Abstract
ATP-binding cassette transporter A1 (ABCA1) plays a central role in promoting cholesterol efflux from macrophages, thereby reducing the risk of foam cell formation and atherosclerosis. The expression of ABCA1 is induced by members of the nuclear receptor family of transcription factors, including retinoic acid receptors (RARs). A key innate immunity signaling kinase, IRAK-1, has been associated with an increased risk of atherosclerosis in humans and mice. This prompted us to investigate the potential connection between IRAK-1 and the expression of ABCA1. Here, we demonstrate that nuclear RARα levels are dramatically elevated in IRAK-1−/− macrophages. Correspondingly, IRAK-1−/− macrophages exhibit increased expression of ABCA1 mRNA and protein, as well as elevated cholesterol efflux in response to the RAR ligand ATRA. Analysis of the ABCA1 proximal promoter revealed binding sites for both RAR and NFAT. Chromatin immunoprecipitation assays demonstrated increased binding of RARα and NFATc2 to the ABCA1 promoter in IRAK-1−/− macrophages compared to wild-type macrophages. Additionally, lipopolysaccharide pretreatment reduced the nuclear levels of RARα and decreased ABCA1 expression and cholesterol efflux in wild-type but not in IRAK-1−/− cells. In summary, this study reveals a novel connection between innate immunity signaling processes and the regulation of ABCA1 expression in macrophages and defines a potential therapeutic target for treating atherosclerosis.
ABCA1 is widely expressed in diverse cells and tissues and mediates cholesterol and phospholipid trafficking from cells to plasma lipid poor apolipoproteins, such as apoA1, resulting in the formation of nascent high-density lipoprotein particles (HDL) (19, 50). Humans harboring a mutated ABCA1 gene develop the rare Tangier disease, characterized by low plasma HDL levels, accumulation of cholesterol in macrophages, and increased risk of cardiovascular diseases (6, 32, 39). Targeted disruption of the ABCA1 gene in mice produces a phenotype similar to that of Tangier disease in humans (40). Conversely, overexpression of ABCA1 in the transgenic mouse model increased plasma HDL levels and protected against atherosclerosis (53). In addition to exporting cholesterol and preventing foam cell formation, ABCA1 expressed in macrophages is also implicated in decreasing inflammatory responses (59, 60). ABCA1 expression in macrophages can also facilitate the uptake and clearance of apoptotic cells, by a process termed efferocytosis (16, 18). Collectively, proper function of ABCA1 in macrophages is essential to prevent both metabolic and inflammatory alterations contributing to the pathogenesis of atherosclerosis.
The expression of ABCA1 is regulated by the family of nuclear receptors, including retinoic acid receptor (RAR), retinoid X receptor (RXR), and liver X receptor (LXR) (11, 42, 46, 48, 55). All-trans-retinoic acid (ATRA) and other agonists can induce the binding of RAR and related transcription factors to the direct repeat sequences spaced by 1 to 5 nucleotides (DR1 to -5) on the proximal promoter of ABCA1 (1). Using murine models, LXR agonists have been shown to promote cholesterol efflux and cause substantial regression of atherosclerosis (25, 51). Earlier studies have also demonstrated roles for RARα and RARγ in the upregulation of ABCA1 expression and cholesterol efflux (10). On the other hand, inflammatory mediators and conditions can suppress ABCA1 expression in macrophages and exacerbate the pathogenesis of atherosclerosis (9, 37), likely through decreasing the nuclear levels of RAR-related transcription factors. However, the underlying molecular mechanism is not yet clearly understood.
