Abstract
The Borrelia burgdorferi BmpA outer surface protein plays a significant role in mammalian infection by the Lyme disease spirochete and is an important antigen for the serodiagnosis of human infection. B. burgdorferi adheres to host extracellular matrix components, including laminin. The results of our studies indicate that BmpA and its three paralogous proteins, BmpB, BmpC, and BmpD, all bind to mammalian laminin. BmpA did not bind mammalian type I or type IV collagens or fibronectin. BmpA-directed antibodies significantly inhibited the adherence of live B. burgdorferi to laminin. The laminin-binding domain of BmpA was mapped to the carboxy-terminal 80 amino acids. Solubilized collagen inhibited BmpA-laminin binding, suggesting interactions through the collagen-binding domains of laminin. These results, together with previous data, indicate that BmpA and its paralogs are targets for the development of preventative and curative therapies for Lyme disease.
Early during the course of Lyme disease, humans frequently produce antibodies directed against a Borrelia burgdorferi antigen originally described as “P39” (66). Antibodies recognizing P39 are considered to be specific and diagnostic for Lyme disease spirochete infection (5, 18, 30, 62, 64). The antigenic protein was subsequently identified as BmpA (Borrelia membrane protein A) (65). The bmpA gene is located on the main borrelial chromosome, adjacent to three paralogous genes named bmpB, bmpC, and bmpD, which together form a complex operon (3, 4, 28, 32, 55, 56, 65). These other Bmp proteins are also often antigenic in infected humans (14). In addition to the serological data described above, examination of B. burgdorferi within skin and joint tissues confirmed the production of BmpA protein during mammalian infection (21, 49). BmpA is located in the borrelial outer membrane (46), where it is exposed to the external environment and can be a target of bactericidal antibodies (49, 63; F. Cabello, personal communication). BmpA and its paralogs have been implicated as playing roles in some symptoms of Lyme disease (49, 72). B. burgdorferi mutants in which bmpA or bmpB is specifically deleted are unable to persist in mouse joint tissues (49), indicating an important role for these proteins in the maintenance of mammalian infection. Despite the extensive research conducted on these important antigens, functions for the Bmp proteins had not been determined previously.
B. burgdorferi is an extracellular organism, frequently found associated with its hosts' connective tissues (6-9, 16, 17, 24, 26, 31, 36, 39, 48). In the laboratory, B. burgdorferi shows affinity for various host extracellular matrix (ECM) components, such as type I collagen, fibronectin, and decorin (16, 33, 34, 50, 74). We recently determined that B. burgdorferi also adheres to mammalian laminin, an important component of many mammalian ECMs (13). Ligand affinity blot analyses of a B. burgdorferi cell fraction enriched for outer membrane components revealed that the type strain, B31, can produce several distinct laminin-binding proteins, one of which we previously identified as being the surface-exposed outer membrane lipoprotein ErpX (11, 13, 69). We now present data indicating that BmpA and its paralogs are also laminin-binding proteins.
MATERIALS AND METHODS
Bacteria.
An infectious clone of the sequenced culture of B. burgdorferi type strain B31, named B31-MI-16, was used for all studies (44). Bacteria were cultured at 34°C in Barbour-Stoenner-Kelly II medium supplemented with 6% rabbit serum (75). After reaching mid-logarithmic phase (107 bacteria/ml), bacteria were harvested for either Triton X-114 extraction (see below) or isolation of chromosomal DNA (58).
Cellular fractionation.
An outer membrane-enriched fraction of B. burgdorferi B31-MI-16 was extracted by Triton X-114 solubilization and phase partitioning as described previously (22, 51, 53). Briefly, cultured bacteria were washed in phosphate-buffered saline (PBS) and then gently extracted in 1% protein-grade Triton X-114 (EMD-Calbiochem, San Diego, CA) at 4°C for 12 h. Protoplasmic cylinders were pelleted by centrifugation at 15,000 × g for 10 min, and the supernatant, consisting of periplasmic and outer membrane contents, was retained. The supernatant was warmed to 37°C to induce phase separation, followed by centrifugation for 15 min at 15,000 × g. The outer membrane component-enriched detergent phase was extracted two additional times with PBS, and proteins contained in that phase were precipitated with methanol-chloroform.
Two-dimensional electrophoresis.
The B. burgdorferi outer membrane-enriched Triton X-114 fraction was separated by 2-dimensional electrophoresis using the MultiPhor II system (GE Healthcare, Piscataway, NJ). The detergent-phase pellet was resuspended in ReadyPrep rehydration buffer (Bio-Rad, Hercules, CA) and allowed to rehydrate ReadyStrip immobilized pH gradient strips (pH 3 to 10; Bio-Rad) overnight. Isoelectric focusing was performed for 3,000 V-h (500 V, 6 h, 10°C). After the completion of isoelectric focusing, strips were equilibrated and then separated by conventional sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-PAGE). Gels were either stained with SYPRO Ruby (Molecular Probes, Eugene, OR) or transferred to nitrocellulose membranes for a laminin immunoaffinity assay. Protein spots of interest were extracted and analyzed by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry (University of Louisville, Louisville, KY). Spectrometry results were compared with the known sequence of B. burgdorferi strain B31 using Mascot (Matrix Science, Boston, MA).
Recombinant proteins.
