Abstract
Anthrax lethal and edema toxins (LeTx and EdTx, respectively) form by binding of lethal factor (LF) or edema factor (EF) to the pore-forming moiety protective antigen (PA). Immunity to LF and EF protects animals from anthrax spore challenge and neutralizes anthrax toxins. The goal of the present study is to identify linear B-cell epitopes of EF and to determine the relative contributions of cross-reactive antibodies of EF and LF to LeTx and EdTx neutralization. A/J mice were immunized with recombinant LF (rLF) or rEF. Pools of LF or EF immune sera were tested for reactivity to rLF or rEF by enzyme-linked immunosorbent assays, in vitro neutralization of LeTx and EdTx, and binding to solid-phase LF and EF decapeptides. Cross-reactive antibodies were isolated by column absorption of EF-binding antibodies from LF immune sera and by column absorption of LF-binding antibodies from EF immune sera. The resulting fractions were subjected to the same assays. Major cross-reactive epitopes were identified as EF amino acids (aa) 257 to 268 and LF aa 265 to 274. Whole LF and EF immune sera neutralized LeTx and EdTx, respectively. However, LF sera did not neutralize EdTx, nor did EF sera neutralize LeTx. Purified cross-reactive immunoglobulin G also failed to cross-neutralize. Cross-reactive B-cell epitopes in the PA-binding domains of whole rLF and rEF occur and have been identified; however, the major anthrax toxin-neutralizing humoral responses to these antigens are constituted by non-cross-reactive epitopes. This work increases understanding of the immunogenicity of EF and LF and offers perspective for the development of new strategies for vaccination against anthrax.
Infectious agents with biological-weapon potential have become the focus of intense interest since the malicious release of anthrax spores through the U.S. postal system in 2001. Bacillus anthracis, the etiological agent of anthrax infection, is a gram-positive, rod-shaped, spore-forming bacterium that is commonly found in soil (37). The use of this agent as a bioterror weapon has highlighted the importance of developing improved vaccination strategies.
The virulence of B. anthracis is attributable to a tripartite protein complex consisting of the receptor binding component protective antigen (PA) and two catalytic components, lethal factor (LF) and edema factor (EF). Combination of PA and LF forms lethal toxin (LeTx), and combination of PA and EF forms edema toxin (EdTx) (26). Interestingly, the PA-binding domains of both EF and LF, corresponding to the N-terminal regions, have been shown to share large regions of structural and amino acid similarities that have been implicated in binding to PA (6, 9, 17, 20). The simultaneous addition of an excess of LF to cells treated with EF plus PA (EdTx) prevented an increase of cyclic AMP (cAMP) in vitro (21). Monoclonal antibodies have also been shown to inhibit the binding of EF to PA, and these antibodies also recognize epitopes within the PA-binding domain of EF (22). In addition, binding of LF-neutralizing antibodies to EF by enzyme-linked immunosorbent assays (ELISAs) suggests that host immune responses against these domains may prevent toxin components from entering target cells (23). While studies have shown that even when aggressive, early antibiotic therapy eradicates bacterial load within 72 h, anthrax toxins are still present in concentrations sufficient to cause death (8, 16). Since death can result even with bacterial clearance, vaccine- or toxin-directed immunotherapeutic development is essential to prevent or stop infection at an early stage.
The human vaccine currently available in the United States, anthrax vaccine absorbed (AVA), contains mainly PA as the protective component. AVA has many disadvantages, including a complicated dosing schedule (five intramuscular injections with yearly boosters), batch-to-batch variation of the protective bacterial components, limited duration of protection, requirement for containment facilities for production, and transient reactogenicity in many vaccinees (14, 15, 30, 37). Second-generation vaccines based on recombinant PA are currently in development; however, these vaccines will not elicit antibodies to LF and EF (2). Although PA has been shown to be the main protective component in the currently licensed vaccine, studies in which mice were immunized with strains of mutant B. anthracis revealed the significant individual contributions of antibodies to EF and LF toward immunoprotection (27, 29). Further studies have shown that immunization with a DNA construct encoding the N-terminal fragment of EF elicited protective immunity against a subcutaneous challenge of A/J mice with the B. anthracis Sterne strain (38). Moreover, our studies have demonstrated that immunization with recombinant LF (rLF) can induce high-titer protective antibodies in vivo and in vitro (28).
Despite significant achievements toward understanding the contribution of EF and LF antibodies to protection, considerable gaps remain in understanding the fine specificity of the protective responses to these components of the tripartite toxin. The purposes of this study were to identify sequential B-cell epitopes within EF and to determine the relative contributions of cross-reactive antibodies in the conserved PA-binding domains of EF and LF to LeTx and EdTx neutralization. Host immune responses against these cross-reactive domains may prevent both EF and LF from gaining access into cells. We hypothesized that the protective host immune response following EF and LF vaccination would include antibodies directed to cross-reactive epitopes that prevent binding to PA and thus entry into target cells. We found that immunization of A/J mice with recombinant EF (rEF) generated high-antibody titers capable of neutralizing EdTx in vitro and in vivo. Furthermore, cross-reactive B-cell epitopes in the PA-binding domains of whole rLF and rEF have been identified. However, there was no evidence for contribution of these epitopes to toxin neutralization.
MATERIALS AND METHODS
Mice.
Six-week-old A/J strain female mice were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were housed in the Oklahoma Medical Research Foundation (OMRF) Chapman Animal Facility. All mouse experiments were approved by the OMRF Institutional Animal Care and Use Committee.
Immunizations and blood sampling.
For antibody studies, experimental mice (18 to 20 mice per independent mapping experiment) were immunized subcutaneously with rEF protein (100 μg) emulsified 1:1 in complete Freund's adjuvant (Difco, Lawrence, KS) on day 0 according to a previously described immunization schedule (11). Mice were boosted three times with 50 μg of the immunizing protein emulsified 1:1 in incomplete Freund's adjuvant on days 10, 24, and 38. Control mice (18 to 20 mice per independent mapping experiment) were similarly immunized and boosted with adjuvant alone. Retro-orbital bleeds were performed on anesthetized mice on days 14, 28, 42, and 111. Immunization and blood sampling were carried out on a total of three separate and independent occasions to ensure reproducibility of data, in which groups of mice (18 to 20 mice per group) were immunized with either rEF or adjuvant alone on each independent occasion.