IRAK-1 is an intracellular kinase utilized by multiple inflammatory signaling pathways and triggered by a diverse array of ligands, including interleukin-1 and Toll-like-receptor (TLR) agonists (13, 15, 31). The activation of IRAK-1 in macrophages and monocytes contributes to elevated expression of proinflammatory mediators (17, 49). In addition, IRAK-1 has been linked to the pathogenesis of atherosclerosis in both mice and humans (29, 52). Elevated expression of IRAK-1 has been observed in atherosclerotic plaques of human patients (29). In addition, genetic variants of the human IRAK-1 gene are associated with a higher risk of atherosclerosis and related cardiovascular complications (29). Furthermore, IRAK-1 is constitutively active in blood mononuclear cells isolated from patients with atherosclerosis (23, 29). Our recent study using ApoE−/−/IRAK-1−/− mice also demonstrated that IRAK-1 deletion renders protection from high-fat diet-induced atherosclerosis (56). Despite compelling evidence linking IRAK-1 with the pathogenesis of atherosclerosis, the underlying molecular mechanism is not fully defined.
In the present study, we examined the regulation of ABCA1 expression as well as cholesterol efflux using bone marrow-derived macrophages (BMDMs) from wild-type (WT) and IRAK-1−/− mice. We demonstrate that ATRA induces significantly higher levels of ABCA1 and elevated cholesterol efflux in macrophages harvested from IRAK-1−/− mice compared to the WT mice. Nuclear RARα protein levels were also significantly higher in IRAK-1−/− macrophages. Additionally, lipopolysaccharide (LPS) pretreatment caused a significant reduction of nuclear RARα levels, ABCA1 expression, and cholesterol efflux in WT but not in IRAK-1−/− macrophages. Mechanistically, we demonstrate that the elevated induction of ABCA1 by ATRA in IRAK-1−/− cells is mediated by the synergistic actions of NFATc2 and RARα. This study defines IRAK-1 as a key inflammatory signaling component modulating the expression of ABCA1. Therefore, IRAK-1 may serve as a potential therapeutic target in the prevention of foam cell formation and thus the progression of atherosclerosis.
MATERIALS AND METHODS
Reagents.
ATRA, cyclosporine A (CsA), leptomycin B, LPS, and apoA1 were commercially obtained from Sigma. Recombinant transforming growth factor β (TGF-β) was purchased from R&D Systems. The antibody against murine ABCA1 was obtained from Novus Biologicals. Antibodies specific for lamin B, β-actin, RARα, LXRα, RXRα/β, RARγ, and NFATc2 were purchased from Santa Cruz Biotechnology. [3H]cholesterol (40 Ci/mmol) was obtained from Perkin-Elmer.
Isolation of murine BMDMs.
Wild-type C57BL/6 mice were obtained from Charles River Laboratories. IRAK-1−/− mice with a C57BL/6 background were kindly provided by James Thomas from the University of Texas Southwestern Medical School. All mice were housed and bred at Derring Hall Animal Facility in compliance with approved Animal Care and Use Committee protocols at Virginia Polytechnic Institute and State University. BMDMs were isolated from the tibias and femurs of WT and IRAK-1−/− mice by flushing the bone marrow with Dulbecco's modified Eagle's medium (DMEM). The cells were cultured in non-tissue-culture-treated petri dishes with 50 ml DMEM containing 30% L929 cell supernatant, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and 2 mM glutamine. On the third day of culture, the cells were fed with an additional 20 ml fresh medium and cultured for another additional 3 days. Cells were harvested with phosphate-buffered saline (PBS), resuspended in DMEM supplemented with 1% fetal bovine serum, and allowed to rest overnight before further treatments.
Cholesterol efflux assays.
The cholesterol efflux assay involves the uptake of radiolabeled cholesterol into cells and the measurement of the amount of labeled cholesterol transferred to the medium (38). Briefly, the BMDMs were radiolabeled with cholesterol for 16 h in medium containing 1% fetal bovine serum. Following incubation, the cells were washed with PBS and equilibrated with 0.2% bovine serum albumin overnight before treatment with ATRA for 16 h in the presence of apoA1 or HDL, isolated from human plasma. Following treatments, the medium was removed after centrifugation at 3,000 rpm in a benchtop microcentrifuge to remove cellular debris. The remaining cells were washed with PBS and lysed in 0.5% sodium dodecyl sulfate (SDS) and 0.1 N NaOH. Aliquots of medium and cell lysates were mixed with scintillation liquid, and radioactivity was measured on a beta scintillation counter. Efflux was calculated using the following formula: percent efflux = 100 × cpmmedia/(cpm media + cpmcell), where cpmmedia is the counts per minute calculated for all the conditioned media and cpmcell is the counts per minute in the entire cell lysate.
