Abstract
Cryptosporidium spp. are a cause of self-limited diarrhea in immunocompetent hosts. In immunocompetent rats, Cryptosporidium parvum infection induced digestive hypersensitivity, a key pathophysiological factor in functional digestive disorders such as irritable bowel syndrome (IBS). In such a rat model, we sought to document whether jejunal hypersensitivity depends on C. parvum isolate and is associated with a mast cell accumulation. Five-day-old rats were orally administered 105 oocysts of either Nouzilly (NoI) or Iowa (IoI) C. parvum isolate. NoI-infected rats exhibited the lowest food intake on days 7 and 14 postinfection (p.i.). On day 7 p.i., small intestine villus atrophy, crypt hyperplasia, and inflammatory cell infiltration were prominent in NoI-infected rats, with higher numbers of Cryptosporidium forms than in IoI-infected rats. Compared to uninfected control rats, jejunal intraepithelial lymphocytes (IELs) were increased only in NoI-infected rats on day 14 p.i. On day 50 p.i., jejunal hypersensitivity to distension was found only in NoI-infected rats; this hypersensitivity is associated with activated mast cell accumulation. The number of mast cells in the jejunal lamina propria was increased from day 36 p.i. in NoI-infected rats and only at day 120 p.i. in IoI-infected rats. Our data suggest that both the severity of infection (weight loss, reduced food intake, villus atrophy, and IEL accumulation) and the onset of a jejunal hypersensitivity after infection in association with an activated mast cell accumulation are isolate dependent and related to NoI infection. This cryptosporidiosis rat model is a relevant model for the study of underlying mechanisms of postinfectious IBS-like symptoms.
Cryptosporidium spp. are obligate intracellular protozoans able to infect the gastrointestinal tract of both immunocompetent and immunodeficient animals and humans (37, 51). Symptoms of human cryptosporidiosis include gastrointestinal upset, diarrhea, abdominal pain, fluid loss, cramping, and fever (46). It is worth nothing that the severity of acute experimental human cryptosporidiosis varies among different Cryptosporidium parvum isolates (17, 39). After an acute episode of cryptosporidiosis, a substantial subset of patients describes the onset of gastrointestinal symptoms despite recovery with parasite clearance (27). Symptoms following C. parvum infection are similar to those described by patients suffering from irritable bowel syndrome (IBS), which suggests that C. parvum could be a potential agent for postinfectious IBS. This syndrome occurs in 7 to 31% of patients after a prolonged intestinal infection (16, 22, 26, 47) by either bacterial (due to Campylobacter spp., Salmonella spp., Shigella spp., and Escherichia coli) or protozoan species (i.e., Giardia duodenalis) (31, 38, 45, 49, 51, 37).
An enhanced visceral perception of pain with decreased pain threshold during intestinal distension appears to be a major pathophysiological mechanism of IBS and was proposed as a functional marker (5, 8). In a subgroup of patients, peripheral mechanisms are involved in the pain transmission to the brain (5). The peripheral mechanisms include the sensitization of primary afferent endings by inflammatory mediators released by immune cells and particularly mast cells (13).
In an unweaned immunocompetent rat model, we have previously reported that C. parvum infection-induced jejunal hypersensitivity to distension lasted more than 100 days after spontaneous clearance of the parasites (30). The present study aims to investigate in this model whether C. parvum-triggered intestinal hypersensitivity to distension in association with mast cell infiltrates depends on parasite isolate.
MATERIALS AND METHODS
C. parvum isolates.
Oocysts of the C. parvum Iowa isolate (IoI) were obtained from Waterborne Inc (New Orleans, LA). Oocysts of the Nouzilly isolate (NoI) maintained and kindly gifted by R. Mancassola and M. Naciri (Laboratoire de Pathologie Aviaire, Institut National de Recherche Agronomique, Nouzilly, France) were purified as previously described from feces obtained from experimentally infected calves and stored in a 2.5% K2Cr2O7 solution for less than 3 months (6). Oocysts from both isolates were bleached and rinsed in phosphate-buffered saline (PBS) before infection (4, 29).
Preparation of Nouzilly sporozoite crude extract.
To investigate the role of immune responses in antigen-induced pre- and postinfection alterations, a Nouzilly isolate sporozoite crude extract (NSCE) was prepared from sporozoites as previously described (19). After purification, oocysts were counted in an hemacytometer and resuspended in a 1.5% taurocholic acid solution (Sigma, St. Louis, MO) in BHK 21 medium for 90 min at 37°C in a humidified 5% CO2 atmosphere. Parasite suspensions were sieved through 5-μm-pore-size cellulose acetate filters (Sartorius, Göttingen, Germany) to remove nonexcysted oocysts, empty shells, and debris, which were microscopically absent from final sporozoite suspensions. A total of 120 million purified sporozoites were resuspended in PBS, subjected to 10 freeze-thaw cycles, and disrupted by ultrasonication until <1% was observed to be intact by microscopy to prepare 1 ml of total (i.e., particulate and water-soluble fraction) sporozoite extract.
Infection of unweaned rats.
Five-day-old suckling Sprague-Dawley rats (Janvier, Le Genest Saint Isle, France) were used to evaluate C. parvum pathogenicity as previously described (30). Dams and their litters were maintained free of Cryptosporidium spp. under specific-pathogen-free conditions, held separately in plastic cages, and given heat-sterilized food and water ad libitum. Suckling rats were orally gavaged with 100 μl of PBS containing 105 oocysts of NoI or IoI (100 μl of PBS in control rats), which resulted in preliminary experiments in a maximum ileal parasite burden and onset of clearance of parasite at days 6 to 8 p.i. and 14 p.i, respectively (54). Another group were gavaged with a solution of NSCE corresponding to 105 oocysts. Animals were handled according to the regulations enforced by the French Ministry of Agriculture and the University ad hoc ethical committee.