Production of rEF and rLF proteins.
rEF and rLF proteins were produced as amino-terminal His6-tagged proteins as previously described (31, 36). Cultures of BL-21/pET15b (containing LF and EF cDNA from B. anthracis) were grown in Luria broth containing ampicillin (50 μg/ml) to an optical density at 600 nm (OD600) of 0.6 to 0.8, and protein expression was induced by addition of 1 mM isopropyl β-d-thiogalactoside (IPTG) overnight at 16°C. Cells were then pelleted and disrupted by using a French press. The lysed cells were then centrifuged, and the supernatant was passed over a Ni2+-charged column equilibrated with a binding buffer (Novagen, Gibbstown, NJ). The bound fusion protein was removed with 0.5 M imidazole in accordance with the manufacturer's instructions (Novagen, Gibbstown, NJ). The eluted protein was then dialyzed against 20 mM Tris-HCl (pH 7.5). The protein concentration was determined by a standard Bradford assay (Bio-Rad, Hercules, CA), and the protein was stored at 4°C on ice. Purified EF and LF (20 μg) were electrophoresed in 12.5% sodium dodecyl sulfate-polyacrylamide gels and visualized by Coomassie stains to ensure the purity of the samples.
Standard ELISA.
Ninety-six-well microtiter plates (Corning CoStar, Lowell, MA) were coated with 2.5 μg of antigen/ml in carbonate coating buffer (0.125 μg of antigen/well; pH 9.6) overnight at 4°C. The plates were then washed four times with 0.05% Tween 20 in phosphate-buffered saline (PBS) and blocked with 0.1% bovine serum albumin-0.2% NaN3 in PBS for 1 h at room temperature (RT). Serial twofold dilutions of the samples were carried out, starting at 1:50 and ending at 1:25,600, in PBS. Plates were washed again, and appropriately diluted samples were added to the plates and incubated for 2 h at RT. After the wash, alkaline phosphatase-labeled goat anti-mouse immunoglobulin G (IgG [H+L]; Sigma, Saint Louis, MO) diluted at 1:5,000 was added. After a 2-h incubation at RT, ρ-nitrophenyl phosphate (Sigma, St Louis, MO) in substrate buffer (0.167 M NaHCO3, 0.012 M Na2CO3, 0.001 M MgCl2, 0.02% NaN3) was added to the plates, and the ODs of the plates were measured at 410/490 nm. Titer was defined as the inverse of the last serum dilution giving a positive signal. Positive signals were defined as OD values exceeding 3 standard deviations (SD) above the mean 1:50 dilution values for samples from control mice immunized with adjuvant alone.
In vitro LeTx neutralization assay.
RAW 264.7 mouse macrophages were grown in Dulbecco's modified Eagle's medium containing 4 mM l-glutamine (ATCC) supplemented with 10% fetal calf serum at 37°C in a 5% CO2-95% air atmosphere. To determine toxin neutralization, 1 × 105 cells were plated per well of a 96-well flat-bottom tissue culture plate and cultured overnight at 37°C with 5% CO2. The next day, serum samples were diluted at twofold dilutions starting at 1:2 and ending at 1:2,048 in culture medium. These serum samples were then incubated for 1 h at RT with LeTx (composed of a 3:1 PA-to-LF ratio [75 ng/well PA and 25 ng/well LF; final well volume = 100 μl]). Following incubation, the medium was removed from the cultured cells and 100 μl of the serum-toxin mixture was added per well. Cells treated with medium alone served as negative controls. An additional set of controls involved treatment of cells with LF alone, PA alone, and LeTx alone. All experiments were carried out in duplicate. Following addition of the serum-toxin mixture, the cells were incubated for 2 h at 37°C with 5% CO2. After 2 h, 10 μl of WST-8 (CCK-8; Dojindo Molecular Technologies, Inc., Gaithersburg, MD) was added to each well and incubated for an additional 3 h at 37°C with 5% CO2. The OD450 was detected using a Dynex MRX II microplate reader (Dynex Technologies, Chantilly, VA). Percent viability was calculated based on the absorbance reading from the control wells containing cells only (medium alone). Neutralization titer was defined as the inverse of the last serum dilution giving detectable neutralization compared to the level for wells exposed to active toxin alone.
In vitro EdTx neutralization assay.
RAW 264.7 mouse macrophages were grown in Dulbecco's modified Eagle's medium containing 4 mM l-glutamine (ATCC) supplemented with 10% fetal calf serum at 37°C in a 5% CO2-95% air atmosphere. To determine toxin neutralization, we utilized a technique adapted from Taft and Weiss (33). RAW 264.7 cells were harvested to 2 × 106 cells/ml, and 100 μl was added to a 96-well flat-bottomed tissue culture plate for 2 h. Serum dilutions were then prepared similarly to those for the in vitro LeTx neutralization assay, starting at 1:50 and ending at 1:1,600, and incubated with EdTx (composed of a 3:1 PA-to-EF ratio [75 ng/well PA and 25 ng/well EF; final well volume = 100 μl]) for 1 h at RT. Following incubation, the medium was removed from the cultured cells and 100 μl of the serum-toxin mixture was added per well. The plates were incubated for 4 h at 37°C in 5% CO2. Cells treated with medium served as controls. An additional set of controls involved treatment of cells with EF alone, PA alone, EdTx alone, and serum from mice immunized with adjuvant only. All experiments were carried out in duplicate. After 4 h, intracellular cAMP was measured utilizing a cAMP ELISA kit in accordance with the manufacturer's protocol (Cayman Chemical Company, Ann Arbor, MI). Briefly, the medium was removed, and the cells were lysed with 60 μl of 0.1 M HCl for 20 min at RT. After lysis, cells were spun at 1,000 × g for 10 min. Resulting supernatant (50 μl) or cAMP standards (50 μl), tracer (50 μl), and anti-cAMP antibody (50 μl) were added to a 96-well microplate coated with mouse monoclonal anti-rabbit IgG. The plate was incubated overnight at 4°C. The plate was then rinsed five times with washing buffer and then incubated with 200 μl of Ellman's reagent for 90 to 120 min. OD was determined using a Dynex MRX II microplate reader (Dynex Technologies, Chantilly, VA) at 405 and 420 nm as a reference wavelength. Percent neutralization was calculated based on the absorbance reading relative to the level for the control wells containing cells plus toxin only (toxin alone).
In vivo EdTx challenge.
rEF and rPA were produced as described above. rEF-immunized mice and mice immunized with adjuvant alone were challenged with four times the A/J 50% lethal dose (LD50) of EdTx (360 μg PA plus 144 μg EF, experimentally determined). Mice were then monitored for 7 days, and mortality was recorded. Survival curves and percent survival were generated using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Solid-phase peptide ELISAs.
