Abstract
Vibrio cholerae O1 can cause diarrheal disease that may be life-threatening without treatment. Natural infection results in long-lasting protective immunity, but the role of T cells in this immune response has not been well characterized. In contrast, robust B-cell responses to V. cholerae infection have been observed. In particular, memory B-cell responses to T-cell-dependent antigens persist for at least 1 year, whereas responses to lipopolysaccharide, a T-cell-independent antigen, wane more rapidly after infection. We hypothesize that protective immunity is mediated by anamnestic responses of memory B cells in the gut-associated lymphoid tissue, and T-cell responses may be required to generate and maintain durable memory B-cell responses. In this study, we examined B- and T-cell responses in patients with severe V. cholerae infection. Using the flow cytometric assay of the specific cell-mediated immune response in activated whole blood, we measured antigen-specific T-cell responses using V. cholerae antigens, including the toxin-coregulated pilus (TcpA), a V. cholerae membrane preparation, and the V. cholerae cytolysin/hemolysin (VCC) protein. Our results show that memory T-cell responses develop by day 7 after infection, a time prior to and concurrent with the development of B-cell responses. This suggests that T-cell responses to V. cholerae antigens may be important for the generation and stability of memory B-cell responses. The T-cell proliferative response to VCC was of a higher magnitude than responses observed to other V. cholerae antigens.
Vibrio cholerae is a gram-negative bacterium that can cause a severe, acute secretory diarrhea. Serological differentiation of V. cholerae strains is based on the O-side chain of the lipopolysaccharide (LPS) component of the outer membrane. Of the more than 200 serogroups of V. cholerae identified, only the O1 and O139 serogroups can cause epidemic cholera (44). These pathogens are noninvasive and colonize the mucosal surface of the small intestine (44).
Natural infection with V. cholerae is known to provide protection against subsequent disease, but the mechanism of this protective immunity is not fully characterized. The vibriocidal antibody is a complement-dependent bactericidal antibody that is associated with protection from infection. However, no known threshold level of the vibriocidal antibody confers complete protection from V. cholerae infection, and some individuals with low serum vibriocidal antibody titers are still protected. This suggests that the vibriocidal titer may be a surrogate marker (16, 45). Elevated serum immunoglobulin A (IgA) antibody levels specific for the B subunit of cholera toxin (CTB), the major structural subunit of a type IV pilus (TcpA), and LPS are also associated with protective immunity in areas where cholera is endemic (19). However, after natural infection, the serum levels of these antibodies wane more rapidly than protective immunity (19). Patients with cholera develop memory B-cell responses of both the IgG and the IgA isotype to at least two V. cholerae protein antigens, CTB and TcpA. These responses are detectable for at least 1 year after infection and persist even after V. cholerae antigen-specific antibody-secreting cells and serum antibody titers have returned to baseline (18). B-cell memory responses also develop for the T-cell independent antigen LPS, but these responses wane more rapidly than memory B-cell responses to protein antigens, suggesting that durable memory B-cell responses to some V. cholerae antigens may be T-cell dependent (18).
We have recently demonstrated that cholera patients mount a primed T-cell response in the mucosa after V. cholerae O1 infection (6). We hypothesize that protection from cholera may be mediated by memory B cells capable of an anamnestic response in the gut mucosa and that these memory B cells may depend on stimulation provided by memory T cells for their development and maintenance. T cells may contribute to the activation of B cells during V. cholerae infection by secreting stimulatory cytokines and direct contact with B cells in lymph nodes. Therefore, T cells may have an important role in protective immunity to V. cholerae infection.
We characterized the memory T-cell responses to V. cholerae antigens following natural V. cholerae infection and compared these with serological responses to the same antigens. Previously, our group has studied various V. cholerae antigens, including mannose-sensitive hemagglutinin, TcpA, CTB, and LPS (22, 33, 37). We also included in the present study responses to a novel antigen, V. cholerae cytolysin/hemolysin (VCC) (31, 32). The hly gene that encodes the VCC protein is widespread across both pathogenic and environmental strains of V. cholerae, suggesting that VCC may impart an advantage to the organism (42). Although the precise role of VCC in V. cholerae infection is unknown, VCC is the primary virulence factor in V. cholerae infection with non-O1, non-O139 strains that do not produce cholera toxin (12, 46). The immune response to VCC is not well understood; however, recent studies suggest that VCC may promote a Th2 response in V. cholerae infection (2). In addition, the cytolytic activity of VCC may generate epithelial destruction that allows other V. cholerae antigens to penetrate the mucosa and promote the inflammatory response observed in V. cholerae infection (35, 39).
MATERIALS AND METHODS
Study subjects and overview.
The International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B) Dhaka hospital cares for over 20,000 cholera patients annually, most of whom are residents of Dhaka city. Patients older than 6 months who had a positive stool culture for V. cholerae and were without significant comorbid conditions were eligible for inclusion in the present study. All of the participants included presented with severe, acute watery diarrhea and required treatment with intravenous fluids. The study participants were also treated with antibiotics. Individuals with similar socioeconomic backgrounds as patients and who experienced no diarrheal illness in the previous 3 months were selected as healthy controls. For patients, blood samples were obtained during acute infection (the second day of hospitalization) and on days 7 and 30 during the convalescent period. At each time point, T-cell lymphoblast proliferation was measured in response to ex vivo antigenic stimulation with a membrane preparation from V. cholerae O1 (MP), TcpA, VCC, or V. cholerae LPS. These antigens are described in detail below. In addition, vibriocidal titers and serologic responses to antigens TcpA, VCC, and the homologous LPS (V. cholerae-specific serotypes Inaba or Ogawa) were performed. Study participants were enrolled after providing written informed consent. The Research and Ethical Review Committee of the ICDDR,B and the Institutional Review Board of Massachusetts General Hospital approved the present study. The human experimentation guidelines of the U.S. Department of Health and Human Services were followed during the conduct of this research.