Body weight index.
Body weight indices were expressed for each group and postinfection (p.i.) day as follows: the present mean weight/the mean weight at the day of infection.
Food intake.
At days 7 and 14 p.i., pups were isolated from their dams for 4 h, weighed, given back to their dams for 2 h for suckling, and weighed again. On day 26 p.i., rats were placed in metabolic cages and given food and water ad libitum, and the 24-h food intake was recorded.
Immunohistochemistry.
Ten rats in each group at days 7, 14, 36, and 50 p.i., and five rats of the IoI-infected, NoI-infected, and control uninfected groups at day 120 p.i. were killed for histocytological examination. Pieces from the distal jejunum or ileum (heavily infected segment) were fixed in 10% formalin and embedded in paraffin (52).
At days 7 and 14 p.i., 5-μm sections of ilea were Giemsa stained to check the C. parvum infection. The parasite burden was calculated by counting the C. parvum mucosal forms in the ileum in each section on 10 well-oriented villus-crypt units (VCU) (45). The parasite burden is expressed as the number of parasites per millimeter of villus. Villus heights (VH) and crypt depths (CD) were measured in each ileal section on 20 well-oriented VCU by using the image analysis software Histolab 5.12.0 (Microvision, Evry, France). For each group, the VH/CD ratio was calculated as the mean VH divided by the mean CD.
Intestinal intraepithelial lymphocytes (IELs) were labeled in 5-μm sections with a monoclonal mouse anti-CD3 antibody (Dako, Glostrup, Denmark) revealed using a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Dako). Intestinal IELs were counted in each section on 20 well-oriented VCU. Aggregates in the submucosa were not included in the counting (59).
Mast cells were labeled in 5-μm sections with a polyclonal sheep anti-RMCP II antiserum (Moredun Scientific, Moredun, Scotland, United Kingdom) and revealed using a horseradish peroxidase-conjugated goat anti-sheep secondary antibody (Dako). Mucosal mast cells were counted in each section on 20 well-oriented VCU.
Jejunal sensitivity to distension.
On day 50 p.i., 10 rats from each group were anesthetized with intraperitoneal sodium pentobarbitone (Abbott Diagnostic, Rungis, France), a midline abdominal incision was made to expose the small intestine, a cut was made on the antimesenteric side at one end of a jejunum segment, i.e., 7 cm from Treitz ligament, and arterial embolectomy catheters were used as balloons to perform distension (Fogarty-Edwards Life Sciences, Saint-Prex, Switzerland). The intestinal segment was replaced in the peritoneal cavity, and the abdomen was closed. Distending the jejunum by rapid inflation with volumes increasing in 0.1-ml steps, from 0.1 to 0.3 ml, for 25 s every 5 min resulted in a stimulus-related decrease in systemic blood pressure, which was recorded from a side arm of the carotid cannula by using a pressure transducer (P10EZ) connected to a window graph 240 (Gould, Courtaboeuf, France).
Levels of jejunal rat mast cell protease II (RMCP-II).
On day 50 p.i., the RMCP-II levels were measured in full-thickness, 2-cm jejunal fragments from six rats/group. Jejunal samples were homogenized in 1.0 ml of ice-cold 0.15 mol of KCl/liter for 30 s, and the supernatants were collected after centrifugation at 36,000 × g for 30 min at 4°C (42). The levels of jejunal RMCP-II were assayed with a commercially available enzyme-linked immunosorbent assay kit (Moredun Animal Health, Edinburgh, United Kingdom) (61). The protein concentration was determined by using a Bradford assay.
Jejunal MPO activity.
On day 50 p.i., myeloperoxidase (MPO) activity was measured in full-thickness, 2-cm jejunal fragments from six rats/group as described previously (12). MPO from human neutrophils (Sigma, l'Isle d'Abeau Chesnes, France) was used as a standard. One MPO unit was defined as the activity able to convert 1 μmol of H2O2 to H2O min−1 at 25°C. The results were expressed in MPO units/g of protein.
Expression of results and statistical analysis.
Data were expressed as means ± one standard error of the mean (SEM; 95% confidence interval). One-way or two-way analysis of variance (ANOVA) Bonferroni post tests and Student t tests were used (GraphPad Prism 5 software). P values of <0.05 were considered significant.
RESULTS
Isolate dependence of body weight, food intake, and intestinal mucosal alterations during infection.