Total chromosomal DNA from B. burgdorferi B31-MI-16 was used as a template to PCR amplify wild-type bmpA, bmpB, bmpC, bmpD, and amino- and carboxy-terminal truncations of bmpA, using the gene-specific primers listed in Table 1. In order to facilitate maximal expression and solubility of recombinant proteins, all amplicons lacked the amino-terminal leader polypeptide- and lipidation site-encoding sequences (29). Amplicons were cloned into pET200 (Invitrogen, Carlsbad, CA), and inserts were completely sequenced on both strands to ensure against the accidental introduction of mutations during cloning processes. Recombinant plasmids thus produced were transformed into Escherichia coli Rosetta(DE3)(pLysS) (Novagen, Madison, WI). Expression of polyhistidine-tagged recombinant proteins was induced by the addition of 1 mM isopropyl thiogalactopyranoside to mid-exponential-phase cultures grown at 37°C. Induced E. coli bacteria were harvested after 3 h and lysed by sonication, and debris was cleared by centrifugation. Recombinant proteins were purified from cleared lysates using MagneHis nickel-conjugated magnetic beads (Promega, Madison, WI). The purities of recombinant proteins were assessed by separation by SDS-PAGE, followed by staining with Coomassie brilliant blue. Concentrations of protein preparations were determined by a bicinchoninic acid assay (Pierce, Rockford, IL).
TABLE 1.
Oligonucleotides used during this worka
| Primer name | Primer pair sequences (5′ to 3′) |
|---|---|
| pBmpA | CAC CGG TAA AGG TAG TCT TGG GA |
| CCA TTT CAA TTA TTC AAA CAA AAC CAA TGT | |
| pBmpA-N2 | CAC CCC CGA TAT GAA ATA TGC AAT TAT TG |
| CCA TTT CAA TTA TTC AAA CAA AAC CAA TGT | |
| pBmpA-N3 | CAC CGG TAG AAG CGT TGC AAC TAG |
| CCA TTT CAA TTA TTC AAA CAA AAC CAA TGT | |
| pBmpA-N4 | CAC CGA TGT TGG TAG AGC TTT AAA TAT |
| CCA TTT CAA TTA TTC AAA CAA AAC CAA TGT | |
| pBmpA-I | CAC CCC CGA TAT GAA ATA TGC AAT TAT TG |
| TTA AAT TCC TCC AAG GCC TGC AGC | |
| pBmpA-C2 | CAC CGG TAA AGG TAG TCT TGG GA |
| TTA GGT CAT GCC CAC CAA ATT TGC | |
| pBmpB | CAC CTT TAG TAG AAA TGG AAT AGA ATC TAG |
| GCA AAA TCC TCT AAA ACA ACA GAA ATG | |
| pBmpC | CAC CTT TAA ATC TAA TAA AAA GTC TAT TAA ATC TG |
| CCC TTT ACA AAC AAA GCT ATA TTT AAG TAG | |
| pBmpD | CAC CTC TAG CTC TGA TGA TGG CAA GTC G |
| GAA TTA AAA AGA TTT TTC ACA AAT CAG CTC |
All amplicons were cloned into pET200, and the resultant plasmids were used to produce recombinant proteins.
Assays of protein binding to laminin.
Ligand affinity blot analyses were performed essentially as described previously (13, 70). Briefly, recombinant proteins were separated by SDS-PAGE, electrotransferred to nitrocellulose membranes, and then blocked overnight at 4°C with 5% bovine serum albumin (BSA) in Tris-buffered saline-Tween 20 (TBS-T; 20 mM Tris [pH 7.5], 150 mM NaCl, 0.05% Tween 20). Membranes were washed with TBS-T and then incubated for 1 h at room temperature with 13 mg/ml Engelbreth-Holm-Swarm (EHS) mouse sarcoma laminin (Sigma-Aldrich) in TBS-T. This source of laminin is particularly relevant to investigations of B. burgdorferi, in that mice and other rodents are natural reservoir hosts of the Lyme disease spirochete, particularly in the northeastern United States, where strain B31 was isolated (42). After extensive washing with TBS-T, membranes were incubated for 1 h at room temperature with an affinity-isolated rabbit anti-EHS laminin polyclonal antiserum (Sigma-Aldrich), diluted 1:5,000 with TBS-T. Following a wash with TBS-T, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated protein G (Invitrogen), diluted 1:20,000 in TBS-T. Membranes were then developed with the SuperSignal West Pico enhanced chemiluminescence substrate (Pierce), and bands were visualized with BioMax Light film (Kodak).
For enzyme-linked immunosorbent assay (ELISA)-based binding assays, wells of Maxisorp 96-well plates (Nalge-Nunc, Rochester, NY) were coated overnight with 13 μg/ml EHS laminin in 50 mM NaCO3 (pH 9.6) at 4°C. Plates were brought to room temperature and washed three times with PBS plus 0.5% Tween 20 (PBS-T). Wells were blocked for 2 h at room temperature with 2% BSA in PBS-T and then washed three times with PBS-T. Wells were incubated for 1 h at 37°C with various concentrations of recombinant BmpA (rBmpA) in PBS-T. Following three washes with PBS-T, wells were incubated for 1 h at 37°C with a 1:500-diluted BmpA-specific rabbit polyclonal antiserum (gifts of Tom Schwan and Felipe Cabello). Plates were washed three times with PBS-T, and then wells were incubated for 1 h at room temperature with horseradish peroxidase-conjugated protein G, diluted 1:5,000. Wells were again washed three times with PBS-T; 100 μl/well ready-to-use 3,3′,5,5′-tetramethyl benzidine substrate solution (1-Step Turbo TMB-ELISA; Thermo Scientific, Rockford, IL) was added; then reactions were stopped by addition of 2 N H2SO4 at 50 μl/well. Absorbance was read at 450 nm with a Spectramax plate reader using SoftMax Pro (Molecular Devices, Sunnyvale, CA). Statistical analyses were performed using Student's t test by assuming unequal variances.
