa coincided in a dose-dependent manner with apoptosis and necrosis at concentrations which were nondeleterious for dendritic cell-like immune cells. Moreover, sublethal doses of both signaling molecules induced premature loss of the acrosome, a cap-like structure of the sperm head which is essential for fertilization. Addressing their mechanism of action, we found that the bacterial molecule, but not the fungal molecule, actively induced the acrosome reaction via a calcium-dependent mechanism. This work uncovers a new facet in the interaction of microorganisms with human gametes and suggests a putative link between microbial communication systems and host infertility.
The phenomenon of quorum sensing (QS) has gained intensive attention not only in the study of microbial communication within defined bacterial populations but also in the study of interkingdom signaling and pathogenicity. QS is defined as a means for microorganisms to sense their population density via the release of signaling molecules, to which they in turn respond. Reaching a threshold concentration in a bacterial population, these molecules can coordinately regulate a multitude of different effects, such as bioluminescence, biofilm formation, and virulence gene expression. Different QS molecules, such as the autoinducing peptides or N-acylhomoserine lactones, have been described in the past for many bacterial species (17, 40).
A eukaryotic QS system was first evidenced in Candida albicans, a major human fungal pathogen and frequent commensal of the gastrointestinal and genitourinary tracts (10, 23). The isoprenoid alcohol farnesol was shown to inhibit the transition of C. albicans yeast cells to filamentous growth forms, and an opposite effect was recorded for tyrosol, another QS molecule in this fungus (5). Both these molecules are detectable in the supernatants of dense C. albicans yeast cell cultures in micromolar amounts. Recent observations indicate that the effects of farnesol are much more complex, since physiological farnesol concentrations of approximately 35 μM favor stress resistance in C. albicans (38) and even support antagonistic properties on other microbes. For example, farnesol was shown to induce apoptosis in the filamentous fungus Aspergillus nidulans (29) and to inhibit biofilm formation in the bacterial pathogen Staphylococcus aureus (13). There has also been particular interest in the impact of QS molecules on human cells, because evidence suggests implications in immunomodulation and the proliferation of distinct immune and malignant cells (11, 22, 26).
Genital infections caused by various microbial pathogens are frequently associated with infertility in men and women worldwide (24). Little attention has been paid, however, to potential direct influences of commensal or pathogenic microorganisms on human gametes, and therefore their interaction with human spermatozoa remains largely elusive. Nevertheless, adverse effects of microbes on sperm could be observed during in vitro coincubation experiments with uropathogenic bacteria and yeasts (12), even in the presence of cell-free supernatants of C. albicans cultures (37). Since QS molecules are expected to be released by microorganisms in substantial amounts in vitro as well as in the human host, we raised for the first time the question whether such molecules can directly affect human spermatozoa. To address this possibility, we monitored the impact of selected QS molecules on sperm parameters which are crucial for fertilization. Here we studied not only the impact of C. albicans farnesol but also that of 3-oxododecanoyl-l-homoserine lactone (3-oxo-C12-HSL), which is employed by the gram-negative, opportunistic pathogenic bacterium Pseudomonas aeruginosa, a frequent inducer of urinary tract infections (34). These studies revealed that distinct microbial QS molecules elicit multiple detrimental effects on human spermatozoa. They not only can impair sperm motility but also can induce spermatozoal cell death and, most notably, at sublethal doses can cause premature acrosome loss, a phenotype which is known to prevent the penetration of the oocyte by the sperm (19).
MATERIALS AND METHODS
Human spermatozoa, C. albicans strains, chemicals, and culture conditions.
Human semen from healthy donors, provided with informed consent and local ethics committee approval, were analyzed according to WHO guidelines (39). In some of the experiments the isolated spermatozoa were pooled, and in other cases they were derived from individual donors. The observed effects were consistent despite the identity of the donor or whether they were derived from a single donor or from a pool. Sperm motility was assessed microscopically by scoring the percentage of progressive motile (A+B), nonprogressive (C), and immotile (D) spermatozoa (39). Progressive motile sperm were recovered by a two-step pure sperm gradient (Nidacon) and adjusted to 1 × 108/ml sperm preparation medium (MediCult). To monitor sperm motility in the presence of QS molecules, 4 × 106 sperm were incubated with different concentrations of farnesol (Fluka), tyrosol (Fluka), or 3-oxo-C12-HSL (Cayman) in 100 μl of SynVitroFlush medium (MediCult). Control samples were incubated with the solvent as recommended by the manufacturers. Sterile filtered supernatant aliquots of the C. albicans wild-type-strain SC5314 (8) and the Δtup1 mutant Bca2-10 (2) were obtained from cultures grown in synthetically defined medium (6.7 g yeast nitrogen base with ammonium sulfate [MP Biomedicals] and 20 g glucose per liter) for 48 h at 30°C. Thirty-microliter C. albicans supernatant aliquots were mixed with 4 × 106 sperm in a volume of 100 μl SynVitroFlush medium.
Sperm assays for the evaluation of spermatozoal membrane integrity, acrosome status, and DNA fragmentation.