Bacteriological examination of patient stools.
Cases were confirmed by culturing stool onto taurocholate-tellurite-gelatin and MacConkey agar (28). After overnight incubation of plates, suspected V. cholerae colonies were serologically confirmed by slide agglutination with specific monoclonal antibody for Ogawa or Inaba serotypes (34, 41).
V. cholerae-specific stimulating antigens and controls.
A V. cholerae MP was made from the sequenced O1 El Tor strain N16961 grown in vitro in AKI medium (21). Specifically, a culture of N16961 was inoculated into 1 liter of AKI medium (21). The culture was grown in nonaerating conditions to an optical density at 600 nm of 0.3 and then transferred to shaking conditions for growth to stationary phase. Bacteria were pelleted by centrifuging and then sonicated. The sonicated mixture was centrifuged at 1,400 × g for 10 min, and the remaining supernatant was then centrifuged at 14,900 × g for 30 min. The pellet containing the membrane fraction was then suspended in MgCl2-Tris buffer (5 mM MgCl2, 10 mM Tris [pH 8.0]) for subsequent experiments. Mass spectrometry analysis indicated that the MP contains a mixture of bacterial proteins, and the most abundant of these proteins are listed in Table 1.
TABLE 1.
Proteins represented in the V. cholerae membrane preparation and abundance of selected virulence proteins
| Protein identifier | Protein name | Gene no. | Quantity rank | Spectral count |
|---|---|---|---|---|
| Most abundant proteins | ||||
| OMPU_VIBCH | Outer membrane protein U precursor porin OMPU | VC0633 | 1 | 1,897 |
| EFTU2_VIBCH | Elongation factor tu-b | VC0362 | 2 | 435 |
| CH601_VIBCH | Chaperonin 1 (protein cpn60 1) | VC2664 | 3 | 314 |
| ATPA_VIBCH | ATP synthase subunit α | VC2766 | 4 | 299 |
| O31154_VIBCH | Putative outer membrane porin OMPA | VC2213 | 5 | 295 |
| ATPB_VIBCH | ATP synthase subunit β | VC2764 | 6 | 282 |
| Q9KUB8_VIBCH | Aconitate hydratase 2 | VC0604 | 7 | 281 |
| PPCK_VIBCH | Phosphoenolpyruvate carboxykinase | VC2738 | 8 | 212 |
| DNAK_VIBCH | Chaperone protein DNAK | VC0855 | 9 | 206 |
| Q9KT62_VIBCH | Long-chain fatty acid transport protein | VC1043 | 10 | 199 |
| Q9KQB1_VIBCH | Succinate dehydrogenase, flavoprotein subunit | VC2089 | 11 | 195 |
| Q9KNS5_VIBCH | Fumarate reductase, flavoprotein subunit | VC2656 | 12 | 194 |
| MDH_VIBCH | Malate dehydrogenase | VC0432 | 13 | 192 |
| Q9KUF5_VIBCH | Protease do | VC0566 | 14 | 191 |
| LAMB_VIBCH | Maltoporin precursor (maltose-inducible porin) | VCA1028 | 15 | 158 |
| EFG2_VIBCH | Elongation factor G2 | VC2342 | 16 | 155 |
| Q9KNN3_VIBCH | Aspartate ammonia-lyase | VC2698 | 17 | 150 |
| ENO_VIBCH | Enolase | VC2447 | 18 | 145 |
| Q9KTZ9_VIBCH | Antioxidant, ahpc/tsa family | VC0731 | 19 | 142 |
| DLDH_VIBCH | Dihydrolipoyl dehydrogenase | VC2412 | 20 | 139 |
| Selected virulence proteins | ||||
| HLYA_VIBCH | Hemolysin precursor | VCA0219 | 90 | 21 |
| TCPA1_VIBCH | Toxin-coregulated pilin precursor | VC0828 | 100 | 11 |
| TCPC_VIBCH | Toxin-coregulated pilus biosynthesis outer membrane protein C | VC0831 | 106 | 5 |
The VCC monomer was used for V. cholerae-specific antigenic stimulation at a concentration of 2.5 ng/ml. We used VCC purified from a non-O1 clinical strain of V. cholerae; this protein is known to be immunologically and biochemically identical to V. cholerae O1 VCC (50). Isolation and purification was conducted as previously described (10). Recombinant TcpA, prepared as described previously, was used at a concentration of 2.5 μg/ml (3). V. cholerae O1 Inaba or Ogawa LPS, matched to the case serotype, was used at a concentration of 2.5 μg/ml. Preparation of V. cholerae O1 LPS was conducted as previously described (33). Positive controls for the assay included purified protein derivative (Statens Serum Institut, Copenhagen, Denmark) and phytohemagglutinin (Murex, Remel, Sweden) at concentrations of 5 and 1 μg/ml, respectively. Samples containing unstimulated cells were also included.