Western blot analysis and immunoprecipitation assays.
Isolation of whole-cell lysates, as well as cytoplasmic and nuclear extracts, was performed as described earlier (23). Briefly, untreated or treated BMDMs (WT and IRAK-1−/−) were rinsed in PBS and then lysed on ice in lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin) for 30 min followed by addition of 10% Triton X-100. The samples were centrifuged for 10 min at 5,000 rpm, and the supernatant fractions were transferred and saved as cytoplasmic extracts. Pellets containing the intact nuclei were lysed and solubilized with a high-salt buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.4 M NaCl, 0.2 mM EDTA, 0.5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) for 30 min on ice followed by centrifugation at maximum speed for 20 min. The supernatant was saved as the nuclear extract. Western blotting analysis of the protein samples was performed as described previously (23). Immunoblots were developed by using the Amersham ECL Plus chemiluminescent detection system (GE Healthcare). The intensities of the bands were quantified using the Fujifilm Multi Gauge software and then normalized against β-actin levels.
For immunoprecipitation (IP) assays, the cell lysates were treated with 2 μg primary antibody or normal immunoglobulin G (IgG) in the presence of 35 μl of protein A/G PLUS-agarose (Santa Cruz Biotechnology) and rotated at 4°C overnight. The samples were centrifuged briefly, and the pellet was washed three times in IP buffer containing protease inhibitors. The bound proteins were eluted from the agarose beads by boiling with 2× Laemmli sample buffer for 5 min. The eluted proteins were loaded on a 7.5% SDS-polyacrylamide gel for electrophoresis followed by Western analysis with the specified antibodies.
Real-time RT-PCR.
Total RNA was prepared from untreated or ATRA-treated BMDM cells using TRIzol (Invitrogen) according to the manufacturer's protocol. Reverse transcription (RT) was carried out using the High-Capacity cDNA reverse transcription kit (Applied Biosystems), and subsequent real-time RT-PCR analyses were performed using the SYBR green supermix on an IQ5 thermocycler (Bio-Rad). The relative levels of ABCA1, ABCG1, and RARα transcripts were calculated using the ΔΔCt method after normalizing with GAPDH as the internal control. The relative level of ABCA1/ABCG1 mRNA in untreated wild-type cells was adjusted to 1 and served as the basal reference value.
Chromatin immunoprecipitation (ChIP) assays.
Murine BMDMs were either untreated or treated with 50 nM ATRA for 16 h followed by cross-linking with 1% formaldehyde in complete medium for 15 min with gentle rocking at room temperature. Cells were then washed twice with ice-cold PBS and treated with glycine solution for 5 min to stop the cross-linking reaction. Cells were then lysed in buffer containing SDS and protease inhibitor cocktail. Samples were sonicated six times with 30-s pulses at 4°C followed by centrifugation to collect the sheared chromatin. The sheared chromatin was used to set up immunoprecipitation reactions using the CHIP-IT Express kit (Active Motif) as per the manufacturer's recommendations. The immunoprecipitated DNA fragments were analyzed by PCR using the primers spanning the binding sites of the specified transcription factors on the ABCA1 promoter.
siRNA interference assays.
For small interfering RNA (siRNA) interference assays, WT BMDM cells (2 × 106 cells) were plated in six-well plates and transfected the following day by using Lipofectamine 2000 (Invitrogen) with the indicated siRNA oligos (Santa Cruz Biotechnology). After 36 h posttransfection, the cells were treated with ATRA (50 nM) for 16 h. Whole-cell extracts were prepared followed by Western blot analysis using the specified antibodies.
Statistical analyses.