To determine the fine specificity of the anti-LF, anti-EF, and cross-reactive antibodies, a solid-phase peptide ELISA was used. Peptides that were 10 amino acids (aa) in length overlapped by 8 aa and covered the entire length of the EF (GenBank accession number AAA79215) and LF (GenBank accession number AAM26117) proteins were covalently synthesized onto polyethylene pins in a 96-well format as previously described (24, 25). Wash steps and incubations were carried out using sealed plastic containers. Other assay steps were performed by lowering the solid-phase peptides into microtiter plate wells. First, pins were blocked with 3% low-fat milk in PBS for 1 h at RT. Solid-phase peptides were then incubated in 1:200 dilutions of sera in 3% milk-PBS with 0.05% Tween for 2 h at RT, washed with PBS-Tween, and then incubated with a 1:20,000 dilution of horseradish peroxidase-labeled anti-mouse IgG conjugate (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, MD) at 4°C overnight. Following washing, solid-phase peptides were incubated with SureBlue Reserve TMB microwell peroxidase substrate (KPL) for 5 min at RT, and the reaction was terminated by the addition of TMB stop solution (KPL). The OD was measured at 450 nm by using a Dynex MRX II microplate reader (Dynex Technologies). Results for each plate were then standardized by comparison with standard positive peptides. The same control peptides were used for all plates and were allowed to develop to a specific OD with a known concentration of a standard control serum. After completion of an assay, solid-phase peptide supports were sonicated for 2 h in sonication buffer (40 g sodium dodecyl sulfate, 4 ml β-mercaptoethanol, and 62.4 g sodium phosphate to give 4 liters) to remove antibodies, conjugate, and substrate. After sonication, solid-phase peptides were washed twice in hot water and boiled in methanol for 2 min. Pins were then allowed to air dry for 15 min and were stored with desiccant or used for another assay. An epitope was defined as two or more consecutive solid-phase peptide responses exhibiting an OD450 greater than or equal to the average plus 3 SD of “adjuvant only” sera on all decapeptides.
Column absorption.
rEF and rLF antigens were both produced as described above. These antigens were bound onto cyanogen bromide-preactivated Sepharose 4B medium individually, according to the manufacturer's protocol (GE Healthcare, Piscataway, NJ). To absorb the antigen-specific or cross-reactive antibodies, serum was passed at least three times over the columns to ensure depletion of antigen-specific or cross-reactive IgG. Columns were than washed with running buffer (0.02 M Tris, 0.15 NaCl, pH 7.4) and subsequently eluted with 0.2 M glycine-HCl (pH 2.5). Eluates were collected into neutralization buffer (1 M Tris-HCl, pH 9). All samples were then subjected to buffer exchange and concentrated to the original start volumes (0.5 ml). Column-absorbed (retained) and three serial unabsorbed (unretained; UR #1, UR #2, and UR #3) fractions were then used in standard ELISAs to test for reactivity with rEF or rLF, solid-phase ELISAs were used to test for reactivity to overlapping decapeptides of LF or EF, and in vitro LeTx or EdTx neutralization assays were used as described above.
RESULTS
rEF immunization results in high-titer, neutralizing anti-EF antibodies and protection from EdTx challenge.
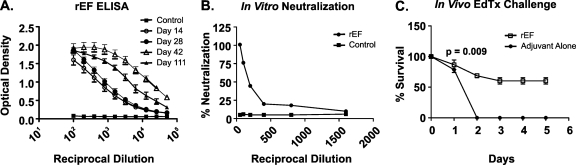
The antibody titers of female A/J mice immunized with rEF were evaluated. Groups (18 to 20 mice/group) of female A/J mice were immunized with rEF in complete Freund's adjuvant subcutaneously on day 0 and boosted with rEF in incomplete Freund's adjuvant on days 10, 24, and 38. Sera were collected 3 to 4 days after each boost to determine the anti-EF antibody levels by ELISA. Elevated antibody titers were observed in sera collected 2 weeks after the initial immunization and continued to intensify (>25,600) by day 42 (Fig. 1A). The titers of anti-rEF IgG were minimally diminished on day 111, nearly 73 days after the final booster immunization. Sera collected from mice immunized with adjuvant alone (control group) failed to bind EF antigen, as expected.
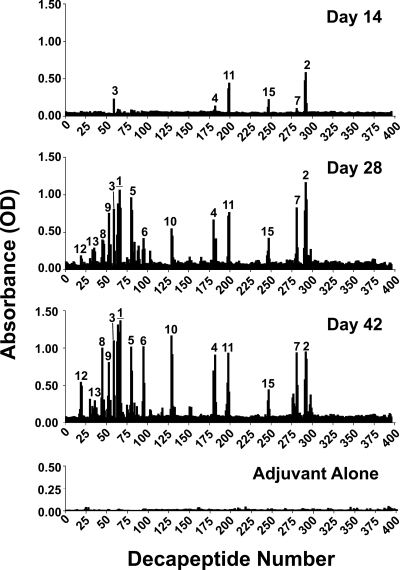
FIG. 1.
Immunization of A/J mice results in high-titer anti-EF antibodies that are neutralizing in vitro and protective in vivo. A/J mice were immunized with either rEF or adjuvant alone on days 10, 24, and 38 and bled on days 14, 28, 42, and 111. (A) IgG anti-EF antibody titers of pooled sera from A/J mice immunized with rEF or adjuvant alone were assessed at specified time points to quantitate the IgG anti-EF response. Antibody levels were measured via standard ELISA at OD410/490. (B) Day 111 sera collected were subjected to an in vitro neutralization assay using a 3:1 ratio of PA to EF (EdTx). Serial twofold dilutions (1:50 to 1:1,600) of anti-EF sera were incubated with a 3:1 ratio of PA to EF (EdTx) for 1 h and added to RAW 264.7 mouse macrophages for 4 h. The cells were lysed, and intracellular cAMP was monitored by ELISA. Percent neutralization was calculated relative to the level for the control wells containing toxin alone. Sera demonstrate near-maximal neutralization of toxin activity on day 111 (73 days past the final booster immunization). The neutralization titer of rEF antisera was 800. (C) A/J mice immunized with rEF are partially protected from an in vivo EdTx challenge using four times the experimentally derived A/J LD50 (360 μg PA plus 144 μg EF, experimentally determined) for EdTx (P = 0.009).
To determine the functionality of these antibodies elicited by immunization with rEF, an in vitro neutralization assay with anthrax EdTx was performed. Neither EF nor PA alone is capable of elevating cAMP concentrations in RAW 264.7 mouse macrophages. Only EF combined with PA (EdTx) increased intracellular cAMP (data not shown). These values were then used to calculate the percent neutralization relative to the level for control wells containing toxin only. Anti-EF sera were diluted at various concentrations and preincubated with an EdTx concentration (3:1 molar ratio of PA to EF) previously shown to result in maximal cAMP production in RAW 264.7 macrophages and then added to RAW 264.7 cells. In comparison to what was found for control wells receiving toxin only, we observed that anti-EF sera diluted 50-fold could completely neutralize EF and inhibit EdTx from generating elevated cAMP levels in RAW 264.7 macrophages. This response was measured 73 days after the last booster immunization. As expected, sera collected from controls immunized with PBS-adjuvant alone failed to neutralize EdTx (Fig. 1B).