Measurement of V. cholerae antigen-specific antibodies in serum and lymphocyte supernatants.
Vibriocidal antibody assays were performed as previously described using guinea pig complement and the homologous matched serotype of V. cholerae El Tor Ogawa (X-25049) or El Tor Inaba (T-19479) as the target organism (38). The vibriocidal titer was defined as the reciprocal of the highest serum dilution resulting in >50% reduction of the optical density compared to the optical density of control wells without serum. Seroconversion was defined as a ≥4-fold increase in vibriocidal titer after acute dehydrating diarrhea.
LPS-, TcpA-, and VCC-specific IgG and IgA in serum samples were quantified by using standardized enzyme-linked immunosorbent assay (ELISA) procedures as previously described (36, 40). For anti-TcpA detection, ELISA plates were coated with TcpA (1 μg/ml) in carbonate buffer (50 mM, pH 9.6). For LPS- and VCC-specific responses, ELISA plates were coated with 2.5 μg of antigen/ml in phosphate buffered saline (PBS; 10 mM, pH 7.2). Sera were diluted 1:100 for TcpA and VCC antibody testing and 1:50 for LPS, with 0.1% bovine serum albumin in PBS-Tween (10 mM PBS [pH 7.2] containing 0.05% Tween 20). Then, 100 μl of diluted serum/well was added for each antigen. Horseradish peroxidase-conjugated secondary antibodies to human IgG or IgA were applied in separate wells. Plates were washed and developed with ortho-phenylene diamine substrate (Sigma, St. Louis, MO) and 0.012% hydrogen peroxide in 0.1 M sodium citrate buffer. The optical density was measured kinetically at 450 nm for 5 min. The maximal rate of optical density change was expressed as milli-optical density absorbance units per minute. ELISA units were normalized by calculating the ratio of the test sample to a standard of pooled convalescent-phase serum from recovered cholera patients added to each plate (33).
For the collection of lymphocyte supernatants, heparinized blood was diluted in PBS (1:1). Peripheral blood mononuclear cells (PBMC) and serum were isolated by differential centrifugation with Ficoll-Isopaque (Pharmacia, Piscataway, NJ). PBMC were resuspended at a concentration of 107 cells/ml in RPMI medium (Gibco, Carlsbad, CA) and supplemented with 1% penicillin, 1% streptomycin, 1% l-glutamine, 1% sodium-pyruvate, and 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT). PBMC were incubated for 48 h at 37°C in 5% CO2 in 24-well tissue culture plates without mitogen. Plates were centrifuged at 1,200 × g for 10 min, and the supernatants were collected. A protease inhibitor cocktail containing aprotinin (0.15 μM), leupeptin (10 μM), sodium azide (15 μM), and 4-(amino-ethyl) benzene sulfonyl fluoride (0.2 μM) was added at a concentration of 10 μl/ml of supernatant, and sample aliquots were preserved at −70°C. Supernatants were assayed at a 1:2 dilution in 0.1% bovine serum albumin in PBS-Tween in an ELISA as described above, and responses were detected with rabbit anti-human IgA- and IgG-horseradish peroxidase conjugate. Responses were measured kinetically as previously described (33).
FASCIA.
The FASCIA (flow cytometric assay of specific cell-mediated immune response in activated whole blood) method was used as previously described to determine lymphoblast formation in response to antigenic stimulation (14, 48). Briefly, whole blood was collected in a lithium heparin-coated tube and diluted 1:8 in Dulbecco modified Eagle medium supplemented with 1% gentamicin, 1% mercaptoethanol, and 10% heat-inactivated fetal calf serum. Then, 100 μl of stimulating antigen, control antigen, or additional medium was added to 400 μl of diluted blood. After 6 days of in vitro culture at 37°C in 5% CO2, supernatant was preserved for cytokine analysis, and cells were stained with anti-CD3-FITC, anti-CD4-PerCP, anti-CD8-APC, and anti-CD45R0-PE monoclonal antibodies (Becton Dickinson Immunocytometry Systems [BD], Stockholm, Sweden). An erythrocyte lysing solution of 1.0 μl of ammonium chloride (Orthumune lysing reagents; Ortho Diagnostics, Stockholm, Sweden) was added for 6 min, followed by incubation at room temperature, followed in turn by centrifugation, the removal of supernatants, and washing. Cells were suspended in 2% paraformaldehyde and stored in the dark; within 12 h, acquisition was conducted by fluorescence-activated cell sorting (FACSCalibur [BD]) for standardized 2-min intervals using CellQuest Pro software (Becton Dickinson, San Jose, CA). Analysis was performed with FlowJo software (TreeStar, Inc.). The results are presented as the ratio of lymphoblast count with antigenic stimulation to the count without stimulation (stimulation index). A value of “1” indicates that stimulation is equal in samples with or without V. cholerae antigenic stimulation.
Additional investigation of memory T-cell phenotype.
To further define the character of lymphoblasts proliferating in response to V. cholerae-specific antigens, we examined the peripheral blood of five cholera patients for additional T-cell phenotypic markers. On day 2 and day 7 after infection, we used the FASCIA method as described above and identified cell surface markers with anti-CD4-PerCP, anti-CD45R0-PE, anti-CD45RA-FITC, and anti-CCR7-APC monoclonal antibodies (BD).
Statistical analyses.