The hypoosmotic swelling test (14) was used to classify spermatozoa as osmotically intact by tail swelling. Briefly, the swelling test was performed by diluting 20 μl of sperm suspension (2 × 106 sperm) with 200 μl of hypoosmotic solution (7.35 g sodium citrate and 13.51 g fructose in 1 liter of distilled water). After incubation for 60 min at 37.5°C with 5.7% CO2, spermatozoa were centrifuged for 5 min at 900 × g. The pellet was resuspended in 20 μl hypoosmotic solution and smeared on a glass slide, and subsequently 200 spermatozoa were scored. Spermatozoa were classified as osmotically intact if tail swelling was observed. Spermatozoa were classified as osmotically incompetent if a straight tail was observed (14). Acrosome staining with fluorescein isothiocyanate (FITC)-Pisum sativum agglutinin (PSA) (Sigma) was used to identify intact acrosomes as follows. An aliquot of 20 μl of sperm suspension (2 × 106 sperm) was centrifuged for 5 min at 900 × g, resuspended in 2-μg/ml Hoechst solution, and incubated for 10 min in the dark. The sperm suspension was centrifuged again to remove excess stain, and the sperm pellet was resuspended in 20 μl SynVitroFlush medium. Twenty-microliter droplets of the sperm suspension were smeared on glass slides and allowed to dry. The slides were fixed in ice-cold methanol for 30 s. After drying, the fixed sperm cells were incubated for 30 min with 30 μl of 50 μg/ml FITC-PSA (Sigma) in water. After washing in water and mounting with Moviol, acrosome staining was classified as follows: intact acrosomes displayed a uniform green fluorescence in the acrosomal region of the sperm head, whereas acrosome loss was indicated by absent fluorescence or equatorial segment staining. A total of 200 spermatozoa were scored per sample. To monitor the effect of farnesol and 3-oxo-C12-HSL on acrosome loss under conditions of low calcium levels, SynVitroFlush medium with 5 mM EDTA was used. A 10 μM concentration of the calcium ionophor calcimycin (A23187) (Sigma), dissolved in dimethyl sulfoxide, was used as a positive control to induce the acrosomal reaction in spermatozoa. The Halosperm kit (Halotech DNA), a sperm chromatin dispersion test, was used to detect DNA fragmentation in sperm as follows. Aliquots of 25 μl of sperm suspension (2.5 × 106 cells) were mixed with 50 μl of low-melting-point agarose at 37°C. Fifty microliters of the mixture was pipetted onto a glass slide provided with the Halosperm kit, covered with a glass coverslip, and left to dry at 4°C for 5 min. Glass coverslips were removed carefully, and slides were immersed horizontally in a tray with freshly prepared denaturant solution for 7 min at room temperature and then transferred in a tray with lysis solution for 25 min at room temperature. After washing with water, slides were dehydrated in 70%, 90%, and 100% ethanol (2 min for each step) and allowed to dry. Sperm cells were stained with Wright's stain and classified by bright-field microscopy. Five hundred spermatozoa per sample were scored. Spermatozoa without DNA fragmentation show a large halo, which is absent or very small in spermatozoa with fragmented DNA (6).
Preparation of human DCs.
Human monocyte-derived dendritic cells (DCs) were obtained from peripheral blood mononuclear cells by a standard protocol (27). In brief, after dilution with 50 ml phosphate-buffered saline-0.1 M citrate, blood was distributed over a density gradient (leukocyte separation medium; PAA Laboratories) and centrifuged at 400 × g for 30 min at room temperature. Monocytes were collected from the interface; washed with phosphate-buffered saline; resuspended in RPMI 1640 medium (PAA Laboratories) supplemented with 10% fetal bovine serum, 2 mM glutamine, and 50 μg/ml gentamicin; and incubated for 1 h at 37°C with 5.7% CO2 on plastic cell culture dishes (Greiner). The nonadherent cells were removed after 1 h. The adherent fractions (monocytes) were cultured for 2 to 3 days in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 50 μg/ml gentamicin, 20 U/ml granulocyte-macrophage colony-stimulating factor, and 16 U/ml interleukin-4. Cytokines and medium were replaced every second day. The purity of immature DCs (iDCs) was indicated by fluorescence-activated cell sorter (FACS) analysis using HLA-DR antibody.
FACS analysis.
FACS analyses detected apoptotic and necrotic sperm and iDCs by use of the annexin V-FITC kit (Bender Medsystems). In brief, an aliquot of 2 × 106 cells was incubated with 195 μl of prediluted binding buffer and 5 μl of annexin V-FITC. The cells were incubated in the dark at room temperature for 10 min. The suspension was centrifuged at 900 × g for 5 min and resuspended in 190 μl prediluted binding buffer with 10 μl propidium iodide. The cells were detected using log forward- and log side-scatter dot plots and density plots. FACS analysis was performed by use of a FACScan 2.0 cytometry system equipped with an argon laser emitting at 488 nm (Becton Dickinson). The fluorescence was measured on the FL1 fluorescence channel equipped with a 530-nm band-pass filter. Ten thousand cells were analyzed per sample and counted at low flow rate. Fluorescence data were collected by using logarithmic amplifiers, and forward scatter data were collected using linear amplifiers. The mean fluorescence values were determined with CellQuest 33 (Becton Dickinson) software.
Microscopy.
Fluorescence microscopy was performed with a Zeiss LSM 510 inverted confocal laser scanning microscope equipped with a Zeiss Axiovert 100 microscope. Imaging scans were acquired with an argon laser with a wavelength of 488 nm and corresponding filter settings for FITC and parallel transmission images. The cells were observed with a ×63 immersion oil objective. Light microscopy was performed with an Olympus IX 51 microscope equipped with an Octax Eye USB2 camera. The cells were observed with a ×40 achromat objective. The imaging software was Octax EyeWare Mx.
Statistics.
All data were expressed as the mean ± standard deviation (SD). Differences were analyzed by the two-tailed unpaired Student t test. In all analyses, a P value of <0.05 was considered statistically significant.
RESULTS
A secretory factor from C. albicans culture supernatants reduces sperm motility.