To determine whether immunization with rEF can provide protection against EdTx challenge in vivo, the rEF-immunized A/J mice were challenged intraperitoneally with four times the experimentally determined A/J LD50 of EdTx (360 μg PA plus 144 μg EF) (Fig. 1C and data not shown). Figure 1C shows that approximately 60% of A/J mice immunized with rEF were protected from EdTx challenge but that none of the A/J controls immunized with adjuvant alone survived the challenge (P = 0.009). These results demonstrate that anti-EF antibodies elicited by immunization with rEF are functional neutralizing antibodies against EF and have the capacity to inhibit the activity of EdTx in vivo and in vitro, further supporting previous studies (22, 38) suggesting that humoral immunity directed to EF is protective.
Sequential mouse B-cell epitopes cluster in PA- and calmodulin-binding domains of EF.
To characterize the fine specificity of the neutralizing antisera from rEF-immunized A/J mice, we subjected pooled samples (pools contained equal volumes of sera taken from 18 to 20 mice per immunization group per independent experiment) to sequential humoral epitope mapping using a series of solid-phase, overlapping decamer peptides, representing the whole protein. Reproducible epitopes were defined by performing three independent sets of immunization and mapping experiments. Day 14, 28, and 42 bleeds were mapped as shown in Fig. 2. These results were compared to the day 42 results for mice immunized with adjuvant alone (control group). An antigenic epitope was defined as described in Materials and Methods. These epitopes were ranked in order of reproducibility and reactivity on the basis of results from three separate immunization and mapping experiments. The reproducibility and OD (average ± standard error of the mean) values are shown for each defined epitope in Table 1. These studies revealed 15 major antigenic regions of rEF. All epitopes defined on day 42 were still present on day 111, with increases in the intensities of epitopes 4, 5, 8, 9, 13, and 14 (data not shown). Mice immunized with adjuvant alone did not significantly bind any of the decapeptides.
FIG. 2.
Fine specificity of the humoral anti-EF response following A/J immunization. Antibody binding to decapeptides of EF overlapping by 8 aa was measured using a solid-phase ELISA. Shown is the antibody response in sera from rEF/adjuvant-immunized mice on days 14, 28, and 42 and sera from mice immunized with adjuvant alone. Data represent the average of results from three independent immunization/mapping experiments. Mapping within each experiment was performed using sera pooled from 18 to 20 mice. Epitopes were numbered in order of reproducibility and reactivity on day 42, as shown in Table 1. Epitope numbers correspond to epitope numbers in Tables 1 and 2. Horizontal lines indicate extended antigenic regions of epitope 1 as defined on day 42.
TABLE 1.
EF epitope reproducibility
| Epitope | Decapeptide(s) | Amino acids | Epitope reproducibility valuea | OD (avg ± SEM)b |
|---|---|---|---|---|
| 1 | 63-67 | 125-142 | 3 | 1.17 ± 0.21103 |
| 2 | 291-293 | 580-593 | 3 | 0.883 ± 0.09214 |
| 3 | 59, 60 | 117-128 | 3 | 0.674 ± 0.06339 |
| 4 | 180-182 | 359-372 | 3 | 0.639 ± 0.05979 |
| 5 | 80-82 | 159-172 | 3 | 0.634 ± 0.02611 |
| 6 | 94-96 | 187-200 | 3 | 0.532 ± 0.11416 |
| 7 | 280-282 | 558-571 | 3 | 0.520 ± 0.05566 |
| 8 | 44-47 | 87-102 | 3 | 0.442 ± 0.05572 |
| 9 | 52-55 | 103-118 | 3 | 0.418 ± 0.06338 |
| 10 | 129-131 | 257-270 | 2 | 0.592 ± 0.25024 |
| 11 | 196-199 | 391-406 | 2 | 0.611 ± 0.20085 |
| 12 | 19, 20 | 37-48 | 2 | 0.522 ± 0.12608 |
| 13 | 30 | 59-68 | 1 | 0.320 ± 0.15948 |
| 14 | 35, 36 | 69-80 | 1 | 0.276 ± 0.02943 |
| 15 | 246-248 | 490-503 | 1 | 0.258 ± 0.13478 |
This value is the number of independent immunization and mapping experiments out of three in which a region was identified as an epitope. An epitope was defined as described in Materials and Methods.
Values shown were obtained using the results from three independent epitope mapping studies.
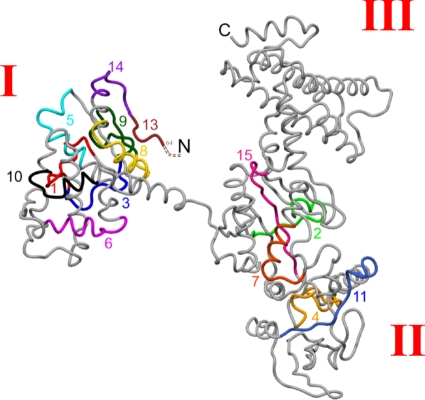
Superimposition of these 15 identified epitopes onto the EF crystal structure, shown in Fig. 3, reveals clustering of epitopes in the PA- and calmodulin-binding domains. Sequences, domain locations, and secondary structures of the epitopes are shown in Table 2. Specifically, 10 epitopes were in the PA-binding domain and 5 epitopes were in the calmodulin-binding domain (Table 2). Significantly, 4 of the 10 epitopes (epitope numbers 5, 6, 8, and 10) located in the PA-binding domain occur within reported regions of amino acid similarity between EF and LF, and the remaining 6 epitopes have amino acid similarities to LF ranging from 67 to 92% based on the alignment of Bragg and Robertson (6). Interestingly, no epitopes were located in the helical domain (domain III) (Fig. 3 and Table 2).
FIG. 3.
Sequential epitopes of EF. The 15 most reactive A/J epitopes are superimposed onto the EF crystal structure (Protein Data Bank access code 1J7N) (5). Roman numerals indicate structural domain numbers. Epitope numbers correspond to those in Fig. 2 and Tables 1 and 2.
TABLE 2.