The Wilcoxon signed-rank test was used to compare immunologic responses of cholera patients on different study days, and the Mann-Whitney U test was used to compare immune responses between patients and healthy controls. All reported P values are two tailed. A cutoff of P ≤ 0.05 was the predetermined threshold for statistical significance. Analyses and figure preparation were performed on GraphPad Prism 4.0 and Stata version 9.0 (Stata Corp., Inc., College Station, TX). Geometric means with 95% confidence intervals are shown in figures unless otherwise stated.
RESULTS
Study population.
Sixteen patients were enrolled in the study, and fifteen completed 30 days of follow-up. All participants had cholera and severe dehydration upon initial clinical evaluation. Ten apparently healthy adults were included as healthy controls. Demographic and clinical features comparing cholera patients and healthy controls are shown in Table 2.
TABLE 2.
Demographic and clinical characteristics of cholera patients and healthy controls
| Demographics and clinical characteristics | Patients (n = 16) | Healthy controls (n = 10) |
|---|---|---|
| No. of female (% total) | 8 (50) | 5 (50) |
| Median age in yr (range) | 34 (13-50) | 33 (18-45) |
| V. cholerae O1 serotype | Ogawa (n = 11), Inaba (n = 5) | |
| Mean ± SD | ||
| Duration of diarrhea (h) | 21 ± 3.7 | |
| Amt of fluids required (liters) | 8.6 ± 1.6 | |
| Duration of hospitalization (h) | 31 ± 3.3 |
Vibriocidal and antigen-specific antibody responses.
VCC, LPS, and TcpA-specific antibody responses and vibriocidal titers were measured in sera on days 2, 7, and 30 and are shown in Table 3. On day 2 of infection, the geometric mean vibriocidal titer was 35 (95% confidence interval, 13 to 97), and this increased to 4,300 (3,000 to 6,300) by day 7 after infection (P < 0.001). All 16 patients demonstrated seroconversion.
TABLE 3.
Serum vibriocidal and antibody responses in patients and healthy controls
| Immune response | Antigen | Geometric mean antibody level (95% CI) fora: |
|||||
|---|---|---|---|---|---|---|---|
| Healthy controls | Study patients |
||||||
| Day 2 | Day 7 | P (day 2 vs day 7) | Day 30 | P (day 2 vs day 30) | |||
| Vibriocidal | NAb | 42 (20-91) | 35 (13-97) | 4,300 (3,000-6,300)* | 0.0004 | 1,280 (900-1,800)* | 0.0007 |
| IgA | TcpA | 16 (12-21) | 13 (8.3-20) | 38 (21-67)* | 0.001 | 22 (15-35) | 0.0007 |
| LPS | 29 (15-56) | 15 (9.4-22) | 100 (62-180)* | 0.001 | 34 (23-50) | 0.0037 | |
| VCC | 35 (21-61) | 52 (41-65) | 150 (110-200)* | 0.0004 | 88 (70-100)* | 0.0008 | |
| IgG | TcpA | 52 (39-70) | 47 (34-64) | 87 (64-120)* | 0.0009 | 84 (58-120) | 0.0008 |
| LPS | 69 (29-46) | 42 (29-62) | 150 (110-220)* | 0.0005 | 110 (86-152)* | 0.0007 | |
| VCC | 150 (110-200) | 190 (160-220) | 230 (190-280)* | 0.056 | 260 (220-300)* | 0.027 | |
See Materials and Methods. CI, confidence interval. *, P ≤ 0.05 compared to healthy controls.
NA, not applicable.
We observed significant humoral responses to V. cholerae-specific antigens. VCC-specific IgG antibody titers peaked on day 30 and were significantly higher than titers on day 2 (P = 0.027). TcpA- and V. cholerae O1 LPS-specific IgG levels were highest on day 7 (P < 0.001 and P < 0.001 compared to day 2). Patients demonstrated peak IgA responses on day 7 for all antigens, and these levels were significantly higher than day 2 measurements (P = 0.001 for TcpA- and LPS-specific antibodies, P < 0.001 for VCC-specific antibodies). LPS-, VCC-, and TcpA-specific antibody responses and vibriocidal titers were significantly greater on day 7 compared to responses observed in healthy controls.
Memory T-cell responses.
Using the FASCIA method to measure lymphoblast proliferation, we observed significant increases in V. cholerae-specific memory T cells on day 7 after infection; these results are shown in Fig. 1. T-cell memory responses after stimulation by VCC and MP peaked on day 7 and decreased by day 30 (P = 0.013 and P = 0.001 for day 7 compared to day 2). Proliferation in response to TcpA increased by day 7 (P = 0.013) and remained elevated until day 30. Compared to healthy controls, the proliferation of memory T cells in response to VCC was significantly elevated on day 7 and day 30 (P < 0.001 and P < 0.001). Differences in proliferation between HC and patient lymphoblasts were significant on both day 7 (P < 0.001) and day 30 (P = 0.035). Stimulation with V. cholerae O1 LPS, a T-cell independent antigen, did not generate any significant differences in memory T-cell proliferation during the 30-day period after infection.
FIG. 1.
Memory T-cell responses to V. cholerae antigens. Memory T cells are defined as CD4+/CD45R0+ cells. *, P ≤ 0.05 for day 2 compared to day 7 or day 30; ∧, P ≤ 0.05 for healthy controls (HC) compared to day 7 or day 30.