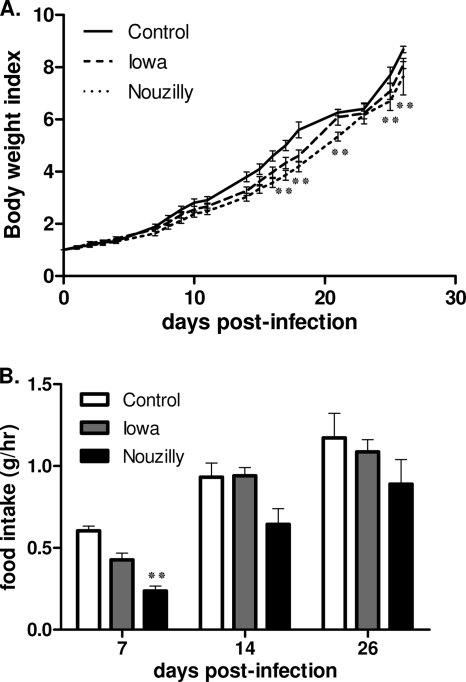
As shown in Fig. 1A, C. parvum-infected rats showed decreased body weight gain over time compared to uninfected controls counterparts, and this difference became significant for NoI-infected rats from day 17 p.i. (P < 0.01). Compared to uninfected rats, rats infected with NoI exhibited the lowest food intake on day 7 (i.e., peak of infection) (P < 0.01) and on day 14 p.i. (i.e., the onset of the clearance of infection). On day 26, this difference had lost it statistical significance (Fig. 1B). After the onset of clearance of infection, food intake for infected rats became similar to control uninfected rats. Administration of NCSE did not alter body weight gain or food intake compared to uninfected control rats (P > 0.05 [data not shown]).
FIG. 1.
Follow-up of body weight indices and food intakes in the course of C. parvum infection. (A) Mean body weight indices. Body weight indices were calculated for each group and p.i. day as follows: present mean weight/mean weight at the day of infection. C. parvum-infected rats showed decreased body weight gain over time compared to uninfected rats, and this difference became significant only for NoI-infected rats from day 17 p.i. (B) Mean daily food intakes on days 7, 14, and 28 p.i. Rats infected with NoI exhibited the lowest food intake on day 7 (i.e., peak of infection). After clearance of infection, the food intakes of infected rats became similar to those of control uninfected rats. One bar represents 1 SEM. Values are means ± the SEM (n = 10 in each group). **, P < 0.01 (significantly different from uninfected control rats, as calculated by two-way ANOVA).
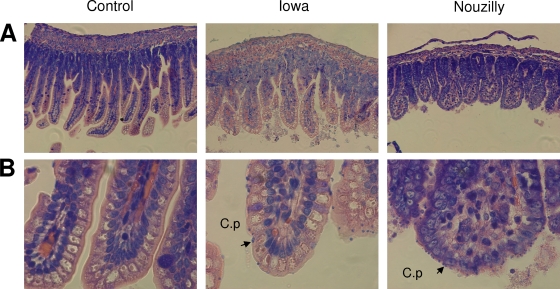
At peak of infection (day 7 p.i.), histopathology revealed in both NoI- and IoI-infected animals epithelium damages of small intestine with villus atrophy, crypt hyperplasia, and inflammatory cell infiltration that were more pronounced with NoI. As shown in Table 1, C. parvum both NoI- and IoI-infected rats exhibited significant decreases in VH and increases in CD with a decreased VH/CD ratio compared to uninfected control rats.
TABLE 1.
C. parvum mucosal form counts and mean VH, CD, and VH/CD ratios on day 7 p.i, in the ilea of control uninfected and infected rats
| Rat group | Mean ± SEMa |
|||
|---|---|---|---|---|
| No. of C. parvum mucosal forms/mm | VH (μm) | CD (μm) | VH/CD | |
| Control (uninfected) | 0 | 264.5 ± 16.2 | 24.7 ± 1.3 | 11.08 ± 1.3 |
| IoI infected | 30 ± 11 | 178.8 ± 8.8† | 35 ± 2.2‡ | 5.34 ± 0.4‡ |
| NoI infected | 52 ± 13* | 111.9 ± 9.4§ | 35.1 ± 2.7‡ | 3.72 ± 0.5§ |
*, P < 0.05 NoI- versus IoI-infected rats (Student t test). †, P < 0.05; ‡, P < 0.01; §, P < 0.001 (significantly different from the control [one-way ANOVA]).
Sections of ilea from NoI-infected rats exhibited, at day 7 p.i., higher numbers of C. parvum mucosal developing forms than did similar sections from IoI-infected rats (Fig. 2): 52 (±11)/mm and 30 (±10)/mm, respectively (Table 1). At day 14 p.i., no parasite was present in the ilea of IoI- and NoI-infected rats.
FIG. 2.
Histology of ilea from rats at 7 days p.i. and from control uninfected rats. Pieces from each ileum were fixed in 10% formalin and embedded in paraffin, and 5-μm sections were Giemsa stained to check C. parvum infection. Representative aspects of ileal mucosa showing consistent features observed in each group (i.e., uninfected, IoI-infected, and NoI-infected rats, with 10 rats/group). Arrows show typical C. parvum mucosal forms. (A) Original magnification, ×200; (B) original magnification, ×400.
Isolate dependence of jejunal IEL accumulation during and after C. parvum infection.
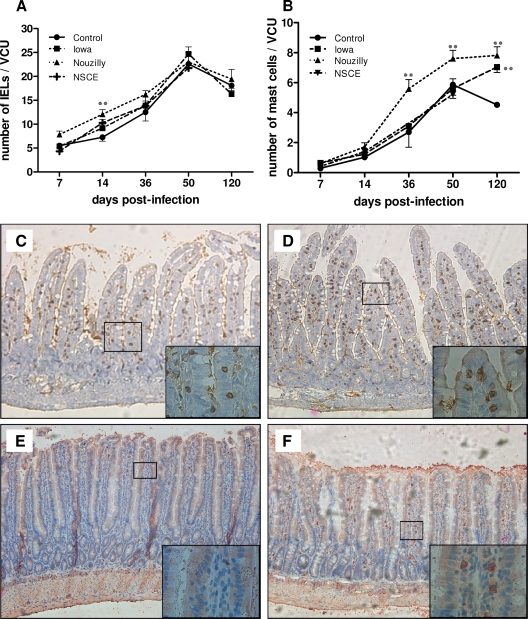
As shown in Fig. 3A, the numbers/VCU of jejunal IELs regularily increased until day 50 in uninfected control rats. Compared to controls, these levels were significantly higher only in NoI-infected rats at day 14 p.i. (i.e., the onset of clearance of infection) (P < 0.01 versus controls). Rats that received NSCE did not differ from controls in IEL numbers. After clearance of infection, the numbers/VCU of IELs were similar in all groups of rats. Figure 3C and D shows typical patterns of jejunal IELs in control uninfected rats and in NoI-infected rats, respectively, at day 14 p.i.