Most-reactive EF epitopesa
| Epitope | Sequence | Domain | Domain function | Secondary structure |
|---|---|---|---|---|
| 12 | HYTESDIKRNHK | I | PA binding | NA |
| 13 | FKDSINNLVK | I | PA binding | Beta |
| 14 | TEFTNETLDKIQ | I | PA binding | Beta/loop/alpha |
| 8 | KKIPKDVLEIYSELGG | I | PA binding | Alpha helix |
| 9 | EIYFTDIDLVEHKELQ | I | PA binding | Beta/beta/loop |
| 3 | LQDLSEEEKNSM | I | PA binding | Loop |
| 1 | KNSMNSRGEKVPFASRFV | I | PA binding | Loop/alpha/beta |
| 5 | YAINSEQSKEVYYE | I | PA binding | Loop/alpha |
| 6 | SLDPEFLNIKSLS | I | PA binding | Loop/alpha |
| 10 | LELYAPDMFEYMNK | I | PA binding | Alpha helix |
| 4 | PVAGYIPFDQDLSK | II | Catalytic core (CaM binding) | Loop/beta/loop |
| 11 | ITEHEGEIGKIPLKLD | II | Catalytic core (CaM binding) | Alpha/beta/loop |
| 15 | DYDLFALAPSLTEI | II | Catalytic core (CaM binding) | Loop/beta/alpha |
| 7 | RLNEAVKYTGYTGG | II | Catalytic core (CaM binding) | Alpha/loop |
| 2 | QDNEEFPEKDNEIF | II | Catalytic core (CaM binding) | Loop |
The bolded sequence represents a portion of the published amino acid sequence implicated in binding to PA (22). NA, not available.
Cross-reactive humoral epitopes of EF and LF are identified but do not constitute major anthrax toxin neutralization responses following EF and LF vaccination.
In order to measure the levels of cross-reactive IgG titers present in EF- and LF-immunized mice, we subjected their sera to EF and LF standard ELISAs (Fig. 4A). For these studies, we utilized day 42 sera from LF-immunized mice and day 111 sera from EF-immunized mice, which were available in sufficient quantities. The IgG titers of both LF sera on EF antigen (Fig. 4A, upper right) and EF sera on LF antigen (lower left) were up to 3,200. In contrast, EF and LF sera tested on their respective antigens contained titers up to 25,600, indicating that cross-reactive IgG constitutes only a small portion of the total antigen-specific IgG.
FIG. 4.
Cross-reactivity assays using sera from rEF- and rLF-immunized mice. (A) IgG anti-EF and anti-LF antibody titers of pooled sera from A/J mice immunized with rEF (day 111 sera) or rLF (day 42 sera) were assessed to quantitate the levels of cross-reactive IgG in each sample. Antibody levels were measured by using a standard ELISA at OD410 and OD490. (B) The fine specificity of the humoral cross-reactive response was measured using overlapping decapeptides of EF or LF. The data are the antibody responses in sera from rEF (day 111 sera) and rLF (day 42 sera) immunized mice on both EF and LF decapeptide pins. Significant epitopes are defined as the average for the background plus 3 SD. (C) EF (day 111) and LF (day 42) sera (serial twofold dilutions from 1:2 to 1:2,048) were both subjected to LeTx and EdTx in vitro neutralization assays as described in Materials and Methods. EF and LF immune sera neutralized EdTx and LeTx, respectively. The LeTx neutralization titer for LF immune sera was 1,024, and the EdTx neutralization titer for EF immune sera was 256. However, EF sera did not neutralize LeTx, nor did LF sera neutralize EdTx. Control sera are sera from mice immunized with adjuvant alone.
To examine the fine specificity of these cross-reactive IgG, we performed a standard solid-phase peptide mapping assay as described above. As shown in Fig. 4B, LF sera on EF decapeptides (upper right) and EF sera on LF decapeptides (lower left) bound sequential epitopes of EF and LF, respectively. Specifically, four cross-reactive epitopes were identified in LF sera (Fig. 4B, upper right) and six in EF sera (Fig. 4B, lower left). Furthermore, the decapeptides bound by the pooled sera were located in regions of amino acid similarity (data not shown) (6). To determine if these antisera could cross-neutralize toxin activity, both EF and LF serum samples were than subjected to EdTx and LeTx in vitro neutralization assays. As shown in Fig. 4C, whole LF and EF immune sera neutralized LeTx and EdTx, respectively. However, LF sera did not cross-neutralize EdTx, nor did EF sera cross-neutralize LeTx, even at concentrated 1:2 serum dilutions (Fig. 4C).
Given that cross-neutralization of LeTx by sera from mice immunized with an adenovirus encoding the N-terminal fragment of EF was reported in studies conducted by Zeng et al., (38) and that the cross-reactive IgG in our whole sera represented only a relatively small fraction of the total anti-EF or anti-LF responses, we utilized whole antigen affinity chromatography to enrich for cross-reactive IgG in the serum samples. We reasoned that isolation and purification of these cross-reactive antibodies may enhance their neutralization capacity, so we absorbed sera from EF-immunized mice over an LF column and sera from LF-immunized mice over an EF column to isolate cross-reactive IgG. Unretained and retained fractions collected from these columns were then tested in the EF and LF standard ELISAs, in vitro neutralization assays, and solid-phase ELISAs.
To ensure successful purification and depletion of cross-reactive IgG from appropriate samples, we tested all fractions in EF and LF standard ELISAs. As shown in Fig. S1 in the supplemental material, retained fractions from EF sera absorbed with the LF column (upper right; “Ret. Pool” LF column samples) and LF sera absorbed with the EF column (lower left; “Ret. Pool” EF column samples) demonstrated successful purification of cross-reactive IgG. Moreover, examination of unretained fractions (UR #1, UR #2, and UR #3) from these columns revealed successful depletion of cross-reactive IgG as anticipated.
Retained fractions, containing cross-reactive IgG, were then mapped onto EF and LF overlapping decapeptides to characterize their fine specificities (Fig. 5). Though multiple cross-reactive epitopes were identified, there were only a few major cross-reactive epitopes that were strongly bound and were also raised by both rEF and rLF immunization. These major epitopes are represented by bolded values in Table 3, in which the appropriate amino acid positions and ODs are also indicated. Interestingly, sequence comparison of these epitopes (EF aa 257 to 270 [LELYAPDMFEYMNK] and LF aa 263 to 278 [RDVLQLYAPEAFNYM]) reveals identical (bolded) or similar (underlined) amino acids that are also located in one of the major regions of known homology (6). There were also other weakly cross-reactive epitopes that were induced only in either rEF- or rLF-immunized mice (Table 3). Most of these epitopes also lie in regions of amino acid similarity in the PA-binding domains of EF and LF (data not shown). In addition, the EF cross-reactive epitopes identified constitute 6 of the 15 identified major EF epitopes (Table 1), and the LF cross-reactive epitopes constitute 9 of the 16 epitopes identified in an earlier study (28).
FIG. 5.
Fine specificity of cross-reactive IgG from column purifications using sera from A/J mice immunized with rEF (day 111 sera) and rLF (day 42 sera). Retained samples containing EF and LF cross-reactive IgG were examined for binding to overlapping decapeptides of both EF and LF by solid-phase ELISA.