Statistical significance was determined using the unpaired two-tailed Student's t test. P values less than 0.05 were considered statistically significant.
RESULTS
IRAK-1−/− macrophages exhibit enhanced cholesterol efflux following ATRA stimulation.
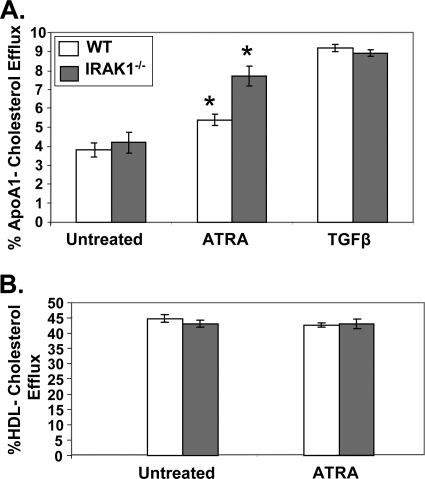
Efficient cholesterol export from macrophages prevents the formation of foam cells and atherosclerotic lesions (38). Since we previously reported that IRAK-1−/− mice had a reduced frequency of atherosclerotic plaques compared to WT mice (56), we studied the effect of IRAK-1 deletion on cholesterol efflux in macrophages. BMDMs harvested from WT or IRAK-1−/− mice were stimulated with ATRA for 16 h, followed by the cholesterol efflux assay as described in Materials and Methods. As shown in Fig. 1A, ATRA induced significantly higher apoA1-mediated cholesterol efflux from macrophages derived from IRAK-1−/− mice (90%) compared to the WT (40%). In contrast, ATRA failed to induce HDL-mediated cholesterol efflux in WT or IRAK-1−/− macrophages (Fig. 1B). As a control, TGF-β induced similar levels of cholesterol efflux between WT and IRAK-1−/− macrophages, indicating that not all signaling pathways regulating ABCA1 expression are affected by IRAK-1 (Fig. 1A). These results indicate that IRAK-1 is selectively involved in the ATRA-mediated apoA1-dependent cholesterol efflux pathway.
FIG. 1.
ATRA stimulation promotes apoA1-mediated cholesterol efflux in IRAK-1−/− macrophages. (A) ATRA induces a significant increase in cholesterol efflux to apoA1 in IRAK-1−/− BMDMs compared to WT. The BMDMs derived from WT and IRAK-1−/− mice were radiolabeled with [3H]cholesterol in cell culture medium for 16 h and then treated with or without ATRA (50 nM) or TGF-β (5 ng/ml) in the presence of apoA1 (10 μg/ml). Cellular cholesterol efflux was analyzed in liquid scintillation counting assays, and the results are expressed as means ± standard deviations from three independent experiments, each performed in triplicate. *, P < 0.05. (B) Effects of ATRA on HDL-mediated cholesterol efflux. WT and IRAK-1−/− BMDMs were radiolabeled with [3H]cholesterol for 16 h and then treated with or without ATRA (50 nM) in the presence of HDL (50 μg protein/ml). Cholesterol efflux to HDL was assayed by liquid scintillation counting as described above.
IRAK-1 selectively modulates ATRA-induced expression of ABCA1 in macrophages.