Immune responses to VCC.
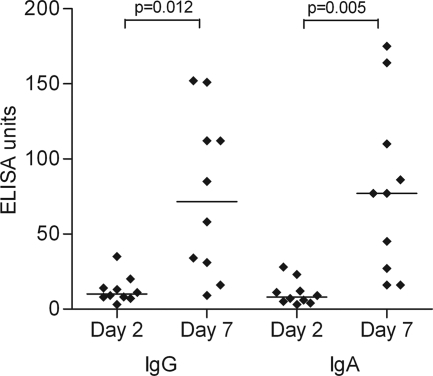
The antigen VCC generated a more robust T-cell memory lymphoblast response than that observed to other V. cholerae antigens assayed (Fig. 1). In addition, high-magnitude humoral responses to VCC were measured (Table 3). We also used the antibody in lymphocyte supernatant assay to assess mucosal immune responses to VCC (9). On day 7 after infection, lymphocytes stimulated in the gut mucosa transiently circulate in the peripheral blood (40). At this time, we observed a significant increase in VCC-specific antibodies secreted from circulating lymphocytes compared to day 2. These results are shown in Fig. 2.
FIG. 2.
VCC-specific antibody responses in supernatants of lymphocytes circulating on days 2 and 7 after infection.
Memory T-cell function and phenotype.
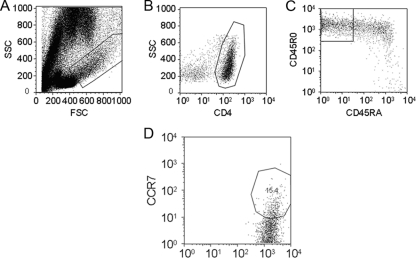
We used known markers of T-cell function to further determine the nature of the memory T-cell populations after V. cholerae infection. The CD45RA marker was used to exclude intermediate memory populations (CD45R0+ CD45RA+ cells) from the analysis (17). The memory T-cell CD4+ CD45R0+ CD45RA− population was on average 80% CCR7− after 6 days ex vivo stimulation, and a representative flow cytometry plot is shown in Fig. 3. Lymphoblast populations displaying these markers indicate an effector memory population. The remaining CD4+ CD45R0+ CD45RA− cells were CCR7+, markers consistent with a central memory T-cell phenotype.
FIG. 3.
Proportion of central memory cells in the proliferating memory T-cell population. On average, 20% of CD4+ CD45R0+ CD45RA− memory T cells had the CCR7+ marker, a finding consistent with the central memory phenotype. Plots above are representative of the five patients sampled. (A) Lymphoblast and lymphocyte population after 6-day ex vivo stimulation with V. cholerae MP. (B) Lymphoblast gated CD4+ population. (C) CD4+ gated CD45R0+ CD45RA− population. (D) CD4+ CD45R0+ CD45RA− gated CCR7+ population. CCR7+ gates were placed by comparing a CCR7 unstained control to a CCR7 stained plot.
DISCUSSION
After natural V. cholerae O1 infection, an adaptive immune response provides protection from subsequent disease, but the mechanism of this protection is incompletely understood. In North American volunteers, infection with V. cholerae provided over 90% specific protection from rechallenge with the same biotype, and this protection lasted for at least 3 years (24). An observational study showed that dehydrating V. cholerae El Tor infection in areas of endemicity conferred significant protection from subsequent disease for approximately 5 years (15).
Compared to natural infection, protective immunity generated by vaccine candidates has been incomplete and often short-lived. In addition, protection mediated by vaccination has declined more rapidly in persons living in areas of endemicity than in V. cholerae-naive persons. An oral, inactivated V. cholerae vaccine containing CTB (rCTB-WC) resulted in 60% protective efficacy in North American volunteers (7). When tested in Bangladesh, this effect waned by 1 year and provided little protection in children (11). Similarly, a live attenuated vaccine strain (CVD-103HgR) gave minimal protection in Indonesian field trials after encouraging results were observed in North American volunteers (43, 49). The reasons for the differences in immune responses between vaccinees and cholera patients and for the discrepancies in the degree of protection between vaccinees in areas where the cholera is endemic versus areas where it is not endemic are not known.
The vibriocidal antibody response is the best characterized of the immune responses to V. cholerae infection. Although the magnitude of vibriocidal titer roughly correlates with protection from disease, this relationship is inconsistent. Since V. cholerae is noninvasive, it is unclear how a serum protein such as the vibriocidal antibody may provide protection. Instead, vibriocidal titers may serve as a proxy measurement for protective immunity. In a study of household contacts of cholera patients, a logistic regression model demonstrated that levels of vibriocidal antibody and CTB-specific IgA were associated with a protective effect independent of other immunologic factors (19). However, the duration and extent of protection mediated by these factors declines more rapidly than the protection observed after natural infection, suggesting that other longer-lasting immunologic responses are necessary for protection.
In order to identify additional factors that may play a role in protective immunity, we studied responses to VCC, an antigen for which immune responses have not previously been studied in humans. We observed that VCC is strongly immunogenic and generates both B- and T-cell responses of a higher magnitude than those observed to other V. cholerae antigens. Further studies are needed to characterize the role of VCC in both infection and protective immunity.