FIG. 3.
Jejunal IELs and mucosal mast cells in the course and after C. parvum infection. Immunostaining for jejunal intraepithelial CD3+ lymphocytes and RMCP-II+ mast cells. (A) Intestinal IELs were counted in each section in 20 well-oriented VCU. Compared to control uninfected rats, the numbers of jejunal IELs/VCU were significantly higher only in NoI-infected rats at day 14 p.i. (i.e., clearance of infection). Values are means ± the SEM (n = 10 in each group). **, P < 0.01 (significantly different from uninfected control rats, as calculated by two-way ANOVA test). (B) Submucosal mast cells were counted in each section on 20 well-oriented VCU. The number of jejunal mast cells was higher in rats infected with NoI-infected than in uninfected control rats from day 36 p.i. to day 120 p.i. Values are means ± the SEM (n = 10 in each group). **, P < 0.01 (significantly different from uninfected control rats as calculated by two-way ANOVA). (C and D) Immunostaining for T IELs with anti-CD3 monoclonal antibody. CD3+ IELs are brown stained. These figures depict representative jejunal sections obtained for control uninfected (C) and NoI-infected (D) rats on day 14 p.i. Original magnification, ×100 (inset, ×1,000). (E and F) Immunostaining for rat RMCP-II+ mast cell protease. RMCP-II+ mucosal mast cells are red stained. These figures depict representative jejunal sections obtained on day 50 p.i. in control uninfected (E) and NoI-infected (F) rats, respectively. Original magnification, ×100 (inset, ×1,000).
Isolate dependence of jejunal hypersensitivity and mucosal mast cell accumulation after infection.
As shown in Fig. 3B, the numbers of jejunal mast cells regularly increased until day 50 in uninfected control rats, and the number of jejunal mucosal mast cells was higher in rats infected with NoI than in controls from day 36 p.i. to day 120 p.i. In contrast, the number of mast cells in rats infected with the IoI was higher than in controls only at day 120 p.i (P < 0.01). Rats that were given NSCE did not differ from controls. Figure 3E and F shows a typical pattern of mucosal mast cell density in control and NoI-infected rats, respectively, on day 50 p.i.
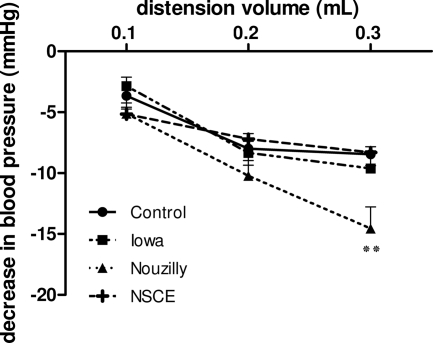
Jejunal distension studies performed on day 50 p.i revealed hypersensitivity for a 0.3-ml distension volume in rats infected with NoI (P < 0.01 versus controls). No difference was seen for rats infected with the IoI compared to control uninfected rats (Fig. 4). Rats challenged orally with NSCE did not differ from control rats.
FIG. 4.
Depressor response to graded jejunal distension in anesthetized 50 days p.i. rats and uninfected rats. Jejunal sensitivity to distension was evaluated on day 50 p.i. by measuring the stimulus related decrease in systemic blood pressure. Hypersensitivity was revealed for a 0.3-ml distension volume in rats infected with NoI-infected rats. Values are means ± the SEM (n = 10 in each group). **, P < 0.01 (significantly different from uninfected control rats, as calculated by two-way ANOVA).
Levels of jejunal RMCP-II on day 50 p.i.
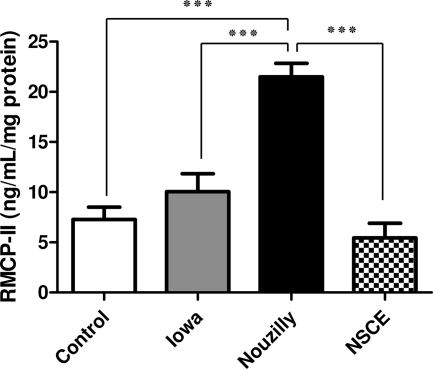
RMCP-II measurement showed that on day 50 postinfection, only NoI-infected rats exhibited significantly higher jejunal RMCP-II levels than control uninfected rats (21.5 ± 3 versus 8.1 ± 3.1 ng/ml/mg of protein [P < 0.001]) (Fig. 5).
FIG. 5.
