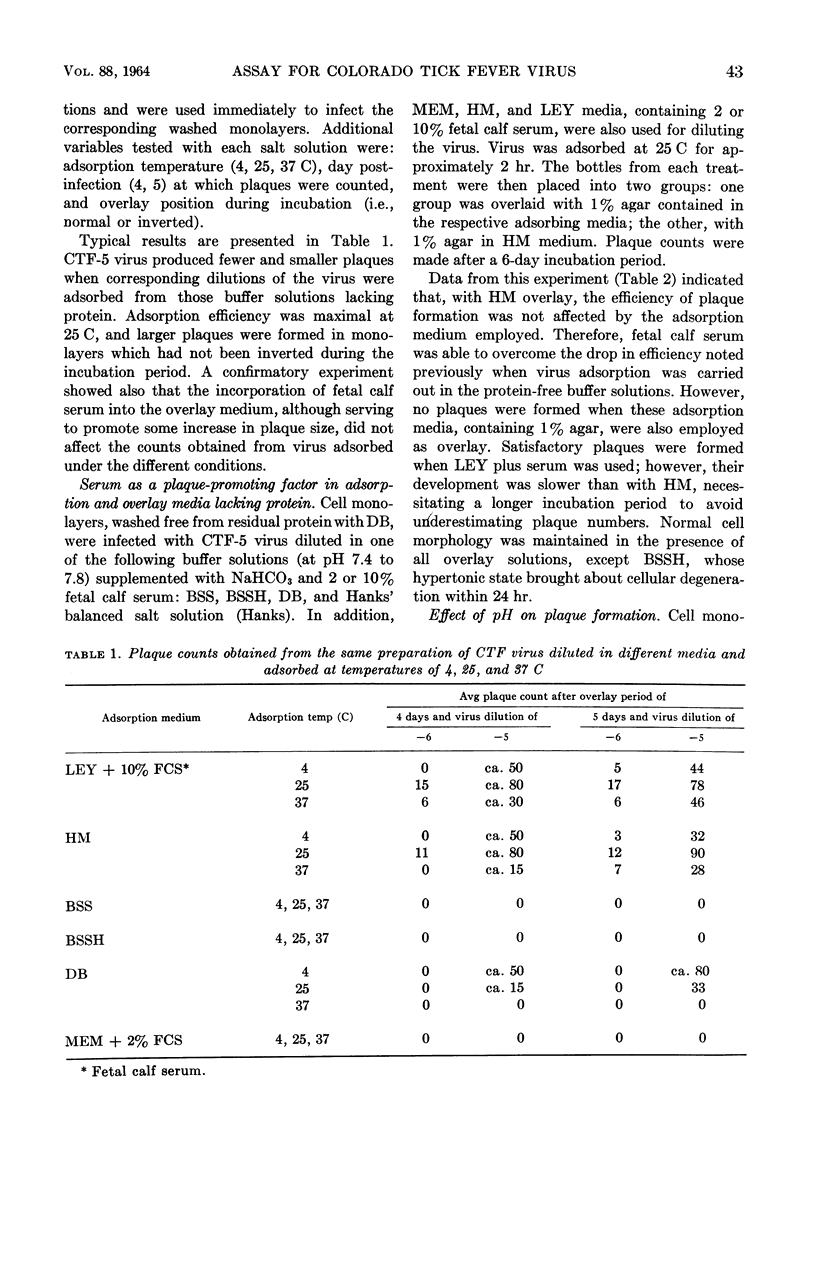
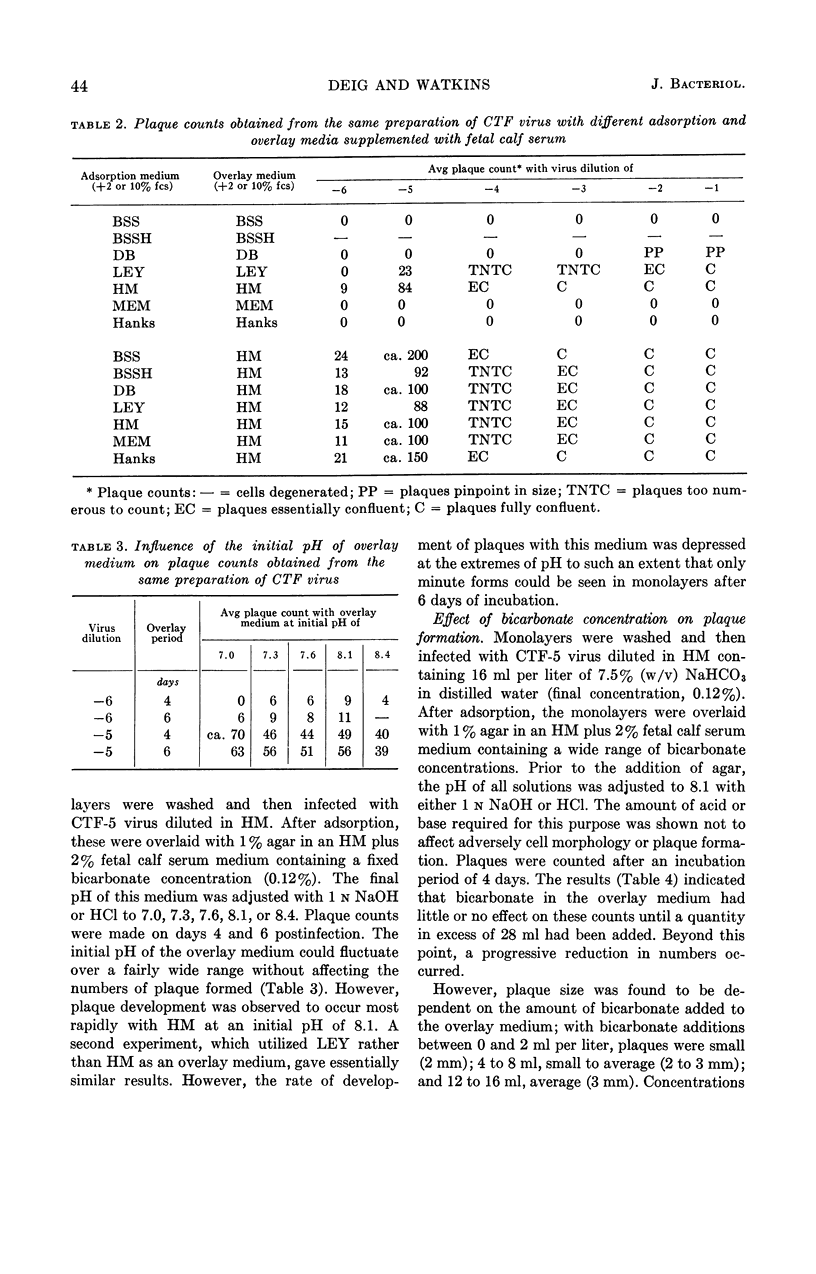
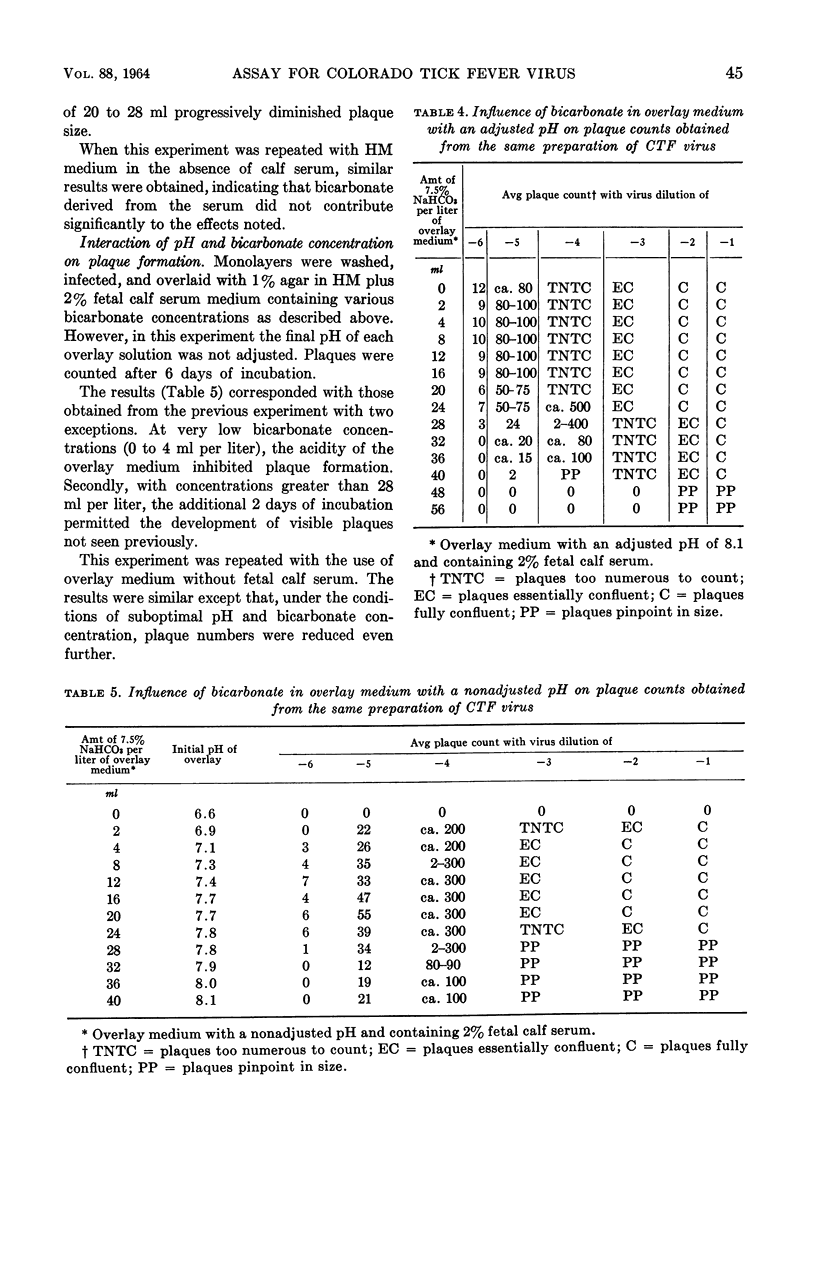
Abstract
Deig, E. Frank (University of California, Berkeley), and H. M. S. Watkins. Plaque assay procedure for Colorado tick fever virus. J. Bacteriol. 88:42–47. 1964.—A reproducible plaque assay procedure is described for the quantitation of Colorado tick fever virus in a cell line established from embryonic hamster tissue. Under the best conditions, plaques approximately 4 mm in diameter were formed after incubation at 37 C of 4 to 6 days. Several environmental variables in the procedure were studied. Efficiency was increased markedly by combining the virus during adsorption with serum proteins, and by carrying out this step at 25 C rather than at 37 C. The overlay medium used contained metabolites which promoted cell viability for periods greater