In addition to antigen-specific antibody responses, patients with cholera develop memory B cells specific for V. cholerae antigens CTB, TcpA, and LPS (18, 22). Responses to protein antigens CTB and TcpA persisted for more than 1 year, whereas memory B-cell responses to LPS, a carbohydrate antigen, waned more quickly after infection (18). Memory B-cell responses to LPS do not require T-cell recognition, and this may explain the more rapidly declining memory B-cell response to LPS compared to responses generated by CTB and TcpA. Development of humoral responses to protein antigens are dependent on T-cell responses (25). CD4+ cell cytokine secretion and costimulation are the primary determinants of the quality and duration of memory B-cell responses to protein antigens (25). The direct binding of T and B cells in secondary lymphoid tissue facilitates CD40 and CD40L interactions critical for B-cell proliferation and isotype switching (13, 26). The stability of memory B-cell responses after exposure to TcpA and CTB, in contrast to T-cell-independent antigens such as LPS, is likely the result of T-cell contributions to memory B-cell activation.
Our results suggest that T-cell responses to V. cholerae infection may play a role in protective immunity, and several recent studies support this hypothesis. Cytokine responses, including an increase in IL-13 secretion by proliferating T cells, have been observed in acute cholera, suggesting a Th2 polarized T-cell response (6). Differences in cytokine levels in the serum and fecal extracts of parasite coinfected cholera patients compared to parasite-uninfected patients suggest that T-cell responses contribute to differences observed between vaccinee responses in areas where cholera is endemic, where parasitic infection is common, versus areas where it is not endemic (20).
Previously observed T-cell responses following V. cholerae infection were hypothesized to represent memory T-cell responses because people living in areas of endemicity develop immune responses to V. cholerae as children (6, 16, 29, 30). Memory T cells are a heterogeneous population and, unlike B cells, changes in T cells induced by exposure to antigen may be reversible (4, 27). Therefore, there is a lack of consensus on the classification of the populations that result from memory T-cell proliferation upon exposure to antigen. Generally, subsets are divided into effector memory cells that secrete cytokines and home to peripheral sites, and central memory cells that secrete fewer cytokines and home to lymph nodes (1, 47). We differentiated these populations using the CCR7 marker, a chemokine receptor active in T-cell migration (8). The absence of CCR7 on memory T cells is associated with the ability to secrete cytokines (1, 23). In our study, CD4+ CD45R0+ CD45RA− CCR7− cells, classified as effector memory cells, were the most abundant T-cell type after 6 days of in vitro stimulation. CD4+ CD45R0+ CD45RA− CCR7+ cells were classified as central memory cells, and this subtype is known to respond to antigen upon subsequent exposure (5, 47). It is uncertain whether the prolonged in vitro stimulation affects the relative abundance of T-cell memory subtypes we observed.
We hypothesize that protective immunity to V. cholerae infection is mediated by an anamnestic memory B-cell response mounted in the intestinal mucosa and that memory T cells are necessary for developing and maintaining this response. Our results show significant memory T-cell responses to a variety of V. cholerae antigens by day 7 after infection, at a time prior to and concurrent with the development of B-cell responses. The majority of the memory T cells observed were of the effector memory phenotype, while a substantial minority were central memory T cells. These results suggest that T-cell responses to V. cholerae antigens may be important for generation and stability of memory B-cell responses.
Acknowledgments
This research was supported by the ICDDR,B Centre for Health and Population Research and by the following grants: U01 AI058935 (S.B.C.), RO3 AI063079 (F.Q.), U01 AI077883 (E.T.R.), International Research Scientist Development Award KO1 TW07144 (R.C.L.), Howard Hughes Medical Institute Physician Scientist Early Career Award (R.C.L.), International Research Scientist Development Award KO1 TW07409 (J.B.H.), and a Fogarty International Center Global Infectious Disease Research Training Program Award in Vaccine Development D43 TW05572 (M.A., A.A.T., and A.S.). A.A.W., A.M.H., E.A.K., F.C., and A.I.K. are recipients of a Fogarty International Clinical Research Scholars award from the Fogarty International Center at the National Institutes of Health (D43 TW005572 and R24 TW007988). F.M. is a recipient of a Harvard Initiative for Global Health SURF Award. F.Q. and T.R.B. are recipients of funds from the Swedish Agency for International Development and Corp. (SIDA/SAREC).
We are grateful for the participation of patients and the work of the field staff at the ICDDR,B. We also thank Mohammad Rasheduzzaman at the ICDDR,B and Richelle Charles at Massachusetts General Hospital for laboratory and technical assistance.
Editor: S. M. Payne
Footnotes
Published ahead of print on 24 August 2009.