Using an in vitro model of freshly isolated, highly motile human spermatozoa, we first investigated whether sperm motility is affected by soluble factors produced by C. albicans yeast cell cultures. For this purpose, the motility of spermatozoa was monitored in the presence of supernatant aliquots of the widely used C. albicans strain SC5314 grown in synthetically defined medium for 48 h. Motility analysis indicated a time-dependent loss of progressive motility for spermatozoa incubated with C. albicans supernatant (Fig. 1A). As a control, spermatozoa were incubated with equal amounts of the growth medium. From these results we hypothesized that C. albicans QS molecules such as farnesol or tyrosol could be potential candidates which might add to the inhibitory effect of C. albicans cultures on sperm motility. Farnesol appeared to be the more likely candidate, since this molecule is known to induce apoptosis in different carcinoma cells (15, 28). We also tested the supernatant of C. albicans strain Bca2-10, a Δtup1 deletion mutant which was recently shown to produce larger amounts of farnesol in the culture supernatant than wild-type cells (16). A stronger inhibition of progressive sperm motility was detected for the supernatant of the C. albicans Δtup1 mutant than for the wild-type control filtrate (Fig. 1A). However, the culture supernatants are probably rather complex and might contain factors other than QS molecules which could inhibit sperm motility. Therefore, we next studied a possible impact of pure farnesol and tyrosol on this phenotype.
FIG. 1.
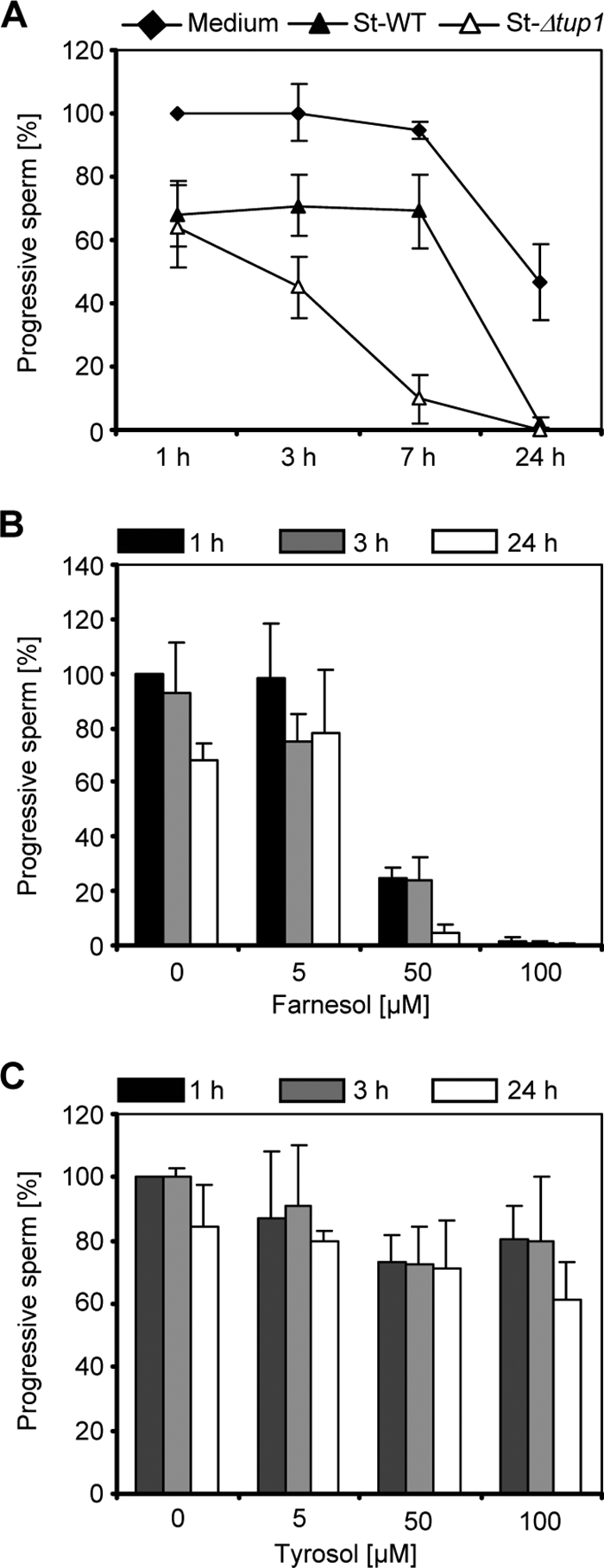
Influence of C. albicans supernatant and farnesol on sperm motility. (A) Highly motile human spermatozoa were incubated in SynVitroFlush medium for 1 h, 3 h, 7 h, and 24 h with the sterile-filtered supernatants of C. albicans growth cultures of the wild type (St-WT) or the C. albicans Δtup1 mutant (St-Δtup1). As a control, spermatozoa were incubated with aliquots of the C. albicans growth medium. The percentage of progressive spermatozoa is reduced in a time-dependent manner in the presence of C. albicans wild-type culture supernatant, and sperm motility is even more decreased in the presence of supernatant of the C. albicans Δtup1 mutant. Progressive spermatozoa in the medium control after 1 h were set to 100%. The results are the means ± SDs from at least three independent experiments. (B and C) The percentage of progressive spermatozoa was determined in the presence of different concentrations of farnesol (B) and tyrosol (C) after incubation for 1 h, 3 h, and 24 h. Increasing concentrations of farnesol, but not tyrosol, inhibit the motility of spermatozoa. Progressive spermatozoa in the medium control (plus solvent) after 1 h were set to 100%. The results are the means ± SDs from at least three independent experiments.
The C. albicans QS molecule farnesol impairs sperm motility.