Essentially the same ELISA protocol was followed to test the binding of other components of mammalian extracellular matrices. Human fibronectin (Sigma-Aldrich), murine collagen I (Sigma-Aldrich), and murine collagen IV (Santa Cruz Biotechnologies, Santa Cruz, CA) were tested, all at 20 μg/ml. Wells were coated with 2 μg/ml rBmpA. Binding of human proteins to rBmpA was assessed using appropriate specific primary antibodies (all from Sigma-Aldrich).
The ability of heparin (Sigma-Aldrich) or solubilized collagen (gelatin; Difco, Becton-Dickinson, Sparks, MD) to compete for the binding of laminin to BmpA was assayed essentially as previously described (35). rBmpA was immobilized to 96-well ELISA plates (100 ng/well) and then incubated with 13 μg/ml EHS laminin plus varying concentrations of either heparin (0 to 50 μM) or solubilized collagen (0 to 100 μg) for 1 h at 37°C. Assay wells were washed three times with PBS-T and were then incubated with a laminin-specific polyclonal antiserum (1:2,500) for 1 h at 37°C. Reaction products were developed and analyzed as described above.
Assays of the adherence of live bacteria to laminin.
The effects of BmpA-directed antibodies or soluble rBmpA protein on laminin binding by live B. burgdorferi bacteria were examined microscopically. For antibody inhibition studies, Fab fragments of immunoglobulin G were generated using Fab preparation kits (Pierce).
Glass microscope slides were first thoroughly washed with deionized water and then coated by overnight incubation with 5 μg/ml EHS laminin (Sigma-Aldrich) in PBS. Control slides were similarly coated with BSA. The following day, slides were washed three times with PBS and then blocked by incubation with 3% (mass/vol) BSA for 2 h at room temperature, followed by another three washes with PBS. Cultured B31-MI-16 (107 bacteria per ml; mid-exponential phase) was harvested by centrifugation, washed three times with PBS, and resuspended in PBS to a final concentration of 2 × 106 bacteria/ml. For antibody inhibition studies, bacteria on slides were incubated for 30 min with purified Fab fragments added to a final concentration of 2.7 μg/ml. For rBmpA inhibition studies, laminin-coated slides were incubated with 50 μg/ml recombinant protein for 30 min prior to the application of bacteria. Slides were covered with suspended bacteria, incubated at 37°C for 2 h, and then gently washed 10 times with PBS. Bacteria were visualized by dark-field microscopy. Numbers of adherent bacteria observed in 10 fields per slide (magnification, ×200) were counted, with two slides per condition. Statistical analyses were performed using Student's t test assuming unequal variances.
RESULTS
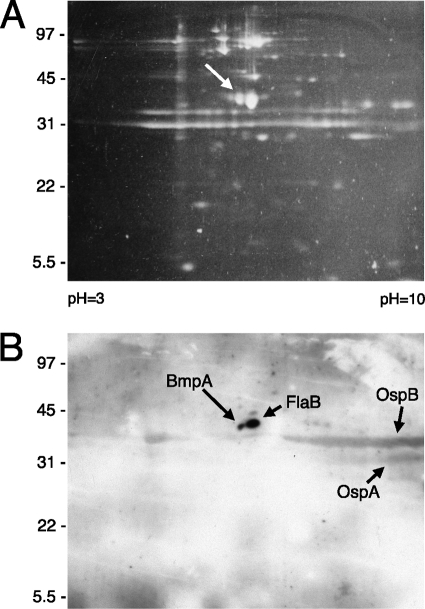
We recently determined that the ErpX protein of B. burgdorferi type strain B31 specifically binds laminin and that cultured B. burgdorferi produces additional proteins that can also bind to laminin (13). Proteomic tools were applied to help identify those other borrelial laminin-binding proteins. An outer membrane-enriched fraction of cultured strain B31-MI-16 was subjected to 2-dimensional electrophoresis and was then either stained with SYPRO Ruby (Fig. 1A) or transferred to nitrocellulose membranes. Transferred proteins were examined for laminin-binding activity by ligand affinity blot analysis. Two relatively intense spots were observed, both with apparent molecular masses of approximately 40 kDa (Fig. 1B). The ligand affinity blot was aligned with the stained gel, and the two corresponding proteins were extracted and then subjected to mass spectrometric analysis. Note that these procedures served only to identify candidate proteins. Note also that the bacterial fraction analyzed included proteins that are not surface localized in intact borreliae. For those reasons, subsequent analyses of purified proteins that are known to be surface exposed in live B. burgdorferi were used to confirm whether or not candidates were able to bind to laminin (see below).
FIG. 1.
Two-dimensional electrophoretic analysis of an outer membrane-enriched fraction of cultured B. burgdorferi B31-MI-16. (A) Polyacrylamide gel stained with the fluorescent dye SYPRO Ruby. The arrow indicates the protein spot that was determined to correspond with BmpA. (B) A second gel was run simultaneously to that shown in panel A; proteins were transferred to a nitrocellulose membrane and then examined for laminin-binding activities through ligand affinity blot analysis. Strong signals that were determined to correspond with BmpA and FlaB are indicated. Relatively weaker signals were produced from OspA and OspB, both of which often form broad smears across 2-dimensional gels (47).