TABLE 3.
Cross-reactive epitopesa
| Sample group | Decapeptide(s) | Amino acids | OD (range) |
|---|---|---|---|
| Edema factor epitopes | |||
| EF sera over LF column | 44, 45 | 87-98 | 0.873-1.696 |
| 67, 68 | 133-144 | 0.289-0.562 | |
| 98 | 195-204 | 0.159 | |
| 129, 130 | 257-268 | 0.948-1.675 | |
| 151-153 | 301-314 | 0.109-0.130 | |
| 184 | 367-376 | 0.152 | |
| LF sera over EF column | 23, 24 | 45-56 | 0.135-0.595 |
| 59 | 117-126 | 0.888 | |
| 80 | 159-168 | 0.487 | |
| 103 | 205-214 | 0.588 | |
| 109 | 217-226 | 0.448 | |
| 117 | 233-242 | 0.479 | |
| 124 | 247-256 | 0.436 | |
| 129-131 | 257-270 | 0.612-1.137 | |
| 133 | 265-274 | 0.551 | |
| 148, 149 | 291-306 | 0.434-0.581 | |
| 151-154 | 301-316 | 0.357-0.772 | |
| 167-173 | 333-354 | 0.437-0.608 | |
| 182-184 | 363-376 | 0.478-0.624 | |
| Lethal factor epitopes | |||
| EF sera over LF column | 50 | 99-108 | 0.264 |
| 97 | 193-202 | 0.182 | |
| 124 | 247-256 | 0.251 | |
| 132, 133 | 263-274 | 0.312-0.389 | |
| 193 | 385-394 | 0.116 | |
| 196 | 391-400 | 0.117 | |
| 204 | 407-416 | 0.102 | |
| LF sera over EF column | 28 | 55-64 | 0.169 |
| 109, 110 | 217-228 | 0.265-0.268 | |
| 133-135 | 265-278 | 0.207-0.718 | |
| 145 | 289-298 | 0.245 | |
| 150 | 299-308 | 0.189 | |
| 160, 161 | 319-330 | 0.168-0.188 | |
| 172-174 | 343-356 | 0.174-0.495 | |
| 182 | 363-372 | 0.249 | |
| 193 | 385-394 | 0.329 | |
| 196 | 391-400 | 0.189 | |
| 220, 221 | 439-450 | 0.168-0.221 |
Bold indicates cross-reactive epitopes raised in both rEF- and rLF-immunized mice.
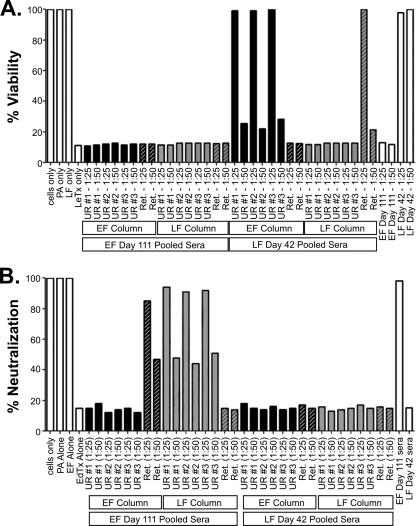
Column samples were then tested in LeTx and EdTx in vitro neutralization assays to determine if these cross-reactive and non-cross-reactive antibodies neutralize toxin activity (Fig. 6). The non-cross-reactive unretained fraction of LF immune serum from the EF column successfully neutralized LeTx as expected (Fig. 6A). Likewise, the non-cross-reactive unretained fraction of EF immune serum from the LF column successfully neutralized EdTx (Fig. 6B). More importantly, cross-reactive fractions (EF immune sera retained on the LF column and LF immune sera retained on the EF column) not only failed to cross-neutralize but, surprisingly, also failed to directly neutralize the toxin activity corresponding to the parental proteins of immunization. These studies have revealed and identified cross-reactive B-cell epitopes in the PA-binding domains of whole rLF and rEF. However, the major anthrax toxin-neutralizing humoral responses to these antigens are constituted by non-cross-reactive epitopes that may reside either within or outside of their PA-binding domains.
FIG. 6.
LeTx and EdTx in vitro neutralization assays with cross-reactive IgG from column purifications. Column-purified sera, serial unretained fractions (UR #1, UR #2, and UR #3), and the retained pool (Ret. Pool) were tested in both in vitro LeTx and EdTx neutralization assays. (A) LeTx toxin neutralization data with sera from both EF-immunized and LF-immunized A/J mice absorbed on EF and LF columns. (B) EdTx toxin neutralization data with sera from both EF-immunized and LF-immunized A/J mice absorbed on EF and LF columns.
DISCUSSION
Infectious agents with biological weapon potential have become the focus of intense interest since the terrorist events and subsequent anthrax attacks of 2001. Indeed, anthrax spores continue to be one of the most commonly identified potential risks for bioterrorism. Several problems with the currently licensed U.S. AVA vaccine, including limited production capabilities, questions regarding safety and efficacy, an onerous vaccination schedule, and lack of induced immunity to EF and LF, have prompted efforts to develop new vaccination approaches that address these issues.
Although PA is the main protective component in the AVA vaccine, studies in which mice were immunized with mutant strains of B. anthracis that expressed each toxin component independently revealed significant individual contributions of antibodies to LF and EF to immunoprotection (3, 4, 29, 35). Moreover, several studies also indicate that antibodies to both EF and LF can protect experimental animals from challenge with anthrax toxins or bacteria (1, 7, 9, 19, 32, 38, 39). Therefore, investigations in our laboratory are focused on defining protective responses to LF and EF components of the tripartite anthrax toxin and using this information to design new vaccination approaches that include well-defined determinants of these proteins. In a previous study, we defined sequential B-cell epitopes of the neutralizing IgG response to rLF (28). In the present study, we aimed to increase our understanding of the neutralizing humoral response to EF by defining sequential B-cell epitopes elicited by rEF immunization. Furthermore, we examined the cross-reactive IgG response between the N-terminal regions of rEF and rLF proteins and assessed the role that these antibodies play in toxin neutralization. The N-terminal regions of EF and LF share structural and amino acid similarities that have been implicated in binding to PA (6, 18, 20). Therefore, the goal of this portion of our study was to identify protective cross-reactive epitopes in the conserved PA-binding domains of EF and LF.
We determined that immunization of mice with rEF elicits anti-EF antibody responses that inhibit the toxicity of EdTx in vitro and in vivo. Using a solid-phase ELISA technique, we identified 15 antigenic regions of EF, all of which were located in the PA-binding and calmodulin-binding domains. Curiously, no epitopes localized to the helical domain (domain III). This domain undergoes significant structural changes upon binding of EF to calmodulin in vivo (10). Although additional studies are required to determine whether the humoral response to rEF includes antibodies that require calmodulin-complexed EF for reactivity, we conclude that the helical domain of EF does not contain sequential B-cell epitopes following rEF immunization.