REFERENCES
- 1.Amyes, E., A. J. McMichael, and M. F. Callan. 2005. Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J. Immunol. 175:5765-5773. [DOI] [PubMed] [Google Scholar]
- 2.Arcidiacono, D., S. Odom, B. Frossi, J. Rivera, S. R. Paccani, C. T. Baldari, C. Pucillo, C. Montecucco, and M. de Bernard. 2008. The Vibrio cholerae cytolysin promotes activation of mast cell (T helper 2) cytokine production. Cell Microbiol. 10:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asaduzzaman, M., E. T. Ryan, M. John, L. Hang, A. I. Khan, A. S. Faruque, R. K. Taylor, S. B. Calderwood, and F. Qadri. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect. Immun. 72:4448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, E. B., and S. M. Sparshott. 1990. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature 348:163-166. [DOI] [PubMed] [Google Scholar]
- 5.Bell, E. B., and J. Westermann. 2008. CD4 memory T cells on trial: immunological memory without a memory T cell. Trends Immunol. 29:405-411. [DOI] [PubMed] [Google Scholar]
- 6.Bhuiyan, T. R., S. B. Lundin, A. I. Khan, A. Lundgren, J. B. Harris, S. B. Calderwood, and F. Qadri. 2009. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect. Immun. 77:1888-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, R. E., M. M. Levine, M. L. Clements, C. R. Young, A. M. Svennerholm, and J. Holmgren. 1987. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect. Immun. 55:1116-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromley, S. K., S. Y. Thomas, and A. D. Luster. 2005. Chemokine receptor CCR7 guides T-cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6:895-901. [DOI] [PubMed] [Google Scholar]
- 9.Chang, H. S., and D. A. Sack. 2001. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay, K., D. Bhattacharyya, and K. K. Banerjee. 2002. Vibrio cholerae hemolysin. Implication of amphiphilicity and lipid-induced conformational change for its pore-forming activity. Eur. J. Biochem. 269:4351-4358. [DOI] [PubMed] [Google Scholar]
- 11.Clemens, J. D., J. R. Harris, D. A. Sack, J. Chakraborty, F. Ahmed, B. F. Stanton, N. Huda, M. R. Khan, M. U. Khan, B. A. Kay, et al. 1988. Field trial of oral cholera vaccines in Bangladesh. Southeast Asian J. Trop. Med. Public Health 19:417-422. [PubMed] [Google Scholar]
- 12.Debellis, L., A. Diana, D. Arcidiacono, R. Fiorotto, P. Portincasa, D. F. Altomare, C. Spirli, and M. de Bernard. 2009. The Vibrio cholerae cytolysin promotes chloride secretion from intact human intestinal mucosa. PLoS ONE 4:e5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durie, F. H., T. M. Foy, S. R. Masters, J. D. Laman, and R. J. Noelle. 1994. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol. Today 15:406-411. [DOI] [PubMed] [Google Scholar]
- 14.Gaines, H., L. Andersson, and G. Biberfeld. 1996. A new method for measuring lymphoproliferation at the single-cell level in whole blood cultures by flow cytometry. J. Immunol. Methods 195:63-72. [DOI] [PubMed] [Google Scholar]
- 15.Glass, R. I., S. Becker, M. I. Huq, B. J. Stoll, M. U. Khan, M. H. Merson, J. V. Lee, and R. E. Black. 1982. Endemic cholera in rural Bangladesh, 1966-1980. Am. J. Epidemiol. 116:959-970. [DOI] [PubMed] [Google Scholar]
- 16.Glass, R. I., A. M. Svennerholm, M. R. Khan, S. Huda, M. I. Huq, and J. Holmgren. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236-242. [DOI] [PubMed] [Google Scholar]
- 17.Hamann, D., P. A. Baars, B. Hooibrink, and R. W. van Lier. 1996. Heterogeneity of the human CD4+ T-cell population: two distinct CD4+ T-cell subsets characterized by coexpression of CD45RA and CD45RO isoforms. Blood 88:3513-3521. [PubMed] [Google Scholar]
- 18.Harris, A. M., M. S. Bhuiyan, F. Chowdhury, A. I. Khan, A. Hossain, E. A. Kendall, A. Rahman, R. C. LaRocque, J. Wrammert, E. T. Ryan, F. Qadri, S. B. Calderwood, and J. B. Harris. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, J. B., R. C. Larocque, F. Chowdhury, A. I. Khan, T. Logvinenko, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris, J. B., M. J. Podolsky, T. R. Bhuiyan, F. Chowdhury, A. I. Khan, R. C. Larocque, T. Logvinenko, J. Kendall, A. S. Faruque, C. R. Nagler, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2009. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl. Trop. Dis. 3:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwanaga, M., and K. Yamamoto. 1985. New medium for the production of cholera toxin by Vibrio cholerae O1 biotype El Tor. J. Clin. Microbiol. 22:405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayasekera, C. R., J. B. Harris, S. Bhuiyan, F. Chowdhury, A. I. Khan, A. S. Faruque, R. C. Larocque, E. T. Ryan, R. Ahmed, F. Qadri, and S. B. Calderwood. 2008. Cholera toxin-specific memory B-cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 198:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, L. A., and D. G. Jackson. 2008. Cell traffic and the lymphatic endothelium. Ann. N. Y. Acad. Sci. 1131:119-133. [DOI] [PubMed] [Google Scholar]
- 24.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-820. [DOI] [PubMed] [Google Scholar]
- 25.McHeyzer-Williams, L. J., L. P. Malherbe, and M. G. McHeyzer-Williams. 2006. Checkpoints in memory B-cell evolution. Immunol. Rev. 211:255-268. [DOI] [PubMed] [Google Scholar]
- 26.McHeyzer-Williams, L. J., and M. G. McHeyzer-Williams. 2005. Antigen-specific memory B-cell development. Annu. Rev. Immunol. 23:487-513. [DOI] [PubMed] [Google Scholar]