Increasing concentrations of farnesol were analyzed for a possible effect on sperm motility at different time points, i.e., 1, 3, and 24 h. The degree of motility inhibition positively correlated with the concentration of farnesol, with a dramatic effect at a 50 μM concentration of the molecule already after 1 h of incubation (Fig. 1B). In contrast, no reduction of progressive sperm motility was seen in the presence of tyrosol under the tested conditions (Fig. 1C).
Farnesol induces membrane damage in human spermatozoa.
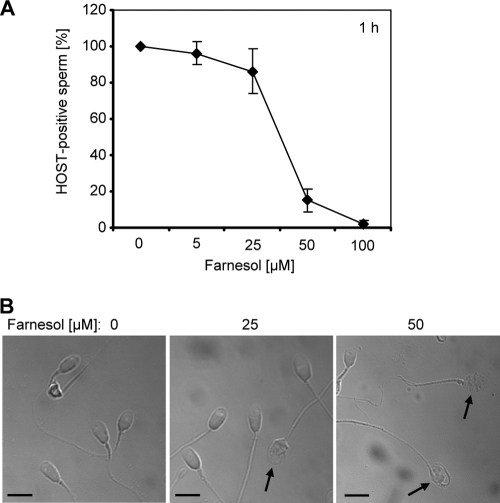
The observed adverse impact of farnesol on spermatozoal motility did not provide evidence about sperm viability. In order to detect viable spermatozoa among nonmotile semen samples, we first employed the hypoosmotic swelling test, which is used in the in vitro fertilization laboratory as an indicator of intact sperm plasma membranes. The assay detected a loss of membrane integrity in the majority of spermatozoa after exposure to 50 μM farnesol for 1 h (Fig. 2A). This result supported the view that the farnesol-induced reduction in motility coincides with spermatozoal membrane damage and killing. Consistently, light microscopic inspection revealed a complete membrane rupture of several sperm heads under these conditions, which was already apparent after 1 hour of treatment with only 25 μM farnesol (Fig. 2B). Control spermatozoa did not reveal significant morphological alterations compared to farnesol-treated samples.
FIG. 2.
Farnesol induces membrane damage in spermatozoa. (A) Spermatozoa were incubated for 1 h in the presence of increasing farnesol concentrations. The percentage of viable spermatozoa with intact cell membranes was determined by use of the hypoosmotic swelling test (HOST). The test is positive only in intact sperm, as indicated by tail swelling in hypoosmotic solution. Farnesol-induced membrane destruction was revealed by the decreased percentage of HOST-positive sperm (control spermatozoa plus solvent were set to 100%). The results are the means ± SDs from at least three independent experiments. (B) Light microscopy after 1 h of incubation with farnesol revealed that the sperm morphology is altered in the presence of increasing concentrations of the molecule (arrows) (scale bars represent 5 μm).
The farnesol-induced spermatozoal membrane damage is a consequence of apoptosis and necrosis.
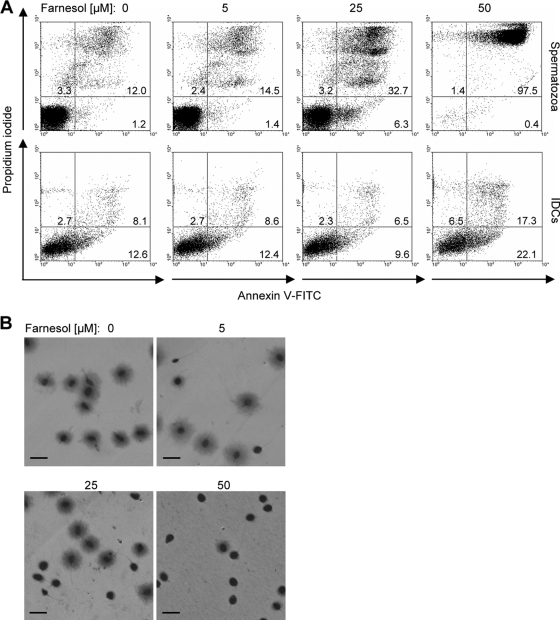
The observed cytotoxic effect on sperm could be a consequence of an apoptosis-like cell death. To investigate this possibility, apoptotic spermatozoa were identified via binding of annexin V-FITC to externalized phosphatidylserine, whereas necrotic cells were visualized with propidium iodide. In the presence of 25 μM farnesol for 3 h, a considerable proportion of necrotic spermatozoa was detected by flow cytometry (32.7%), in contrast to the control (12.0%) (Fig. 3A). Under these conditions, a small yet considerable proportion of spermatozoa (6.3%) was identified to be apoptotic. Almost the entire population of the sperm sample incubated with 50 μM farnesol for 3 h was identified to be necrotic. Addressing the question whether this fast-acting deleterious effect of farnesol is specific for spermatozoa, we also tested human iDCs, which are involved in the defense against cervix-invading pathogens (7). As indicated in the lower panel of Fig. 3A, a strong induction of apoptosis and/or necrosis could not be detected in iDCs under the tested conditions. Because apoptosis and necrosis are assumed to coincide with other cellular alterations (33), we also analyzed whether farnesol induces spermatozoal DNA fragmentation. The assay used identifies spermatozoa with intact DNA by a halo of dispersed DNA loops, which is not visible in sperm with fragmented DNA. After 1 h of treatment with 25 μM farnesol, only 50% of the spermatozoal heads showed an extended halo of DNA (Fig. 3B), indicating a strong impact of farnesol on DNA fragmentation and supporting its role as a trigger of apoptosis. Notably, spermatozoa with fragmented DNA were already detected after treatment with 5 μM farnesol.
FIG. 3.