Levels of jejunal rat mast cell protease II (RMCP-II) at 50 days p.i. in infected and uninfected rats. RMCP-II levels (ng/ml/mg protein) in jejunal tissues were measured by using an enzyme-linked immunosorbent assay. Compared to control uninfected rats, the RMCP-II jejunal levels were found to be significantly higher only in NoI-infected rats at day 50 p.i. Values are means ± the SEM (n = 6 in each group). ***, P < 0.001 (significantly different, as calculated by one-way ANOVA).
Jejunal MPO activity.
No significant difference in jejunal MPO activity was observed at day 50 p.i. between groups (76.4 ± 15.5, 58.3 ± 5, 59.5 ± 16.3, and 86.3 ± 18.2 U/g of protein for NoI-infected, IoI-infected, NCSE-challenged, and control uninfected rats, respectively [P > 0.05]).
DISCUSSION
The data presented here suggest that several parameters, i.e., the severity of infection (assessed by alterations in weight gain, a reduced food intake, and jejunal and ileal histological changes), IEL accumulation, long-term postinfectious jejunal hypersensitivity, and mast cell accumulation and activation are all similarly isolate dependent in a suckling rat model of C. parvum infection.
In contrast to immunocompromised rodent models such as corticosteroid-treated and genetically modified animals, immunocompetent neonatal/unweaned animal models document interactions of Cryptosporidium spp. with physiologically maturing organisms (23, 32, 35, 38, 41, 50, 56, 62). The consequences of the parasitic infection on weight gain and food intake were found to be dependent on C. parvum isolate and notably marked in NoI-infected rats. Eating disorders have been previously reported in association with well-recognized histological features of Cryptosporidium spp. infection, i.e., loss of VH, edema, and inflammatory infiltration by neutrophils, macrophages, and lymphocytes (3, 20). In the present study, small intestine villus atrophy, crypt hyperplasia, and inflammatory cell infiltration were more prominent at day 7 p.i. in NoI-infected rats than in IoI-infected rats. Our findings are consistent with observations of the impact of distal ileal C. parvum infection on rat growth due to an altered amino acid transport in the entire small intestine (9, 14, 31, 52, 53).
An increase in the number of jejunal IELs with time was observed in uninfected control rats. In infected rats, this increase was significantly more marked at the time of the onset of clearance of infection. This suggests that intestinal IELs play a role in host defense against C. parvum infection as reported elsewhere (2, 15).
One of the main results of the present study is the demonstration for the first time that jejunal hypersensitivity following early cryptosporidiosis in unweaned rats is dependent on the C. parvum isolate. Jejunal hypersensitivity was quantified by measuring the decrease in blood pressure during nociceptive distension. This is a pseudoaffective response corresponding to brain stem and/or spinal reflexes that decreases when the noxious stimulus is terminated (43). Hypersensitivity has already been documented during experimental nematode infections (49). Recent studies have also suggested that widespread protozoan parasites such as Blastocystis hominis, Giardia duodenalis, and Dientamoeba fragilis may also promote intestinal hypersensitivity and symptoms of postinfectious IBS (26, 48).
The second main result is the demonstration of a mast cell accumulation in the gut wall at distance of infection. Association of jejunal hypersensitivity with distension and activated mast cell accumulation, quantified by the levels of RMCP-II, a specific product of activated mucosal mast cells (36, 60), was found only in NoI-infected rats. Interestingly, the proximity of activated mast cells to mucosal nerve fibers correlated with the frequency and severity of abdominal pain in human IBS patients (7). Rats infected with the NoI exhibited increased jejunal mucosal mast cell density after clearance of infection. Mast cell recruitment was not involved in the clearance of infection, as reported by others in Cryptosporidium-infected infant mice (24). A similar increase in the number of jejunal mucosal mast cells was reported in association with jejunal hypersensitivity in rats infected with Nippostrongylus brasiliensis (18, 33, 34). A link between postinfectious increase in jejunal mast cells and jejunal hypersensitivity was also supported by the concomitant hypersensitivity to colorectal distension and the increase of mucosal mast cell density observed in adults in a neonatal maternal deprivation rat model (10).
Our two main results led us to consider that this rat model is a relevant model of postinfectious IBS. It has been shown in IBS patients that hypersensitivity is not limited to the expected target organs (i.e., the colon and rectum) but also involves the entire intestine (1, 11) and even the esophagus (55). In patients with IBS, mucosal mast cells were found to be increased at all levels of the gastrointestinal tract such as the ileum (40, 57, 58), jejunum (21), colon (7, 40), cecum (57), and rectum (40). Hypersensitivity seems to result from the sensitization of nerve afferent pathways originating from the gastrointestinal tract, where mucosal mast cells are known to play a key role in the pathogenesis of visceral hypersensitivity associated with both postinfectious IBS and noninfectious IBS by interacting with nerves (57). Interestingly, the neuropeptide substance P was shown to be involved in the pathogenesis in experimental cryptosporidiosis infection models (25, 44). Jejunal MPO measurement in our model reflects on day 50 p.i. the lack of neutrophil infiltration, as noted previously in postinfectious IBS patients. In the present study, no alteration was observed in rats given NoI sporozoite extract which was previously found as inducing antigen-driven T-cell responses (19, 34).
The present data suggest that the severity of infection and long-term postinfectious alterations of jejunal sensitivity and activated mast cell accumulation exhibited the same isolate dependence in neonatal immunocompetent rats. Isolate pathogenicity may relate to fecundity, which was found to be correlated with intracellular levels of the viral symbiont CPV (28).
Acknowledgments
This study was supported by grants from AFSSET (EST-2006/1/30). S.K. was financially supported by a doctoral fellowship from Région Haute-Normandie.