- 27.Michie, C. A., A. McLean, C. Alcock, and P. C. Beverley. 1992. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 360:264-265. [DOI] [PubMed] [Google Scholar]
- 28.Monsur, K. A. 1961. A highly selective gelatin-taurocholate-tellurite medium for the isolation of Vibrio cholerae. Trans. R. Soc. Trop. Med. Hyg. 55:440-442. [DOI] [PubMed] [Google Scholar]
- 29.Mosley, W. H., A. S. Benenson, and R. Barui. 1968. A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull. W. H. O. 38:327-334. [PMC free article] [PubMed] [Google Scholar]
- 30.Mosley, W. H., A. S. Benenson, and R. Barui. 1968. A serological survey for cholera antibodies in rural east Pakistan. 2. A comparison of antibody titres in the immunized and control population of a cholera-vaccine field-trial area and the relation of antibody titre to cholera case rate. Bull. W. H. O. 38:335-346. [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee, G., K. K. Banerjee, and T. Biswas. 2008. Oligomerization of Vibrio cholerae hemolysin induces CXCR3 upregulation and activation of B-1a cell. Cell Mol. Immunol. 5:231-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee, G., A. Biswas, K. K. Banerjee, and T. Biswas. 2008. Vibrio cholerae hemolysin is apoptogenic to peritoneal B-1a cells but its oligomer shepherd the cells for IgA response. Cell Mol. Immunol. 45:266-270. [DOI] [PubMed] [Google Scholar]
- 33.Qadri, F., F. Ahmed, M. M. Karim, C. Wenneras, Y. A. Begum, M. Abdus Salam, M. J. Albert, and J. R. McGhee. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qadri, F., T. Azim, A. Chowdhury, J. Hossain, R. B. Sack, and M. J. Albert. 1994. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin. Diagn. Lab. Immunol. 1:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qadri, F., T. R. Bhuiyan, K. K. Dutta, R. Raqib, M. S. Alam, N. H. Alam, A. M. Svennerholm, and M. M. Mathan. 2004. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 53:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri, F., A. Chowdhury, J. Hossain, K. Chowdhury, T. Azim, T. Shimada, K. M. Islam, R. B. Sack, and M. J. Albert. 1994. Development and evaluation of rapid monoclonal antibody-based coagglutination test for direct detection of Vibrio cholerae O139 synonym Bengal in stool samples. J. Clin. Microbiol. 32:1589-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qadri, F., G. Jonson, Y. A. Begum, C. Wenneras, M. J. Albert, M. A. Salam, and A. M. Svennerholm. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 4:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qadri, F., R. Raqib, F. Ahmed, T. Rahman, C. Wenneras, S. K. Das, N. H. Alam, M. M. Mathan, and A. M. Svennerholm. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qadri, F., E. T. Ryan, A. S. Faruque, F. Ahmed, A. I. Khan, M. M. Islam, S. M. Akramuzzaman, D. A. Sack, and S. B. Calderwood. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman, M., D. A. Sack, S. Mahmood, and A. Hossain. 1987. Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J. Clin. Microbiol. 25:2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman, M. H., K. Biswas, M. A. Hossain, R. B. Sack, J. J. Mekalanos, and S. M. Faruque. 2008. Distribution of genes for virulence and ecological fitness among diverse Vibrio cholerae population in a cholera-endemic area: tracking the evolution of pathogenic strains. DNA Cell Biol. 27:347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richie, E. E., N. H. Punjabi, Y. Y. Sidharta, K. K. Peetosutan, M. M. Sukandar, S. S. Wasserman, M. M. Lesmana, F. F. Wangsasaputra, S. S. Pandam, M. M. Levine, P. P. O'Hanley, S. J. Cryz, and C. H. Simanjuntak. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399-2410. [DOI] [PubMed] [Google Scholar]
- 44.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363:223-233. [DOI] [PubMed] [Google Scholar]
- 45.Saha, D., R. C. LaRocque, A. I. Khan, J. B. Harris, Y. A. Begum, S. M. Akramuzzaman, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318-2322. [DOI] [PubMed] [Google Scholar]
- 46.Saka, H. A., C. Bidinost, C. Sola, P. Carranza, C. Collino, S. Ortiz, J. R. Echenique, and J. L. Bocco. 2008. Vibrio cholerae cytolysin is essential for high enterotoxicity and apoptosis induction produced by a cholera toxin gene-negative V. cholerae non-O1, non-O139 strain. Microb. Pathog. 44:118-128. [DOI] [PubMed] [Google Scholar]
- 47.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 48.Svahn, A., A. Linde, R. Thorstensson, K. Karlen, L. Andersson, and H. Gaines. 2003. Development and evaluation of a flow-cytometric assay of specific cell-mediated immune response in activated whole blood for the detection of cell-mediated immunity against varicella-zoster virus. J. Immunol. Methods 277:17-25. [DOI] [PubMed] [Google Scholar]
- 49.Tacket, C. O., M. B. Cohen, S. S. Wasserman, G. Losonsky, S. Livio, K. Kotloff, R. Edelman, J. B. Kaper, S. J. Cryz, R. A. Giannella, G. Schiff, and M. M. Levine. 1999. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect. Immun. 67:6341-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto, K., Y. Ichinose, N. Nakasone, M. Tanabe, M. Nagahama, J. Sakurai, and M. Iwanaga. 1986. Identity of hemolysins produced by Vibrio cholerae non-O1 and V. cholerae O1, biotype El Tor. Infect. Immun. 51:927-931. [DOI] [PMC free article] [PubMed] [Google Scholar]