Farnesol induces apoptosis, necrosis, and DNA fragmentation in spermatozoa. (A) Spermatozoa were treated for 3 h with increasing concentrations of farnesol. Viable, apoptotic, and necrotic spermatozoa were quantified by flow cytometry using the annexin V-FITC/propidium iodide assay. Representative dot plots demonstrate the dose-dependent increase of apoptotic and necrotic sperm in the presence of farnesol. The detrimental effect of farnesol on spermatozoa is much stronger than in iDCs, which were used as a control (lower panel). (B) Spermatozoa were treated for 1 h with increasing concentrations of farnesol. Light microscopy revealed farnesol-induced DNA fragmentation by use of an assay which detects spermatozoa with intact DNA by a halo of dispersed DNA loops. The number of spermatozoa with fragmented DNA increased in a dose-dependent manner, as indicated by the small or absent halo around the sperm head (scale bars represent 10 μm).
The sperm acrosome displays enhanced sensitivity to farnesol.
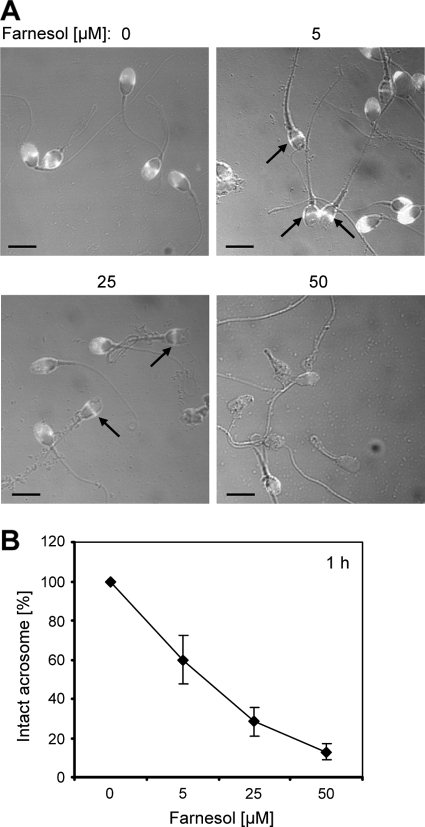
Sperm viability and motility are necessary but not sufficient parameters for successful fertilization. Premature acrosome loss and/or acrosome reaction failure are also important causes of male infertility. In the next experiment, the proportion of intact sperm acrosomes was measured after 1 h of incubation in the presence of farnesol. Here, only those acrosomes which showed a complete positive FITC-conjugated PSA staining were considered to be intact. Acrosome loss was already apparent in a considerable proportion of sperm treated with sublethal doses of 5 μM farnesol (Fig. 4), indicating an enhanced sensitivity of the sperm acrosome to farnesol.
FIG. 4.
Farnesol treatment results in acrosome loss in spermatozoa. (A) Spermatozoa were treated for 1 h with increasing concentrations of farnesol. The sperm acrosomes were detected by fluorescence microscopy using FITC-PSA, which binds to intact acrosomal membranes. Intact acrosomes were identified by a uniform fluorescence in the acrosomal region of the sperm head, whereas acrosome loss was revealed by absent fluorescence or equatorial segment staining (arrows). Note that sperm with defective acrosomes are already visible after treatment with 5 μM farnesol (scale bars represent 5 μm). (B) The proportion of sperm with intact acrosomes after 1 h of incubation with farnesol decreases in a dose-dependent manner. The percentage of spermatozoa with intact acrosomes in the medium controls plus solvent was set to 100%. Results are the means ± SDs from at least three independent experiments.
Apoptosis and acrosome loss in spermatozoa is also induced by a bacterial N-acylhomoserine lactone QS molecule.
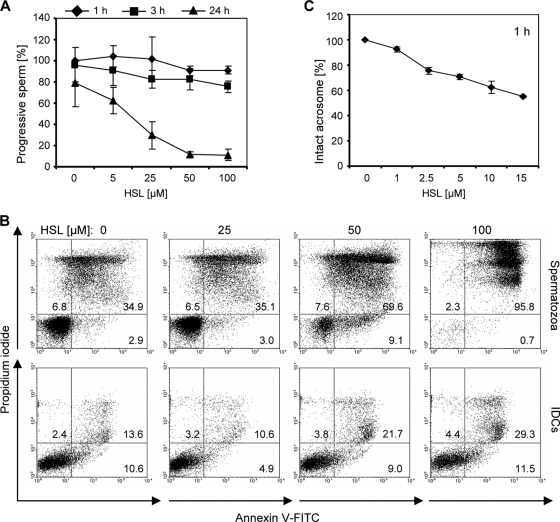
Given that microbial signaling is also assumed to play a key role in host adaptation processes in bacteria, we also investigated whether bacterial QS molecules affect sperm function. As an example, we monitored a potential effect of the widely studied 3-oxo-C12-HSL secreted by P. aeruginosa (26). In the motility assay used, increasing concentrations of 3-oxo-C12-HSL did not significantly affect the progressive motility of spermatozoa after 1 h and 3 h; however, after 24 h a strong reduction of sperm motility was observed for concentrations of ≥25 μM (Fig. 5A). At this time point, a strong increase of apoptotic and necrotic cells in the presence of a 50 μM concentration of the molecule was revealed by flow cytometry (Fig. 5B). Similar to our results obtained with farnesol, the deleterious effect of 3-oxo-C12-HSL was more pronounced on spermatozoa than on iDCs. Nevertheless, treatment with 50 and 100 μM 3-oxo-C12-HSL for 24 h resulted in a detectable proportion of necrotic iDCs. In comparison to the impact on sperm motility and viability, an enhanced adverse effect of 3-oxo-C12-HSL on sperm acrosomes was detected, which was seen already after 1 h of incubation at low concentrations of 1 to 5 μM (Fig. 5C).