The smaller protein spot yielded the highest-probability match to the outer membrane lipoprotein BmpA, with six polypeptides spanning approximately 30% of that protein (data not shown). The location of this protein on our 2-dimensional gels is consistent with previous results (46, 47). Analysis of the larger spot suggested that it was the FlaB component of the borrelial endoflagella. Since spirochete flagella are localized within the periplasm and are completely shielded from the external environment by the outer membrane (38), interactions between FlaB and laminin were not studied further. Appreciably weaker interactions between laminin and OspB and OspA were also observed; however, since neither of those two borrelial proteins are produced at significant levels during normal mammalian infection (27, 45, 60, 61, 73), those proteins also were not studied further. This particular culture of B. burgdorferi did not produce appreciable amounts of ErpX, as has been observed previously (68), so no signal corresponding to the mass and pI of that lipoprotein was detected.
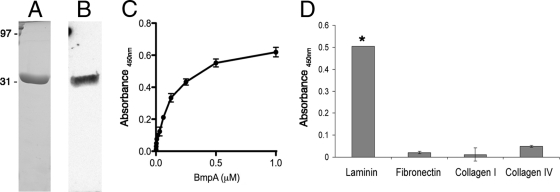
The tentative identification of BmpA as a laminin-binding protein was confirmed through studies using purified recombinant protein (rBmpA) (Fig. 2A). First, ligand affinity blot analyses were performed, in which rBmpA was subjected to SDS-PAGE, transferred to nitrocellulose membranes, and incubated with soluble laminin, and then bound laminin was detected by use of specific antibodies and appropriate secondary antibodies. This method indicated that BmpA is indeed a laminin-binding protein (Fig. 2B). ELISA further supported that conclusion, demonstrating saturable binding and indicating an apparent dissociation constant (Kd) for rBmpA-laminin binding of approximately 0.1 μM (Fig. 2C).
FIG. 2.
BmpA binds laminin. (A) Purified rBmpA, subjected to SDS-PAGE and stained with Coomassie brilliant blue. (B) Ligand affinity blot analysis, indicating the adherence of laminin to rBmpA. (C) ELISA of varying concentrations of rBmpA binding to purified laminin affixed to wells. Saturated binding was observed, with an apparent Kd of 0.1 μM. (D) ELISA results indicating that neither mammalian fibronectin, collagen I, nor collagen IV detectably bound to immobilized BmpA. Error bars indicate 1 standard deviation from the mean. The asterisk indicates ligand binding to rBmpA that was significantly different from binding to the control protein BSA (P < 0.0001).
We then examined rBmpA for the ability to bind additional mammalian ECM components: fibronectin, collagen type I, and collagen type IV. Only laminin yielded ELISA signals that were significantly greater than those obtained for the control protein, BSA (Fig. 2D).
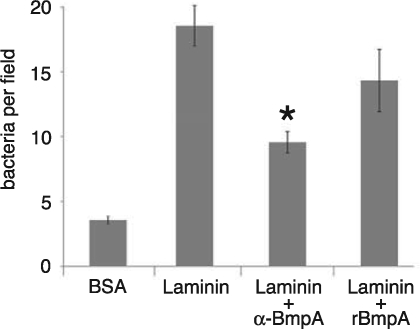
The biological significance of BmpA as a laminin-binding protein was investigated by examining the effects of BmpA-specific antibody Fab fragments or rBmpA protein on the adherence of intact, live B. burgdorferi bacteria to laminin. As assessed by microscopic examinations, anti-BmpA antibodies significantly inhibited the adherence of intact B. burgdorferi to the laminin substrate (Fig. 3). rBmpA also reduced borrelial adhesion, although the changes were not statistically significant. Considering that the cultured B. burgdorferi produced additional laminin-binding outer surface proteins, including OspA and OspB, these results indicate that BmpA plays a substantial role in borrelial adherence to laminin.
FIG. 3.
Microscopic enumeration of bacteria adhering to slides coated with BSA or laminin, showing the effects of BmpA-specific antibodies or soluble rBmpA on adherence. Error bars indicate 1 standard deviation from the mean. Neither antibodies nor recombinant protein was added to control assay mixtures. The asterisk indicates a statistically significant difference from the results of control assays. BmpA-specific antibody Fab fragments significantly inhibited borrelial adherence (P < 0.04). The reduced adherence due to the addition of rBmpA was not statistically significant (P < 0.13).
The laminin-binding domain of BmpA.
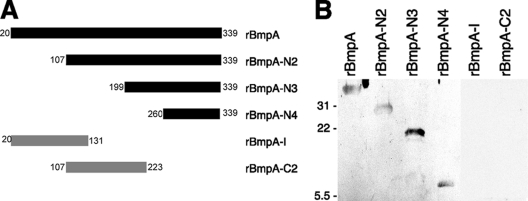
Mutant rBmpA proteins that lacked large regions of the wild-type protein were produced (Fig. 4A). Each truncated recombinant protein was assessed for laminin-binding activity by ligand affinity blot analyses. Three successively shorter amino-terminally truncated mutant proteins, the 233-residue rBmpA-N2, the 141-residue rBmpA-N3, and the 80-residue rBmpA-N4, all bound laminin (Fig. 4B). In contrast, neither the 112-residue amino-terminal fragment rBmpA-C2 nor the centrally located 117-residue rBmpA-I bound the host protein (Fig. 4B). Even with extended film exposure times (>15 min), no signals indicative of laminin binding by rBmpA-I or rBmpA-C2 were ever detected. Taken together, these data suggest that the carboxy-terminal 80 amino acids of BmpA contain all of the residues necessary for binding to laminin.