We are very grateful to R. Mancassola and M. Naciri, INRA, Nouzilly, France, for kindly providing C. parvum-infected calf feces.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 17 August 2009.
REFERENCES
- 1.Accarino, A. M., F. Azpiroz, and J. R. Malagelada. 1992. Symptomatic responses to stimulation of sensory pathways in the jejunum. Am. J. Physiol. 263:G673-G677. [DOI] [PubMed] [Google Scholar]
- 2.Adjei, A. A., A. K. Shrestha, M. Castro, and F. J. Enriquez. 2000. Adoptive transfer of immunity with intraepithelial lymphocytes in Cryptosporidium parvum-infected severe combined immunodeficient mice. Am. J. Med. Sci. 320:304-309. [DOI] [PubMed] [Google Scholar]
- 3.Argenzio, R. A., J. A. Liacos, M. L. Levy, D. J. Meuten, J. G. Lecce, and D. W. Powell. 1990. Villous atrophy, crypt hyperplasia, cellular infiltration, and impaired glucose-Na absorption in enteric cryptosporidiosis of pigs. Gastroenterology 98:1129-1140. [DOI] [PubMed] [Google Scholar]
- 4.Auray, G., S. Lacroix-Lamande, R. Mancassola, I. Dimier-Poisson, and F. Laurent. 2007. Involvement of intestinal epithelial cells in dendritic cell recruitment during Cryptosporidium parvum infection. Microbes Infect. 9:574-582. [DOI] [PubMed] [Google Scholar]
- 5.Azpiroz, F., M. Bouin, M. Camilleri, E. A. Mayer, P. Poitras, J. Serra, and R. C. Spiller. 2007. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol. Motil. 19:62-88. [DOI] [PubMed] [Google Scholar]
- 6.Baishanbo, A., G. Gargala, C. Duclos, A. Francois, J. F. Rossignol, J. J. Ballet, and L. Favennec. 2006. Efficacy of nitazoxanide and paromomycin in biliary tract cryptosporidiosis in an immunosuppressed gerbil model. J. Antimicrob. Chemother. 57:353-355. [DOI] [PubMed] [Google Scholar]
- 7.Barbara, G., V. Stanghellini, R. De Giorgio, C. Cremon, G. S. Cottrell, D. Santini, G. Pasquinelli, A. M. Morselli-Labate, E. F. Grady, N. W. Bunnett, S. M. Collins, and R. Corinaldesi. 2004. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126:693-702. [DOI] [PubMed] [Google Scholar]
- 8.Barbara, G., B. Wang, V. Stanghellini, R. de Giorgio, C. Cremon, G. Di Nardo, M. Trevisani, B. Campi, P. Geppetti, M. Tonini, N. W. Bunnett, D. Grundy, and R. Corinaldesi. 2007. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132:26-37. [DOI] [PubMed] [Google Scholar]
- 9.Barbot, L., E. Windsor, S. Rome, V. Tricottet, M. Reynes, A. Topouchian, J. F. Huneau, J. G. Gobert, D. Tome, and N. Kapel. 2003. Intestinal peptide transporter PepT1 is overexpressed during acute cryptosporidiosis in suckling rats as a result of both malnutrition and experimental parasite infection. Parasitol. Res. 89:364-370. [DOI] [PubMed] [Google Scholar]
- 10.Barreau, F., L. Ferrier, J. Fioramonti, and L. Bueno. 2004. Neonatal maternal deprivation triggers long-term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 53:501-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouin, M., F. Lupien, M. Riberdy, M. Boivin, V. Plourde, and P. Poitras. 2004. Intolerance to visceral distension in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol. Motil. 16:311-314. [DOI] [PubMed] [Google Scholar]
- 12.Bradley, P. P., D. A. Priebat, R. D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 78:206-209. [DOI] [PubMed] [Google Scholar]
- 13.Bueno, L., F. de Ponti, M. Fried, G. A. Kullak-Ublick, M. A. Kwiatek, D. Pohl, E. M. Quigley, J. Tack, and N. J. Talley. 2007. Serotonergic and non-serotonergic targets in the pharmacotherapy of visceral hypersensitivity. Neurogastroenterol. Motil. 19:89-119. [DOI] [PubMed] [Google Scholar]
- 14.Capet, C., N. Kapel, J. F. Huneau, D. Magne, R. Laikuen, V. Tricottet, Y. Benhamou, D. Tome, and J. G. Gobert. 1999. Cryptosporidium parvum infection in suckling rats: impairment of mucosal permeability and Na+-glucose cotransport. Exp. Parasitol. 91:119-125. [DOI] [PubMed] [Google Scholar]
- 15.Chai, J. Y., S. M. Guk, H. K. Han, and C. K. Yun. 1999. Role of intraepithelial lymphocytes in mucosal immune responses of mice experimentally infected with Cryptosporidium parvum. J. Parasitol. 85:234-239. [PubMed] [Google Scholar]
- 16.DuPont, A. W. 2008. Postinfectious irritable bowel syndrome. Clin. Infect. Dis. 46:594-599. [DOI] [PubMed] [Google Scholar]
- 17.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 18.Fioramonti, J., and G. F. Gebhart. 2007. In vivo and transgenic animal models used to study visceral hypersensitivity. Neurogastroenterol. Motil. 19:20-28. [DOI] [PubMed] [Google Scholar]
- 19.Gargala, G., A. Delaunay, L. Favennec, P. Brasseur, and J. J. Ballet. 1997. In vitro interactions of human blood and intestinal intraepithelial lymphocytes with Cryptosporidium parvum and C. parvum permissive enterocytic cell lines. J. Eukaryot. Microbiol. 44:71S-72S. [DOI] [PubMed] [Google Scholar]