FIG. 5.
Adverse influence of 3-oxo-C12-HSL on sperm function and viability. (A) Spermatozoa were incubated with increasing concentrations of 3-oxo-C12-HSL for 1 h, 3 h, and 24 h, and the percentage of progressive motile spermatozoa was determined. The motility of spermatozoa is reduced by increasing concentrations of 3-oxo-C12-HSL after 24 h. The percentage of spermatozoa with progressive motility in the medium control (plus solvent) after 1 h is defined as 100%. The results are the means ± SDs from at least three independent experiments. (B) Spermatozoa and control iDCs were incubated for 24 h with increasing concentrations of 3-oxo-C12-HSL. Viable, apoptotic, and necrotic spermatozoa and iDCs were quantified by flow cytometry using annexin V-FITC and propidium iodide, which detect apoptosis and necrosis, respectively. Representative dot plots demonstrate the stronger decrease of viable spermatozoa treated with 3-oxo-C12-HSL compared to iDCs. (C) Spermatozoa were treated for 1 h with increasing concentrations of 3-oxo-C12-HSL, and the percentage of sperm with intact acrosomes was detected by fluorescence microscopy using FITC-PSA. At this time point, when sperm motility was not yet detectably impaired, the presence of 2.5 μM 3-oxo-C12-HSL already resulted in a detectable proportion of sperm with absent acrosomes. The percentage of spermatozoa with intact acrosomes in the medium control (plus solvent) was set to 100%. The results are the means ± SDs from at least three independent experiments.
3-oxo-C12-HSL specifically induces the acrosome reaction via a calcium-dependent mechanism.
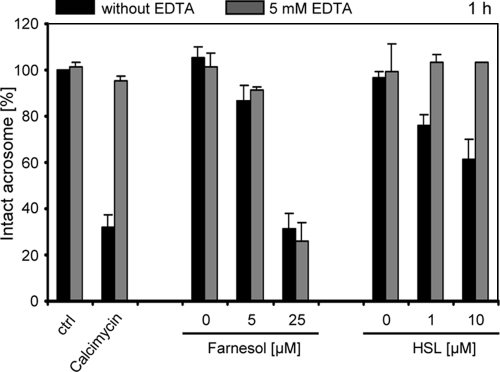
We hypothesized that the acrosome loss detected in the presence of farnesol and 3-oxo-C12-HSL could be either a result of unspecific membrane damage in the acrosomal region of the sperm head or a consequence of an actively induced acrosome reaction. To investigate the latter possibility, the effect of sublethal doses of the QS molecules on sperm acrosomes was monitored in the presence of a calcium chelator. This experiment is based on the knowledge that the oocyte-induced acrosomal reaction is mediated by increased levels of free intracellular calcium in the sperm cytoplasm and is specifically prohibited in the absence of extracellular calcium. The proportion of intact sperm acrosomes was measured after 1 h of incubation in the presence of 5 mM EDTA at increasing concentrations of farnesol or 3-oxo-C12-HSL (Fig. 6). As a positive control, spermatozoa were treated under the same conditions with calcimycin, a known inducer of the acrosome reaction. As expected, the presence of calcimycin reduced the proportion of spermatozoa with intact acrosomes, an effect which was abrogated in the presence of EDTA. Notably, the effect of 3-oxo-C12-HSL on sperm acrosomes was also abolished in the presence of the calcium chelator, suggesting that this molecule also actively induces the acrosome reaction in human spermatozoa via a calcium-dependent mechanism. In contrast, the detrimental effect of farnesol on sperm acrosomes was observed irrespective of the presence of the calcium chelator (Fig. 6).
FIG. 6.
The 3-oxo-C12-HSL-induced acrosome loss depends on calcium. Spermatozoa were incubated with increasing concentrations of farnesol or 3-oxo-C12-HSL for 1 h in SynVitroFlush medium with or without 5 mM EDTA. As a positive control for the calcium-induced acrosome reaction, spermatozoa were incubated in the presence of the calcium ionophor calcimycin. The percentage of sperm with intact acrosomes was determined by FITC-PSA staining, and this was set to 100% for the untreated control (ctrl). Note that the calcimycin- and 3-oxo-C12-HSL-induced acrosome loss was strongly reduced in the presence of 5 mM EDTA, in contrast to the effect of farnesol. The results are the means ± SDs from at least three independent experiments.
DISCUSSION
Altered sperm phenotypes such as reduced motility, acrosome loss, or DNA fragmentation can promptly lead to fertilization failure; e.g., the proportion of sperm with fragmented DNA is negatively correlated with pregnancy, which was determined only for semen samples with less than 30% of fragmentation-positive sperm (18). A first hint that secreted factors of C. albicans might be harmful for spermatozoa was described by Tian and coworkers (37), who demonstrated spermatozoal impairment by C. albicans culture supernatants. Our present findings support this observation and attribute a specific antispermatozoal role to one of the known secreted C. albicans QS molecules, i.e., farnesol, a nonsterol isoprenoid, which is also a catabolite of the cholesterol biosynthetic pathway. We found that a loss of progressive sperm motility in the presence of farnesol coincided with apoptosis and necrosis at molecule concentrations of ≥25 μM, whereas DNA fragmentation and acrosome loss were detected at even lower concentrations. A farnesol concentration of 50 μM induced necrosis in almost the entire sperm population tested. Our results therefore indicate a considerable sensitivity of spermatozoa to the molecule, a finding which is supported by the observation that similar deleterious effects of farnesol were not detected in immature dendritic control cells. However, farnesol was previously shown to induce apoptosis in other fungal organisms and, interestingly, also specifically in distinct human carcinoma cell lines at concentrations of approximately 60 to 250 μM (15, 28). The antitumor activity of farnesol has even led to consideration of its potential use as a therapeutic agent, a proposition which, however, should consider the effects of farnesol on spermatozoa.