FIG. 4.
Laminin-binding analyses of truncated BmpA proteins. (A) Schematic representation of wild-type rBmpA and its truncated mutants. Numbers at either end correspond to the residue numbers of the immature (uncleaved, nonlipidated) wild-type protein. The sequence of rBmpA begins with the first residue downstream of the lipidation site, i.e., residue 20. Filled bars represent recombinant proteins that detectably bound laminin, while shaded bars represent proteins that did not bind laminin. (B) Ligand affinity blot analyses of laminin binding by recombinant wild-type and mutant BmpA proteins. The mobilities of molecular mass markers (in kilodaltons) are given on the left.
Involvement of laminin collagen-binding domains.
Laminins are large, multimeric proteins containing distinctive domains through which they interact with other tissue components (20, 59). Competition assays were performed to examine whether BmpA binds laminin through either of two major domain types: those that bind collagen (gelatin) and those that bind heparin. Addition of solubilized collagen led to dose-dependent inhibition of laminin-BmpA binding, suggesting that BmpA and collagen bind to overlapping regions of laminin (Fig. 5A). Addition of 3.12 μM heparin had a small but significant effect (P < 0.05) on BmpA-laminin binding, but increasing concentrations of heparin had no additional effects (Fig. 5B).
FIG. 5.
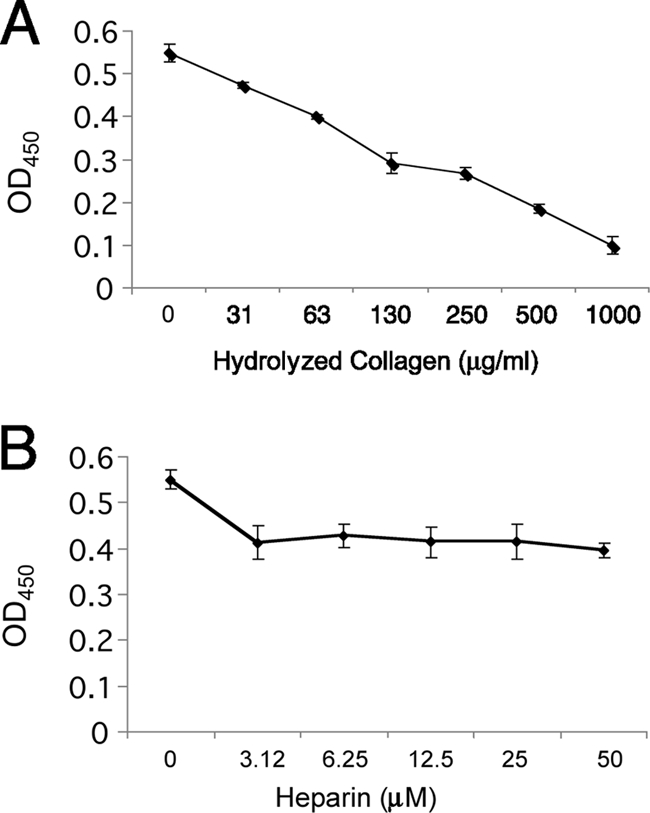
BmpA and collagen compete for binding to laminin. Shown are ELISA results for the adherence of laminin to rBmpA in the presence of increasing concentrations of solubilized collagen (gelatin) (A) or the sulfated glycosaminoglycan heparin (B).
BmpB, BmpC, and BmpD also bind laminin.
The main chromosome of Lyme disease spirochetes contains three additional genes encoding paralogs of BmpA. Overall predicted amino acid similarities among the Bmp proteins of B. burgdorferi strain B31 range from 36 to 64% (Fig. 6A) (56). Since BmpB, BmpC, and BmpD are also produced during mammalian infection, we produced recombinant forms of all three proteins and assessed their abilities to bind laminin. Ligand affinity blot analyses demonstrated that all four members of the Bmp family bound laminin (Fig. 6B).
FIG. 6.
All four members of the B. burgdorferi Bmp protein family bind laminin. (A) Alignment of BmpA, BmpB, BmpC, and BmpD predicted protein sequences from the B. burgdorferi type strain, B31. Identical residues are boxed and shaded. Spaces introduced to maximize alignment are indicated by dashes. The amino-terminal cysteine residue modified by lipidation in each mature protein is indicated by an arrowhead. The carboxy-terminal region of BmpA that is sufficient for adherence to laminin is indicated by a horizontal line over the sequence. (B) Equal masses of recombinant BmpB, BmpC, and BmpD were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and then assessed for the ability to adhere to laminin by ligand affinity blot analysis. The mobilities of molecular mass markers (in kilodaltons) are given on the left. rBmpC consistently migrated more slowly on SDS-PAGE gels than the other rBmp proteins, perhaps reflecting differences in the charges of the proteins (pI values, 5.6 for rBmpB, 5.8 for rBmpD, and 8.7 for rBmpC).