- 20.Gookin, J. L., S. K. Nordone, and R. A. Argenzio. 2002. Host responses to Cryptosporidium infection. J. Vet. Intern. Med. 16:12-21. [DOI] [PubMed] [Google Scholar]
- 21.Guilarte, M., J. Santos, I. de Torres, C. Alonso, M. Vicario, L. Ramos, C. Martinez, F. Casellas, E. Saperas, and J. R. Malagelada. 2007. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 56:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwee, K. A. 2001. Postinfectious irritable bowel syndrome. Curr. Treat. Options Gastroenterol. 4:287-291. [DOI] [PubMed] [Google Scholar]
- 23.Harp, J. A., P. Jardon, E. R. Atwill, M. Zylstra, S. Checel, J. P. Goff, and C. De Simone. 1996. Field testing of prophylactic measures against Cryptosporidium parvum infection in calves in a California dairy herd. Am. J. Vet. Res. 57:1586-1588. [PubMed] [Google Scholar]
- 24.Harp, J. A., and H. W. Moon. 1991. Susceptibility of mast cell-deficient W/Wv mice to Cryptosporidium parvum. Infect. Immun. 59:718-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez, J., A. Lackner, P. Aye, K. Mukherjee, D. J. Tweardy, M. A. Mastrangelo, J. Weinstock, J. Griffiths, M. D'Souza, S. Dixit, and P. Robinson. 2007. Substance P is responsible for physiological alterations such as increased chloride ion secretion and glucose malabsorption in cryptosporidiosis. Infect. Immun. 75:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz, B. J., and R. S. Fisher. 2001. The irritable bowel syndrome. N. Engl. J. Med. 344:1846-1850. [DOI] [PubMed] [Google Scholar]
- 27.Hunter, P. R., S. Hughes, S. Woodhouse, N. Raj, Q. Syed, R. M. Chalmers, N. Q. Verlander, and J. Goodacre. 2004. Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin. Infect. Dis. 39:504-510. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins, M. C., J. Higgins, J. E. Abrahante, K. E. Kniel, C. O'Brien, J. Trout, C. A. Lancto, M. S. Abrahamsen, and R. Fayer. 2008. Fecundity of Cryptosporidium parvum is correlated with intracellular levels of the viral symbiont CPV. Int. J. Parasitol. 38:1051-1055. [DOI] [PubMed] [Google Scholar]
- 29.Lacroix-Lamande, S., R. Mancassola, M. Naciri, and F. Laurent. 2002. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 70:2090-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marion, R., A. Baishanbo, G. Gargala, A. Francois, P. Ducrotte, C. Duclos, J. Fioramonti, J. J. Ballet, and L. Favennec. 2006. Transient neonatal Cryptosporidium parvum infection triggers long-term jejunal hypersensitivity to distension in immunocompetent rats. Infect. Immun. 74:4387-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquet, P., B. Saubamea, L. Snouber-Choucha, V. Gafa, N. Kapel, and L. Barbot-Trystram. 2008. Evidence for intestinal heterogenic expression of di-tripeptides transporter PepT1 during experimental cryptosporidiosis in neonatal rats. Parasitol. Res. 104:985-991. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, V., R. Deer, S. Uni, M. Iseki, and G. J. Bancroft. 1992. Immune responses to Cryptosporidium muris and Cryptosporidium parvum in adult immunocompetent or immunocompromised (nude and SCID) mice. Infect. Immun. 60:3325-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean, P. G., C. Picard, R. Garcia-Villar, R. Ducos de Lahitte, J. More, J. Fioramonti, and L. Bueno. 1998. Role of kinin B1 and B2 receptors and mast cells in post-intestinal infection-induced hypersensitivity to distension. Neurogastroenterol. Motil. 10:499-508. [DOI] [PubMed] [Google Scholar]
- 34.McLean, P. G., C. Picard, R. Garcia-Villar, J. More, J. Fioramonti, and L. Bueno. 1997. Effects of nematode infection on sensitivity to intestinal distension: role of tachykinin NK2 receptors. Eur. J. Pharmacol. 337:279-282. [DOI] [PubMed] [Google Scholar]
- 35.Mead, J. R., M. J. Arrowood, R. W. Sidwell, and M. C. Healey. 1991. Chronic Cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J. Infect. Dis. 163:1297-1304. [DOI] [PubMed] [Google Scholar]
- 36.Miller, H. R., J. F. Huntley, G. F. Newlands, and J. Irvine. 1990. Granule chymases and the characterization of mast cell phenotype and function in rat and mouse. Monogr. Allergy 27:1-30. [PubMed] [Google Scholar]
- 37.Morgan, U. M., L. Xiao, R. Fayer, A. A. Lal, and R. C. Thompson. 2000. Epidemiology and strain variation of Cryptosporidium parvum. Contrib. Microbiol. 6:116-139. [DOI] [PubMed] [Google Scholar]
- 38.Novak, S. M., and C. R. Sterling. 1991. Susceptibility dynamics in neonatal BALB/c mice infected with Cryptosporidium parvum. J. Protozool. 38:103S-104S. [PubMed] [Google Scholar]
- 39.Okhuysen, P. C., C. L. Chappell, J. H. Crabb, C. R. Sterling, and H. L. DuPont. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180:1275-1281. [DOI] [PubMed] [Google Scholar]