A crucial step in the process of fertilization is the acrosome reaction, which is irreversible and takes place in the acrosome of the sperm head as it contacts the zona pellucida of the oocyte (25). Our finding that comparably low and sublethal concentrations of farnesol induce acrosome loss in spermatozoa therefore appears to be highly important and might explain reports which attribute fertilization failures to semen that was contaminated by C. albicans but showed normal sperm parameters in terms of viability and motility (3). This enhanced sensitivity of sperm acrosomes was also observed in coincubation experiments using the P. aeruginosa QS molecule 3-oxo-C12-HSL. Interestingly, 3-oxo-C12-HSL has structural similarity to farnesol, and cross talk between C. albicans and P. aeruginosa via their QS molecules even has been described previously (9). In our studies, significant acrosome loss was visible in spermatozoa treated with comparably low concentrations of 3-oxo-C12-HSL for only 1 hour, whereas motility loss, apoptosis, and necrosis were revealed after treatment for 24 h at higher concentrations. This finding let us raise the question whether acrosome loss caused by sublethal doses of the two QS molecules was a result of unspecific membrane disorder in the acrosomal region of the sperm head or a consequence of an actively induced acrosome reaction. Although components of the oocyte zona pellucida have been suggested to be natural inducers of the acrosome reaction, other external signals, such as progesterone, have also been reported to promote this process, which is triggered by altered levels of free intracellular calcium (21). Interestingly, both the QS molecules 3-oxo-C12-HSL and farnesol have been linked before with a potential impact on intracellular calcium levels. Higher concentrations (100 μM) of 3-oxo-C12-HSL were revealed to induce apoptosis in a detectable proportion of murine fibroblasts via an increase of intracellular calcium (31), and farnesol has been identified as a potent blocker of smooth muscle L-type calcium channels (20). In our experiments, concentrations of 1 to 5 μM 3-oxo-C12-HSL were sufficient to induce the calcium-mediated acrosome reaction in a considerable proportion of sperm, an observation which supports the view that 3-oxo-C12-HSL likely acts as a true signal on human spermatozoa. In contrast, the farnesol-induced acrosome loss was also measured in the presence of the calcium chelator and is hence assumed to be a result of enhanced membrane damage within the region of the sperm acrosome. The cytotoxic effects of 3-oxo-C12-HSL were strongly pronounced in spermatozoa; however, at higher concentration (100 μM), detectable deleterious effects were also observed in iDCs. This finding supports recent data which demonstrated immune modulatory and, at higher concentrations (50 to 100 μM), specific cytotoxic effects for 3-oxo-C12-HSL on macrophages, neutrophils, DCs, and CD4+ T cells but not on CD8+ and different epithelial cells (1, 35, 36).
Addressing the question whether microbial signaling molecules are secreted during host colonization in considerable amounts, efforts were successfully undertaken to detect bacterial QS molecules even in the sputa of cystic fibrosis patients infected with P. aeruginosa (32). The highest concentrations of 3-oxo-C12-HSL up to 600 μM have been measured in P. aeruginosa biofilm cultures grown in vitro, which were thus assumed to be potentially significant (4, 30). However, one has to consider that many host niches are known to be encountered by mixed microbial populations, which likely employ multiple communication systems and consequently produce complex mixtures of signaling molecules. From our data we conclude that soluble QS molecules of different microbial origins may elicit diverse detrimental effects on human spermatozoa. This finding appears to be pathologically important, since many host surfaces of the genitourinary tract are colonized by dense communities of various microbial species, some of which might even synergistically contribute to spermatozoal impairment. Our results provide first insights into a not-yet-understood aspect of host-microbe interaction and present potential consequences thereof.
Acknowledgments
We thank Joachim Morschhäuser and Bernhard Hube for critical reading of the manuscript and Alexander D. Johnson for kindly providing C. albicans strain Bca2-10.
This work was supported by the HKI and the DFG-funded excellence graduate school Jena School for Microbial Communication.
Editor: A. Casadevall
Footnotes
Published ahead of print on 17 August 2009.