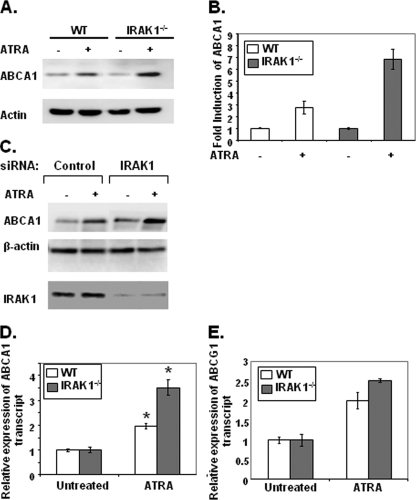
Cholesterol efflux to apoA1 is primarily mediated by the membrane-bound transporter ABCA1 (38). Since IRAK-1−/− macrophages displayed increased cholesterol efflux to ApoA1, we subsequently determined whether ABCA1 expression is differentially induced by ATRA in WT and IRAK-1−/− macrophages. As shown in Fig. 2A, the induced levels of ABCA1 protein were significantly higher in IRAK-1−/− macrophages than in the WT cells in response to ATRA stimulation. After quantitation of the band intensities, the IRAK-1−/− macrophages displayed a 7.5-fold induction of ABCA1 expression in response to ATRA, compared to a 3-fold induction in WT (Fig. 2B). To further confirm the contribution of IRAK-1 in regulating the expression of ABCA1, we used IRAK-1-specific siRNA to knock down its expression in WT BMDMs. As shown in Fig. 2C, knockdown of IRAK-1 resulted in a significantly higher induction of ABCA1 in response to ATRA. The same blots were probed with β-actin as a loading control and with IRAK-1-specific antibody to demonstrate around 90% knockdown of IRAK-1 expression. We further measured the RNA levels of ABCA1 in WT and IRAK-1−/− cells treated with or without ATRA. Consistent with the protein data, we observed that the RNA levels of ABCA1 in IRAK-1−/− BMDMs were significantly induced compared to WT cells following ATRA stimulation (Fig. 2D). These results indicate that increased ABCA1 gene transcription may contribute to the elevated levels of ABCA1. In contrast, the loss of IRAK-1 had no significant effect on ABCG1 transcript levels (Fig. 2E), whose promoter has no apparent binding site for NFAT near the DR4 region. Unlike ABCA1, which uses apoA1 as an acceptor for cholesterol efflux, ABCG1 uses mature HDL as the acceptor (27, 47). This further supports our finding described above that IRAK-1 is selectively involved in modulating the apoA1-mediated macrophage cholesterol efflux pathway induced by ATRA. Furthermore, consistent with the cholesterol efflux data, we observed that stimulation with TGF-β led to similar levels of ABCA1 expression in WT and IRAK-1−/− macrophages (see Fig. S1 in the supplemental material). Taken together, our findings indicate that IRAK-1 selectively modulates ATRA-induced ABCA1 expression in macrophages.
FIG. 2.
Loss of IRAK-1 increases ABCA1 mRNA and protein levels in response to ATRA. (A) Increased levels of ABCA1 protein in BMDMs lacking IRAK-1 in response to ATRA. WT and IRAK-1−/− BMDM cells were either untreated or treated with ATRA (50 nM) followed by Western blot analysis of cell extracts using ABCA1-specific antibodies. Antibodies against β-actin were used as the internal loading control. (B) The band intensities were quantified using the Fujifilm Multi Gauge software, and the fold induction is depicted after normalization against β-actin levels. (C) IRAK-1 siRNA oligo increases ABCA1 induction in response to ATRA. WT BMDM cells were transfected with IRAK-1-specific siRNA oligos by using Lipofectamine 2000. After 36 h, the cells were treated with ATRA for another 16 h, and cellular extracts were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting with the indicated antibodies. (D and E) Higher induction of ABCA1 mRNA by ATRA in IRAK-1−/− BMDMs. The cells were either untreated or treated with 50 nM ATRA for 6 h, and the expression levels of ABCA1 and ABCG1 transcripts were measured by real-time RT-PCR assays and standardized against GAPDH levels. Each experiment was performed in triplicate. *, P < 0.05.
IRAK-1 modulates ATRA-induced ABCA1 expression through RARα and NFATc2.