- 40.Park, J. H., P. L. Rhee, H. S. Kim, J. H. Lee, Y. H. Kim, J. J. Kim, and J. C. Rhee. 2006. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J. Gastroenterol. Hepatol. 21:71-78. [DOI] [PubMed] [Google Scholar]
- 41.Rehg, J. E. 1996. Effect of interferon-gamma in experimental Cryptosporidium parvum infection. J. Infect. Dis. 174:229-232. [DOI] [PubMed] [Google Scholar]
- 42.Rioux, K. P., and J. L. Wallace. 1996. Long-term antigen challenge results in progressively diminished mucosal mast cell degranulation in rats. Gastroenterology 111:1516-1523. [DOI] [PubMed] [Google Scholar]
- 43.Ritchie, J. 1973. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson, P., P. Martin, Jr., A. Garza, M. D'Souza, M. A. Mastrangelo, and D. Tweardy. 2008. Substance P receptor antagonism for treatment of cryptosporidiosis in immunosuppressed mice. J. Parasitol. 94:1150-1154. [DOI] [PubMed] [Google Scholar]
- 45.Serna, H., M. Porras, and P. Vergara. 2006. Mast cell stabilizer ketotifen [4-(1-methyl-4-piperidylidene)-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9H)-one fumarate] prevents mucosal mast cell hyperplasia and intestinal dysmotility in experimental Trichinella spiralis inflammation in the rat. J. Pharmacol. Exp. Ther. 319:1104-1111. [DOI] [PubMed] [Google Scholar]
- 46.Slifko, T. R., D. E. Huffman, and J. B. Rose. 1999. A most-probable-number assay for enumeration of infectious Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 65:3936-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiller, R., and E. Campbell. 2006. Postinfectious irritable bowel syndrome. Curr. Opin. Gastroenterol. 22:13-17. [DOI] [PubMed] [Google Scholar]
- 48.Stark, D., S. van Hal, D. Marriott, J. Ellis, and J. Harkness. 2007. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 37:11-20. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, T., T. Sasaki, H. Takagi, K. Sato, and K. Ueda. 2008. The effectors responsible for gastrointestinal nematode parasites, Trichinella spiralis, expulsion in rats. Parasitol. Res. 103:1289-1295. [DOI] [PubMed] [Google Scholar]
- 50.Theodos, C. M., K. L. Sullivan, J. K. Griffiths, and S. Tzipori. 1997. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 65:4761-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thielman, N. M., and R. L. Guerrant. 2004. Clinical practice: acute infectious diarrhea. N. Engl. J. Med. 350:38-47. [DOI] [PubMed] [Google Scholar]
- 52.Topouchian, A., J. F. Huneau, L. Barbot, S. Rome, J. G. Gobert, D. Tome, and N. Kapel. 2003. Evidence for the absence of an intestinal adaptive mechanism to compensate for Cryptosporidium parvum-induced amino acid malabsorption in suckling rats. Parasitol. Res. 91:197-203. [DOI] [PubMed] [Google Scholar]
- 53.Topouchian, A., N. Kapel, J. F. Huneau, L. Barbot, D. Magne, D. Tome, and J. G. Gobert. 2001. Impairment of amino acid absorption in suckling rats infected with Cryptosporidium parvum. Parasitol. Res. 87:891-896. [DOI] [PubMed] [Google Scholar]
- 54.Topouchian, A., N. Kapel, C. Larue-Achagiotis, L. Barbot, D. Tome, J. G. Gobert, and J. F. Huneau. 2005. Cryptosporidium infection impairs growth and muscular protein synthesis in suckling rats. Parasitol. Res. 96:326-330. [DOI] [PubMed] [Google Scholar]
- 55.Trimble, K. C., R. Farouk, A. Pryde, S. Douglas, and R. C. Heading. 1995. Heightened visceral sensation in functional gastrointestinal disease is not site-specific: evidence for a generalized disorder of gut sensitivity. Dig. Dis. Sci. 40:1607-1613. [DOI] [PubMed] [Google Scholar]
- 56.Urban, J. F., Jr., R. Fayer, S. J. Chen, W. C. Gause, M. K. Gately, and F. D. Finkelman. 1996. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J. Immunol. 156:263-268. [PubMed] [Google Scholar]
- 57.Wang, L. H., X. C. Fang, and G. Z. Pan. 2004. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 53:1096-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weston, A. P., W. L. Biddle, P. S. Bhatia, and P. B. Miner, Jr. 1993. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig. Dis. Sci. 38:1590-1595. [DOI] [PubMed] [Google Scholar]
- 59.Wheatcroft, J., D. Wakelin, A. Smith, C. R. Mahoney, G. Mawe, and R. Spiller. 2005. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol. Motil. 17:863-870. [DOI] [PubMed] [Google Scholar]
- 60.Woodbury, R. G., G. M. Gruzenski, and D. Lagunoff. 1978. Immunofluorescent localization of a serine protease in rat small intestine. Proc. Natl. Acad. Sci. USA 75:2785-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodbury, R. G., and H. R. Miller. 1982. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology 46:487-495. [PMC free article] [PubMed] [Google Scholar]
- 62.You, X., and J. R. Mead. 1998. Characterization of experimental Cryptosporidium parvum infection in IFN-gamma knockout mice. Parasitology 117(Pt. 6):525-531. [DOI] [PubMed] [Google Scholar]