REFERENCES
- 1.Boontham, P., A. Robins, P. Chandran, D. Pritchard, M. Camara, P. Williams, S. Chuthapisith, A. McKechnie, B. J. Rowlands, and O. Eremin. 2008. Significant immunomodulatory effects of Pseudomonas aeruginosa quorum-sensing signal molecules: possible link in human sepsis. Clin. Sci. 115:343-351. [DOI] [PubMed] [Google Scholar]
- 2.Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105-109. [DOI] [PubMed] [Google Scholar]
- 3.Burrello, N., A. E. Calogero, A. Perdichizzi, M. Salmeri, R. D'Agata, and E. Vicari. 2004. Inhibition of oocyte fertilization by assisted reproductive techniques and increased sperm DNA fragmentation in the presence of Candida albicans: a case report. Reprod. Biomed. Online 8:569-573. [DOI] [PubMed] [Google Scholar]
- 4.Charlton, T. S., R. de Nys, A. Netting, N. Kumar, M. Hentzer, M. Givskov, and S. Kjelleberg. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2:530-541. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H., M. Fujita, Q. Feng, J. Clardy, and G. R. Fink. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 101:5048-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez, J. L., L. Muriel, M. T. Rivero, V. Goyanes, R. Vazquez, and J. G. Alvarez. 2003. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J. Androl. 24:59-66. [PubMed] [Google Scholar]
- 7.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 8.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 9.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 10.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes, D. T., and V. Sperandio. 2008. Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol. 6:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huwe, P., T. Diemer, M. Ludwig, J. Liu, H. G. Schiefer, and W. Weidner. 1998. Influence of different uropathogenic microorganisms on human sperm motility parameters in an in vitro experiment. Andrologia 30(Suppl. 1):55-59. [DOI] [PubMed] [Google Scholar]
- 13.Jabra-Rizk, M. A., T. F. Meiller, C. E. James, and M. E. Shirtliff. 2006. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 50:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyendran, R. S., H. H. Van der Ven, M. Perez-Pelaez, B. G. Crabo, and L. J. Zaneveld. 1984. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil. 70:219-228. [DOI] [PubMed] [Google Scholar]
- 15.Joo, J. H., G. Liao, J. B. Collins, S. F. Grissom, and A. M. Jetten. 2007. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res. 67:7929-7936. [DOI] [PubMed] [Google Scholar]
- 16.Kebaara, B. W., M. L. Langford, D. H. Navarathna, R. Dumitru, K. W. Nickerson, and A. L. Atkin. 2008. Candida albicans Tup1 is involved in farnesol-mediated inhibition of filamentous-growth induction. Eukaryot. Cell 7:980-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolter, R., and E. P. Greenberg. 2006. Microbial sciences: the superficial life of microbes. Nature 441:300-302. [DOI] [PubMed] [Google Scholar]
- 18.Larson, K. L., C. J. DeJonge, A. M. Barnes, L. K. Jost, and D. P. Evenson. 2000. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum. Reprod. 15:1717-1722. [DOI] [PubMed] [Google Scholar]
- 19.Liu, D. Y., C. Garrett, and H. W. Baker. 2006. Acrosome-reacted human sperm in insemination medium do not bind to the zona pellucida of human oocytes. Int. J. Androl. 29:475-481. [DOI] [PubMed] [Google Scholar]
- 20.Luft, U. C., R. Bychkov, M. Gollasch, V. Gross, J. B. Roullet, D. A. McCarron, C. Ried, F. Hofmann, Y. Yagil, C. Yagil, H. Haller, and F. C. Luft. 1999. Farnesol blocks the L-type Ca2+ channel by targeting the alpha 1C subunit. Arterioscler. Thromb. Vasc. Biol. 19:959-966. [DOI] [PubMed] [Google Scholar]
- 21.Meizel, S., K. O. Turner, and R. Nuccitelli. 1997. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction. Dev. Biol. 182:67-75. [DOI] [PubMed] [Google Scholar]
- 22.Nickerson, K. W., A. L. Atkin, and J. M. Hornby. 2006. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl. Environ. Microbiol. 72:3805-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh, K. B., H. Miyazawa, T. Naito, and H. Matsuoka. 2001. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. Proc. Natl. Acad. Sci. USA 98:4664-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellati, D., I. Mylonakis, G. Bertoloni, C. Fiore, A. Andrisani, G. Ambrosini, and D. Armanini. 2008. Genital tract infections and infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 140:3-11. [DOI] [PubMed] [Google Scholar]
- 25.Primakoff, P., and D. G. Myles. 2002. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296:2183-2185. [DOI] [PubMed] [Google Scholar]
- 26.Pritchard, D. I. 2006. Immune modulation by Pseudomonas aeruginosa quorum-sensing signal molecules. Int. J. Med. Microbiol. 296:111-116. [DOI] [PubMed] [Google Scholar]
- 27.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheper, M. A., M. E. Shirtliff, T. F. Meiller, B. M. Peters, and M. A. Jabra-Rizk. 2008. Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia 10:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semighini, C. P., J. M. Hornby, R. Dumitru, K. W. Nickerson, and S. D. Harris. 2006. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 59:753-764. [DOI] [PubMed] [Google Scholar]
- 30.Shiner, E. K., K. P. Rumbaugh, and S. C. Williams. 2005. Inter-kingdom signaling: deciphering the language of acyl homoserine lactones. FEMS Microbiol. Rev. 29:935-947. [DOI] [PubMed] [Google Scholar]
- 31.Shiner, E. K., D. Terentyev, A. Bryan, S. Sennoune, R. Martinez-Zaguilan, G. Li, S. Gyorke, S. C. Williams, and K. P. Rumbaugh. 2006. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell. Microbiol. 8:1601-1610. [DOI] [PubMed] [Google Scholar]
- 32.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 33.Steller, H. 1995. Mechanisms and genes of cellular suicide. Science 267:1445-1449. [DOI] [PubMed] [Google Scholar]
- 34.Tabibian, J. H., J. Gornbein, A. Heidari, S. L. Dien, V. H. Lau, P. Chahal, B. M. Churchill, and D. A. Haake. 2008. Uropathogens and host characteristics. J. Clin. Microbiol. 46:3980-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tateda, K., Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro, and K. Yamaguchi. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian, Y. H., J. W. Xiong, L. Hu, D. H. Huang, and C. L. Xiong. 2007. Candida albicans and filtrates interfere with human spermatozoal motility and alter the ultrastructure of spermatozoa: an in vitro study. Int. J. Androl. 30:421-429. [DOI] [PubMed] [Google Scholar]
- 38.Westwater, C., E. Balish, and D. A. Schofield. 2005. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot. Cell 4:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. 1999. Laboratory manual of human semen and semen-cervical mucus interaction, 4th ed. Cambridge University Press, Cambridge, United Kingdom.
- 40.Williams, P. 2007. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153:3923-3938. [DOI] [PubMed] [Google Scholar]