DISCUSSION
Humans and other mammals infected with Lyme disease spirochetes mount rapid and strong antibody responses directed against the BmpA outer surface protein. Such immune responses are so reproducible that the presence of BmpA-directed antibodies in patient serum is a reliable marker of B. burgdorferi infection (5, 18, 30, 62, 64). Despite the apparent ubiquity of BmpA among Lyme disease spirochetes and its known expression during mammalian infection, no function had been defined for the protein prior to this work. We identified BmpA and its three paralogs, BmpB, BmpC, and BmpD, as laminin-binding proteins. This characterization may explain the results from a previous study, which found that a recombinant bacteriophage expressing a fragment of BmpD on its capsid tended to adhere to mouse tissues (2). BmpA and BmpB both play important roles during mammalian infection, as evidenced by the fact that mutants defective in either gene are unable to persistently infect mouse joints (49). The relative importance of BmpC and BmpD has yet to be evaluated. Since those mutant bacteria retained their other bmp paralogs plus the gene for the other infection-associated laminin-binding protein, ErpX (13, 49, 52), those results raise the possibility that borrelial laminin-binding proteins are produced at different levels in different tissues and/or that colonization of some host tissues requires synergy between adhesins. Transcription of the bmp locus involves multiple promoters and terminators (28, 55, 56), suggestive of complex, potentially independent expression patterns for each gene.
The carboxy-terminal 80 amino acids of BmpA were determined to be sufficient for adhesion to laminin. Competition studies point toward the involvement of collagen-binding domains of laminin in its interactions with BmpA. The function of the remainder of the 39-kDa BmpA lipoprotein remains to be determined. BmpA is predicted to fold in a manner similar to that of the Treponema pallidum TmpC (PnrA) lipoprotein, although the two proteins share approximately 30% identical amino acids. TmpC is hypothesized to be involved in purine transport (25), so it is possible that BmpA may perform such a role for B. burgdorferi. Dual-function outer surface proteins, such as the B. burgdorferi p66 porin/integrin adhesin, the subgroup of B. burgdorferi Erp proteins that bind host plasminogen and complement factor H, and the Neisseria gonorrhoeae PorB porin/adhesin, are not uncommon in bacteria (1, 12, 23, 37, 40, 43, 54, 57, 67, 71).
Redundancy of function appears to be the norm for the Lyme disease spirochete. In addition to the four laminin-binding proteins identified in the current work, the B. burgdorferi type strain also produces the unrelated ErpX laminin-binding protein (13). As additional examples, two decorin-binding proteins, three fibronectin-binding proteins, five complement factor H-binding-proteins, and several plasminogen-binding proteins have been identified in the type strain, B31 (10, 12, 19, 34, 41, 50). For some of those examples, it has been shown that different borrelial proteins that adhere to the same host ligand are produced at different times during the spirochete's mammal-tick infectious cycle (15). It remains to be seen whether that is also the case with the multiple borrelial laminin-binding proteins, or if each binding protein is produced only in certain host tissues. Studies of bmpA and bmpB mutants indicate that both encoded proteins play important roles in at least some aspects of mammalian infection. Thus, the Lyme disease spirochete laminin-binding proteins are compelling targets for the development of therapies that disrupt infection processes.
Acknowledgments
This work was supported by NIH grant R01-AI044254 and exploratory funds provided by the University of Kentucky College of Medicine (both to B. Stevenson) and by NIH grant F32-AI081480 (to C. A. Brissette).
We thank Felipe Cabello for providing results of his studies prior to publication; Tom Schwan, Merry Schrumpf, Felipe Cabello, and Henry Godfrey for providing BmpA-specific antisera; and Logan Burns and Michael Woodman for helpful comments and technical advice.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 24 August 2009.
REFERENCES
- 1.Alitalo, A., T. Meri, H. Lankinen, I. Seppälä, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 2.Antonara, S., R. M. Chafel, M. LaFrance, and J. Coburn. 2007. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol. Microbiol. 66:262-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aron, L., M. Alekshun, L. Perlee, I. Schwartz, H. P. Godfrey, and F. C. Cabello. 1994. Cloning and DNA sequence analysis of bmpC, a gene encoding a potential membrane lipoprotein of Borrelia burgdorferi. FEMS Microbiol. Lett. 123:75-82. [DOI] [PubMed] [Google Scholar]
- 4.Aron, L., C. Toth, H. P. Godfrey, and F. C. Cabello. 1996. Identification and mapping of a chromosomal gene cluster of Borrelia burgdorferi containing genes expressed in vivo. FEMS Microbiol. Lett. 145:309-314. [DOI] [PubMed] [Google Scholar]
- 5.Association of State and Territorial Public Health Laboratory Directors. 1995. Recommendations, p. 1-5. In Second National Conference on Serologic Diagnosis of Lyme Disease. Association of Public Health Laboratories, Washington, DC.