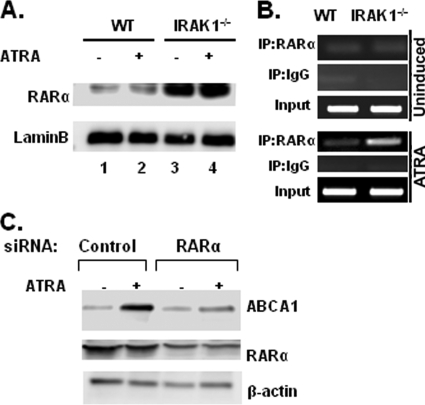
To further dissect the molecular mechanism involved in the elevated induction of ABCA1 transcription in IRAK-1−/− macrophages, we examined the status of key transcription factors responsible for ATRA-induced ABCA1 gene transcription. Earlier studies had shown that ABCA1 expression is upregulated by members of the nuclear receptor family, namely, LXRs, RARs, and RXRs. Since the nuclear receptor RARα is the physiological target for ATRA and has been shown to be involved in the transcription of ABCA1, we compared the nuclear levels of RARα in WT and IRAK-1−/− macrophages by Western blot analysis. As shown in Fig. 3A, the nuclear levels of RARα in IRAK-1−/− macrophages were at least 10-fold higher than in the WT. Therefore, the elevated nuclear RARα levels may enable IRAK-1−/− macrophages to respond more effectively to ATRA stimulation. In contrast, there was no significant difference in the levels of the other members of the nuclear receptor family, like RARγ and RXRα/β, between WT and IRAK-1−/− macrophages following LPS challenge (see Fig. S3A in the supplemental material).
FIG. 3.
In vivo association of RARα and the murine ABCA1 promoter. (A) Higher induction of nuclear RARα levels in IRAK-1−/− BMDMs. Nuclear protein extracts were isolated from untreated and ATRA-treated WT and IRAK-1−/− BMDMs. Harvested nuclear extracts were subjected to SDS-polyacrylamide gel electrophoresis, and the RARα protein levels were determined by Western blot analysis. The same blots were probed with lamin B-specific antibodies as a loading control. (B) Increased binding of RARα to the proximal promoter of murine ABCA1 in IRAK-1−/− BMDMs. The BMDMs were either uninduced (upper three panels) or induced with 50 nM ATRA (lower three panels) for 16 h and subjected to a ChIP assay. The primers spanning the DR4 region on the ABCA1 promoter were used in the ChIP assays. Data are representative of three independent experiments. (C) Loss of RARα when using siRNA oligos decreases ABCA1 induction in response to ATRA. WT BMDM cells were transfected with RARα-specific siRNA oligos by using Lipofectamine 2000. After 36 h, the cells were treated with ATRA for another 16 h, and cellular extracts were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting with the indicated antibodies.
We then carried out ChIP assays to evaluate the induced binding of RARα to the endogenous ABCA1 promoter region in WT and IRAK-1−/− macrophages. Previous studies had identified a well-defined DR4 RAR binding site within the proximal promoter region of ABCA1 (11, 44). As shown in Fig. 3B, the basal binding of RARα to the ABCA1 DR4 promoter region was minimal in both cell types. However, ATRA treatment induced a much stronger association of RARα with the ABCA1 promoter in IRAK-1−/− macrophages compared to the WT (Fig. 3B, lower panel). This is consistent with ligand-induced expression of ABCA1 by RARα.
The contribution of RARα toward ATRA-mediated induction of ABCA1 was further confirmed by siRNA experiments. As shown in Fig. 3C, knockdown of RARα protein levels using specific siRNA oligos decreased ATRA-dependent induction of ABCA1 compared to cells transfected with control siRNAs. The same blots were probed with β-actin as a loading control and with RARα-specific antibody to demonstrate around 70% knockdown of RARα protein expression.