Significant amino acid similarity (44%) between the N-terminal fragments of EF and LF, along with data showing that a LeTx-neutralizing, LF-specific monoclonal antibody can bind to EF (6, 23), suggested to us the hypothesis that LF and EF cross-reactive antibodies may be cross-neutralizing and that these cross-reactive epitopes may be excellent vaccine targets. Indeed, Zeng et al. previously reported that immunization of mice with an adenovirus vector expressing the N-terminal fragment of EF neutralized not only EdTx but also LeTx (38). However, demonstration of this cross-neutralizing activity required concentrated antisera (1:2 serum dilutions), and the effect of control, nonimmune sera in the neutralization assays at similar concentrations was not shown. Moreover, this study did not address the outcome of immunization with intact rEF, which was the subject of our investigation.
We found that rEF immunization induced antibodies cross-reactive with LF and also found the converse to be true, i.e., rLF immunization induced antibodies cross-reactive with EF. The cross-reactive antibody titers in both cases were in the range of 103 and constituted about one-eighth of the titrated response to the respective proteins of immunization. For comparison, the titers of IgG reactive to EF and cross-reactive to LF in the Zeng et al. study were in the range of 103 (38). We also mapped the cross-reactive responses by using overlapping decapeptides of the LF and EF proteins. The most important cross-reactive epitopes were identified as EF aa 257 to 268 and LF aa 265 to 274. Cross-reactive antibodies to these epitopes could be raised either by rEF or by rLF immunization, and these two sequences demonstrated significant homology at the amino acid level. In contrast, several other weakly cross-reactive epitopes were induced only in either rEF- or rLF-immunized mice. As expected, all of the identified cross-reactive epitopes were located in the N-terminal domains of EF and LF, and several occurred in previously noted regions of sequence similarity between the two proteins. Conformational epitopes purified by column chromatography may not have been detected by linear epitope mapping. However, cross-reactive IgG fractions obtained from either rLF- or rEF-immunized mice and containing antibodies recognizing both linear and conformational epitopes failed to neutralize or cross-neutralize toxin activity.
As expected, whole LF and EF immune sera demonstrated neutralization of LeTx and EdTx, respectively. However, despite cross-reactive antibody titers that were in the range of antibody titers reported by Zeng et al., LF sera did not neutralize EdTx, nor did EF sera neutralize LeTx. Cross-neutralization was not observed even at 1:2 concentrated serum dilutions (Fig. 4C). To exclude the possibility that high titers of non-cross-reactive antibodies present in the serum samples somehow impeded cross-neutralization, we also affinity purified the cross-reactive IgG following both rEF and rLF immunization. Examination of these cross-reactive antisera for binding to EF and LF decapeptides revealed similar levels of reactivity to the same cross-reactive epitopes identified using mapping of whole antisera, thus demonstrating that the integrity of the purified antibodies remained intact. However, the purified cross-reactive IgG also failed to demonstrate cross-neutralization activity and thus confirmed that immunization with intact rLF protein fails to provide cross-protection against EdTx and vice versa.
The availability of purified cross-reactive antisera that bound to a limited number of EF and LF peptides provided us with the additional opportunity to assess the capacity of these responses to directly neutralize toxin formed by the protein of immunization. These experiments revealed that these antibodies lacked any measurable neutralization activity. We cannot completely exclude the possibility that some of the weaker cross-reactive antibodies could directly mediate neutralization of the toxin corresponding to the protein of immunization if they were present at higher titers in the absence of competing antibodies or other serum components; however, our data indicate that antibodies binding to the major cross-reactive epitopes identified as EF aa 257 to 268 and LF aa 265 to 274 do not contribute to either direct or cross-toxin neutralization. Although we have excluded these epitopes from contributing to the protective response and demonstrated that the presence of non-cross-reactive antibodies in whole immune sera did not impair potential cross-neutralization, additional studies examining whether immunization with just the N-terminal domains of LF or EF can induce cross-neutralizing antibodies by raising higher titers of IgG to the epitopes that we identified as weakly cross-reactive are warranted. Inhibition of EdTx should remain an important consideration when alternative anthrax vaccine targets are contemplated, not only because systemic EdTx can be lethal (12) but also because it enhances the lethality of LeTx (13) and impairs host immunity on its own and in synergy with LeTx (34).
In summary, we have mapped sequential B-cell epitopes of the EdTx-neutralizing rEF immune response and shown that this response is directed to two of the three domains of this protein. We have further documented and characterized the cross-reactive, humoral immune response between EF and LF that is induced by immunization of mice with these intact, recombinant proteins. We conclude that, although neutralizing responses to the parental toxins of immunization are generated and although cross-reactive antibodies to the PA-binding domains of these proteins are raised, the major neutralizing responses are directed to non-cross-reactive determinants.
Supplementary Material
Acknowledgments
We thank Amber Kuzmic for her technical assistance during the initial stages of this study and Beverly Hurt for formatting figures and tables.