- 6.Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 7.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-972. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 9.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 10.Brissette, C. A., T. Bykowski, A. E. Cooley, A. Bowman, and B. Stevenson. 2009. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 77:2802-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brissette, C. A., A. E. Cooley, L. H. Burns, S. P. Riley, A. Verma, M. E. Woodman, T. Bykowski, and B. Stevenson. 2008. Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int. J. Med. Microbiol. 298(S1):257-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brissette, C. A., K. Haupt, D. Barthel, A. E. Cooley, A. Bowman, C. Skerka, R. Wallich, P. F. Zipfel, P. Kraiczy, and B. Stevenson. 2009. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 77:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brissette, C. A., A. Verma, A. Bowman, A. E. Cooley, and B. Stevenson. 2009. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology 155:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryksin, A. V., H. P. Godfrey, C. A. Carbonaro, G. P. Wormser, M. E. Aguero-Rosenfeld, and F. C. Cabello. 2005. Borrelia burgdorferi BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot reactivity in patients with Lyme disease. Clin. Diagn. Lab. Immunol. 12:935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bykowski, T., M. E. Woodman, A. E. Cooley, C. A. Brissette, V. Brade, R. Wallich, P. Kraiczy, and B. Stevenson. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun. 75:4227-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabello, F. C., H. P. Godfrey, and S. A. Newman. 2007. Hidden in plain site: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 15:350-354. [DOI] [PubMed] [Google Scholar]
- 17.Cadavid, D., Y. Bai, D. Dail, M. Hurd, K. Narayan, E. Hodzic, S. W. Barthold, and A. R. Pachner. 2003. Infection and inflammation in skeletal muscle from nonhuman primates infected with different genospecies of the Lyme disease spirochete Borrelia burgdorferi. Infect. Immun. 71:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 19.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colognato, H., and P. D. Yurchenco. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218:213-234. [DOI] [PubMed] [Google Scholar]
- 21.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 72:5063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham, T. M., D. D. Thomas, S. D. Thompson, J. N. Miller, and M. A. Lovett. 1988. Identification of Borrelia burgdorferi surface components by Triton X-114 phase partitioning. Ann. N. Y. Acad. Sci. 539:376-378. [Google Scholar]
- 23.Defoe, G., and J. Coburn. 2001. Delineation of Borrelia burgdorferi p66 sequences required for integrin αIIbβ3 recognition. Infect. Immun. 69:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Defosse, D. L., P. H. Duray, and R. C. Johnson. 1992. The NIH-3 immunodeficient mouse is a model for Lyme borreliosis myositis and carditis. Am. J. Pathol. 141:3-10. [PMC free article] [PubMed] [Google Scholar]
- 25.Deka, R. K., C. A. Brautigam, X. F. Yang, J. S. Blevins, M. Machius, D. R. Tomchick, and M. V. Norgard. 2006. The PnrA (Tp0319; TmpC) lipoprotein represents a new family of bacterial purine nucleoside receptor encoded within an ATP-binding cassette (ABC)-like operon in Treponema pallidum. J. Biol. Chem. 281:8072-8081. [DOI] [PubMed] [Google Scholar]
- 26.De Koning, J., R. B. Bosma, and J. A. Hoogkamp-Korstanje. 1987. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J. Med. Microbiol. 23:261-267. [DOI] [PubMed] [Google Scholar]
- 27.de Silva, A. M., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrikova, E. Y., J. Bugrysheva, and F. C. Cabello. 2001. Two independent transcriptional units control the complex and simultaneous expression of the bmp paralogous chromosomal gene family in Borrelia burgdorferi. Mol. Microbiol. 39:370-378. [DOI] [PubMed] [Google Scholar]
- 29.Dunn, J. J., B. N. Lade, and A. G. Barbour. 1990. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr. Purif. 1:159-168. [DOI] [PubMed] [Google Scholar]
- 30.Fawcett, P. T., C. Rose, K. M. Gibney, C. A. Chase, B. Kiehl, and R. A. Doughty. 1993. Detection of antibodies to the recombinant P39 protein of Borrelia burgdorferi using enzyme immunoassay and immunoblotting. J. Rheumatol. 20:734-738. [PubMed] [Google Scholar]
- 31.Franz, J. K., O. Fritze, M. Rittig, G. Keyßer, S. Priem, J. Zacher, G. R. Burmester, and A. Krause. 2001. Insights from a novel three-dimensional in vitro model of Lyme arthritis. Arthritis Rheum. 44:151-162. [DOI] [PubMed] [Google Scholar]
- 32.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 33.Grab, D. J., C. Givens, and R. Kennedy. 1998. Fibronectin-binding activity in Borrelia burgdorferi. Biochim. Biophys. Acta 1407:135-145. [DOI] [PubMed] [Google Scholar]
- 34.Guo, B. P., S. J. Norris, L. C. Rosenberg, and M. Höök. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 63:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauk, P., F. Macedo, E. C. Romero, S. A. Vasconcellos, Z. M. de Morais, A. S. Barbosa, and P. L. Ho. 2008. In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect. Immun. 76:2642-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Häupl, T., G. Hahn, M. Rittig, A. Krause, C. Schoerner, U. Schonherr, J. R. Kalden, and G. R. Burmester. 1993. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 36:1621-1626. [DOI] [PubMed] [Google Scholar]
- 37.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppälä, and S. Meri. 2001. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 38.Holt, S. C. 1978. Anatomy and chemistry of spirochetes. Microbiol. Rev. 42:114-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kornblatt, A. N., A. C. Steere, and D. G. Brownstein. 1984. Experimental Lyme disease in rabbits: spirochetes found in erythema migrans and blood. Infect. Immun. 46:220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraiczy, P., K. Hartmann, J. Hellwage, C. Skerka, V. Brade, P. F. Zipfel, R. Wallich, and B. Stevenson. 2004. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int. J. Med. Microbiol. 293(S37):152-157. [DOI] [PubMed] [Google Scholar]