than 1 week, and allowed plaques to develop. Plaque formation was relatively insensitive to a variation in pH between 7.1 and 8.1 (with optimal concentrations of bicarbonate). However, plaque development was inhibited with medium containing greater than 0.22% bicarbonate (at optimal pH), or when the initial pH was less than 7.0.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANG R. S. Participation of bicarbonate in RNA and protein synthesis as indicated by virus propagation in human cells. J Exp Med. 1959 Mar 1;109(3):229–238. doi: 10.1084/jem.109.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIFFORD G. E., ROBERTSON H. E., SYVERTON J. T. Propagation in vitro of polioviruses. VIII. Effect of pH on virus yield and cell metabolism. Proc Soc Exp Biol Med. 1956 Nov;93(2):321–323. doi: 10.3181/00379727-93-22743. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Effect of sodium bicarbonate concentration on plaque formation of virulent and attenuated polioviruses. J Immunol. 1958 Apr;80(4):282–293. [PubMed] [Google Scholar]
- LOCKART R. Z., GROMAN N. B. [Some factors influencing the interaction of Western equine encephalomyelitis and selected host cells]. J Infect Dis. 1958 Sep-Oct;103(2):163–171. doi: 10.1093/infdis/103.2.163. [DOI] [PubMed] [Google Scholar]
- WESTWOOD J. C., APPLEYARD G., TAYLOR-ROBINSON D., ZWARTOUW H. T. The production of high titre poliovirus in concentrated suspensions of tissue culture cells. Br J Exp Pathol. 1960 Apr;41:105–111. [PMC free article] [PubMed] [Google Scholar]
- ZWARTOUW H. T., TAYLOR-ROBINSON D., WESTWOOD J. C. Growth of high-titer poliovirus in nutrient-free saline suspensions of ERK cells. Virology. 1960 Apr;10:393–405. doi: 10.1016/0042-6822(60)90124-0. [DOI] [PubMed] [Google Scholar]