This work was made possible by grant U19 AI062629 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 31 August 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Albrecht, M. T., H. Li, E. D. Williamson, C. S. LeButt, H. C. Flick-Smith, C. P. Quinn, H. Westra, D. Galloway, A. Mateczun, S. Goldman, H. Groen, and L. W. Baillie. 2007. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 75:5425-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie, L. 2001. The development of new vaccines against Bacillus anthracis. J. Appl. Microbiol. 91:609-613. [DOI] [PubMed] [Google Scholar]
- 3.Baillie, L., R. Hebdon, H. Flick-Smith, and D. Williamson. 2003. Characterisation of the immune response to the UK human anthrax vaccine. FEMS Immunol. Med. Microbiol. 36:83-86. [DOI] [PubMed] [Google Scholar]
- 4.Baillie, L. W., K. Fowler, and P. C. Turnbull. 1999. Human immune responses to the UK human anthrax vaccine. J. Appl. Microbiol. 87:306-308. [DOI] [PubMed] [Google Scholar]
- 5.Berman, H. M., J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov, and P. E. Bourne. 2000. The Protein Data Bank. Nucleic Acids Res. 28:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bragg, T. S., and D. L. Robertson. 1989. Nucleotide sequence and analysis of the lethal factor gene (lef) from Bacillus anthracis. Gene 81:45-54. [DOI] [PubMed] [Google Scholar]
- 7.Brossier, F., M. Levy, A. Landier, P. Lafaye, and M. Mock. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 72:6313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 9.Dong, D. Y., J. J. Xu, X. H. Song, L. Fu, and W. Chen. 2005. Expression and analysis of biological activity of the recombination anthrax edema factor. Wei Sheng Wu Xue Bao 45:459-462. (In Chinese.) [PubMed] [Google Scholar]
- 10.Drum, C. L., S. Z. Yan, J. Bard, Y. Q. Shen, D. Lu, S. Soelaiman, Z. Grabarek, A. Bohm, and W. J. Tang. 2002. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 415:396-402. [DOI] [PubMed] [Google Scholar]
- 11.Farris, A. D., L. Brown, P. Reynolds, J. B. Harley, J. A. James, R. H. Scofield, J. McCluskey, and T. P. Gordon. 1999. Induction of autoimmunity by multivalent immunodominant and subdominant T cell determinants of La (SS-B). J. Immunol. 162:3079-3087. [PubMed] [Google Scholar]
- 12.Firoved, A. M., G. F. Miller, M. Moayeri, R. Kakkar, Y. Shen, J. F. Wiggins, E. M. McNally, W. J. Tang, and S. H. Leppla. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firoved, A. M., M. Moayeri, J. F. Wiggins, Y. Shen, W. J. Tang, and S. H. Leppla. 2007. Anthrax edema toxin sensitizes DBA/2J mice to lethal toxin. Infect. Immun. 75:2120-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flick-Smith, H. C., E. L. Waters, N. J. Walker, J. Miller, A. J. Stagg, M. Green, and E. D. Williamson. 2005. Mouse model characterisation for anthrax vaccine development: comparison of one inbred and one outbred mouse strain. Microb. Pathog. 38:33-40. [DOI] [PubMed] [Google Scholar]
- 15.Friedlander, A. M., P. R. Pittman, and G. W. Parker. 1999. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA 282:2104-2106. [DOI] [PubMed] [Google Scholar]
- 16.Guarner, J., J. A. Jernigan, W. J. Shieh, K. Tatti, L. M. Flannagan, D. S. Stephens, T. Popovic, D. A. Ashford, B. A. Perkins, and S. R. Zaki. 2003. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, P., A. Singh, V. Chauhan, and R. Bhatnagar. 2001. Involvement of residues 147VYYEIGK153 in binding of lethal factor to protective antigen of Bacillus anthracis. Biochem. Biophys. Res. Commun. 280:158-163. [DOI] [PubMed] [Google Scholar]
- 18.Juris, S. J., R. A. Melnyk, R. E. Bolcome III, J. Chan, and R. J. Collier. 2007. Cross-linked forms of the isolated N-terminal domain of the lethal factor are potent inhibitors of anthrax toxin. Infect. Immun. 75:5052-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobiler, D., Y. Gozes, H. Rosenberg, D. Marcus, S. Reuveny, and Z. Altboum. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, P., N. Ahuja, and R. Bhatnagar. 2001. Purification of anthrax edema factor from Escherichia coli and identification of residues required for binding to anthrax protective antigen. Infect. Immun. 69:6532-6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little, S. F., S. H. Leppla, J. W. Burnett, and A. M. Friedlander. 1994. Structure-function analysis of Bacillus anthracis edema factor by using monoclonal antibodies. Biochem. Biophys. Res. Commun. 199:676-682. [DOI] [PubMed] [Google Scholar]
- 23.Little, S. F., S. H. Leppla, and A. M. Friedlander. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 58:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClain, M. T., L. D. Heinlen, G. J. Dennis, J. Roebuck, J. B. Harley, and J. A. James. 2005. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 11:85-89. [DOI] [PubMed] [Google Scholar]
- 25.McClain, M. T., B. D. Poole, B. F. Bruner, K. M. Kaufman, J. B. Harley, and J. A. James. 2006. An altered immune response to Epstein-Barr nuclear antigen 1 in pediatric systemic lupus erythematosus. Arthritis Rheum. 54:360-368. [DOI] [PubMed] [Google Scholar]
- 26.Moayeri, M., and S. H. Leppla. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. [Epub ahead of print.] doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed]
- 27.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen, M. L., S. R. Crowe, S. Kurella, S. Teryzan, B. Cao, J. D. Ballard, J. A. James, and A. D. Farris. 2009. Sequential B-cell epitopes of Bacillus anthracis lethal factor bind lethal toxin-neutralizing antibodies. Infect. Immun. 77:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezard, C., M. Weber, J. C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 63:1369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittman, P. R., P. H. Gibbs, T. L. Cannon, and A. M. Friedlander. 2001. Anthrax vaccine: short-term safety experience in humans. Vaccine 20:972-978. [DOI] [PubMed] [Google Scholar]
- 31.Salles, I. I., D. E. Voth, S. C. Ward, K. M. Averette, R. K. Tweten, K. A. Bradley, and J. D. Ballard. 2006. Cytotoxic activity of Bacillus anthracis protective antigen observed in a macrophage cell line overexpressing ANTXR1. Cell. Microbiol. 8:1272-1281. [DOI] [PubMed] [Google Scholar]
- 32.Staats, H. F., S. M. Alam, R. M. Scearce, S. M. Kirwan, J. X. Zhang, W. M. Gwinn, and B. F. Haynes. 2007. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect. Immun. 75:5443-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taft, S. C., and A. A. Weiss. 2008. Neutralizing activity of vaccine-induced antibodies to two Bacillus anthracis toxin components, lethal factor and edema factor. Clin. Vaccine Immunol. 15:71-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tournier, J. N., A. Quesnel-Hellmann, J. Mathieu, C. Montecucco, W. J. Tang, M. Mock, D. R. Vidal, and P. L. Goossens. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 174:4934-4941. [DOI] [PubMed] [Google Scholar]
- 35.Turnbull, P. C., S. H. Leppla, M. G. Broster, C. P. Quinn, and J. Melling. 1988. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med. Microbiol. Immunol. 177:293-303. [DOI] [PubMed] [Google Scholar]
- 36.Voth, D. E., E. E. Hamm, L. G. Nguyen, A. E. Tucker, I. I. Salles, W. Ortiz-Leduc, and J. D. Ballard. 2005. Bacillus anthracis oedema toxin as a cause of tissue necrosis and cell type-specific cytotoxicity. Cell. Microbiol. 7:1139-1149. [DOI] [PubMed] [Google Scholar]
- 37.Wang, J. Y., and M. H. Roehrl. 2005. Anthrax vaccine design: strategies to achieve comprehensive protection against spore, bacillus, and toxin. Med. Immunol. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng, M., Q. Xu, E. D. Hesek, and M. E. Pichichero. 2006. N-fragment of edema factor as a candidate antigen for immunization against anthrax. Vaccine 24:662-670. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, B., C. Carney, and K. D. Janda. 2008. Selection and characterization of human antibodies neutralizing Bacillus anthracis toxin. Bioorg. Med. Chem. 16:1903-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.