- 41.Kraiczy, P., and R. Würzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 42.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 43.Metts, M. S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, J. C., K. von Lackum, K. Babb, J. D. McAlister, and B. Stevenson. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neelakanta, G., X. Li, U. Pal, X. Liu, D. S. Beck, K. DePonte, D. Fish, F. S. Kantor, and E. Fikrig. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowalk, A. J., R. D. Gilmore, Jr., and J. A. Carroll. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 74:3864-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowalk, A. J., C. Nolder, D. R. Clifton, and J. A. Carroll. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 6:2121-2134. [DOI] [PubMed] [Google Scholar]
- 48.Pachner, A. R., J. Basta, E. Delaney, and D. Hulinska. 1995. Localization of Borrelia burgdorferi in murine Lyme borreliosis by electron microscopy. Am. J. Trop. Med. Hyg. 52:128-133. [DOI] [PubMed] [Google Scholar]
- 49.Pal, U., P. Wang, F. Bao, X. Yang, S. Samanta, R. Schoen, G. P. Wormser, I. Schwartz, and E. Fikrig. 2008. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J. Exp. Med. 205:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Probert, W. S., and B. J. B. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 51.Pryde, J. G. 1986. Triton X-114: a detergent that has come in from the cold. Trends Biochem. Sci. 11:160-163. [Google Scholar]
- 52.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radolf, J. D., N. R. Chamberlain, A. Clausell, and M. V. Norgard. 1988. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent Triton X-114. Infect. Immun. 56:490-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramamoorthy, R., N. A. McClain, A. Gautam, and D. Scholl-Meeker. 2005. Expression of the bmpB gene of Borrelia burgdorferi is modulated by two distinct transcription termination events. J. Bacteriol. 187:2592-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramamoorthy, R., L. Povinelli, and M. T. Philipp. 1996. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins in Borrelia burgdorferi. Infect. Immun. 64:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rechner, C., C. Kühlewein, A. Müller, H. Schild, and T. Rudel. 2007. Host glycoprotein Gp96 and scavenger receptor SREC interact with PorB of disseminating Neisseria gonorrhoeae in an epithelial invasion pathway. Cell Host Microbe 2:393-403. [DOI] [PubMed] [Google Scholar]
- 58.Rosa, P. A., and T. G. Schwan. 1989. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J. Infect. Dis. 160:1018-1029. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki, T., R. Fässler, and E. Hohenester. 2004. Laminin: the crux of basement membrane assembly. J. Cell Biol. 164:959-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwan, T. G., M. E. Schrumpf, K. L. Gage, and R. D. Gilmore, Jr. 1992. Analysis of Leptospira spp., Leptonema illini, and Rickettsia rickettsii for the 39-kilodalton antigen (P39) of Borrelia burgdorferi. J. Clin. Microbiol. 30:735-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scriba, M., J. S. Ebrahim, T. Schlott, and H. Eiffert. 1993. The 39-kilodalton protein of Borrelia burgdorferi: a target for bactericidal human monoclonal antibodies. Infect. Immun. 61:4523-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson, W. J., W. Burgdorfer, M. E. Schrumpf, R. H. Karstens, and T. G. Schwan. 1991. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J. Clin. Microbiol. 29:236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simpson, W. J., W. Cieplak, M. E. Schrumpf, A. G. Barbour, and T. G. Schwan. 1994. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol. Lett. 119:381-388. [DOI] [PubMed] [Google Scholar]
- 66.Simpson, W. J., M. E. Schrumpf, and T. G. Schwan. 1990. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J. Clin. Microbiol. 28:1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skare, J. T., T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, J. Bunikis, S. Bergström, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1997. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect. Immun. 65:3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skare, J. T., E. S. Shang, D. M. Foley, D. R. Blanco, C. I. Champion, T. Mirzabekov, Y. Sokolov, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1995. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J. Clin. Investig. 96:2380-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevenson, B., T. Bykowski, A. E. Cooley, K. Babb, J. C. Miller, M. E. Woodman, K. von Lackum, and S. P. Riley. 2006. The Lyme disease spirochete Erp lipoprotein family: structure, function and regulation of expression, p. 354-372. In F. C. Cabello, H. P. Godfrey, and D. Hulinska (ed.), Molecular biology of spirochetes. IOS Press, Amsterdam, The Netherlands.
- 70.Stevenson, B., H. A. Choy, M. Pinne, M. L. Rotondi, M. C. Miller, E. DeMoll, P. Kraiczy, A. E. Cooley, T. P. Creamer, M. A. Suchard, C. A. Brissette, A. Verma, and D. A. Haake. 2007. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS One 2:e1188. doi: 10.1371/journal/pone.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang, X., H. Izadi, A. S. Coleman, P. Wang, Y. Ma, E. Fikrig, J. Anguita, and U. Pal. 2008. Borrelia burgdorferi lipoprotein BmpA activates pro-inflammatory responses in human synovial cells through a protein moiety. Microbes Infect. 10:1300-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zambrano, M. C., A. A. Beklemisheva, A. V. Bryskin, S. A. Newman, and P. C. Cabello. 2004. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect. Immun. 72:3138-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zückert, W. R. 2007. Laboratory maintenance of Borrelia burgdorferi, p. 12C.1.1-12C.1.10. In R. Coico et al. (ed.), Current protocols in microbiology. Wiley, Hoboken, NJ. [DOI] [PubMed]