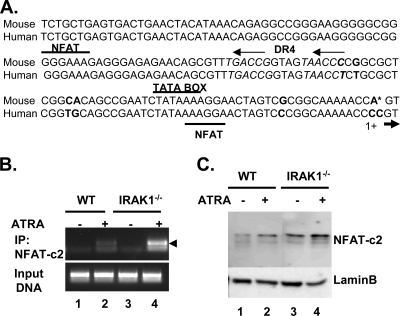
Intriguingly, we also noticed two closely spaced NFAT binding sites near the DR4 region, which are conserved in both the mouse and human ABCA1 promoters (Fig. 4A). Since IRAK-1 has been previously shown to downregulate NFAT nuclear distribution and activity (56), we herein studied whether NFAT may also differentially contribute to the expression of ABCA1 in WT and IRAK-1−/− macrophages. Interestingly, although NFAT family members have been extensively studied in T cells and other nonimmune cells, their functions in macrophages have not been examined. Out of the five members of NFAT, NFATc2 is the predominant form in immune cells, including T cells and macrophages (30, 33). Therefore, we performed ChIP analyses using an NFATc2-specific antibody to determine the binding of NFATc2 with the ABCA1 proximal promoter region. As shown in Fig. 4B, ATRA treatment slightly induced the interaction of NFATc2 with the ABCA1 promoter in WT macrophages (twofold above background). In contrast, NFATc2 binding to the ABCA1 promoter was significantly induced in IRAK-1−/− macrophages (sixfold over background). We subsequently examined the nuclear levels of NFATc2 protein by Western blotting and observed that IRAK-1−/− macrophages had significantly elevated levels of NFATc2 compared to WT (Fig. 4C).
FIG. 4.
NFAT regulates ABCA1 expression. (A) Alignment of human and mouse sequences of the ABCA1 promoter. The mismatched nucleotides between the mouse and human sequences are highlighted in bold. The DR4 and the adjacent NFAT binding sites are indicated. (B) Direct binding of NFATc2 to the proximal promoter of ABCA1 in IRAK-1−/− BMDMs. Cross-linked chromatin from untreated or ATRA-treated BMDMs (WT and IRAK-1−/−) was incubated overnight in the presence of an antibody against NFATc2. Immunoprecipitated DNA was subjected to PCR amplification using primers flanking the potential NFAT binding sites on the ABCA1 promoter. The arrowhead points to the specific band. (C) Elevated levels of NFATc2 in the nuclear fraction of IRAK-1−/− BMDMs. Untreated or ATRA-treated WT or IRAK-1−/− BMDMs were subjected to nuclear fractionation followed by SDS-polyacrylamide gel electrophoresis and Western blot analysis using NFATc2-specific antibodies. The same blots were probed with lamin B-specific antibodies as a loading control.
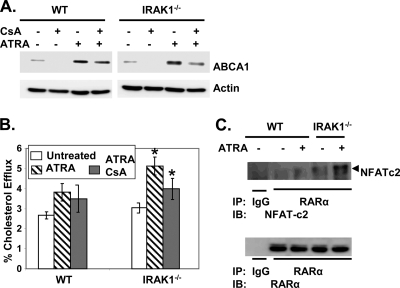
To further confirm the effect of NFATc2 in the regulation of ABCA1, we treated WT and IRAK-1−/− macrophages with an NFAT-specific inhibitor, CsA. As shown in Fig. 5A, CsA reduced ATRA-mediated ABCA1 expression in WT macrophages by ∼20%. In contrast, the reduction was ∼70% in IRAK-1−/− macrophages. Consistent with the Western blot data, CsA treatment reduced ATRA-mediated cholesterol efflux in IRAK-1−/− macrophages, confirming the involvement of NFAT (Fig. 5B).
FIG. 5.
The NFAT inhibitor, CsA, interferes with ATRA-mediated ABCA1 induction and cholesterol efflux in IRAK-1−/− BMDMs. (A) BMDM cells derived from WT and IRAK-1−/− mice were either untreated or treated with CsA alone or in the presence of ATRA. After 16 h of incubation, whole-cell lysates were prepared and subjected to SDS-polyacrylamide gel electrophoresis followed by Western blot analysis with ABCA1-specific antibodies. The blots were also probed with β-actin-specific antibodies as a loading control. (B) WT and IRAK-1−/− BMDMs were radiolabeled with [3H]cholesterol in DMEM cell culture medium containing 1% fetal bovine serum for 16 h and then treated with CsA and ATRA (50 nM) in the presence of apoA1 (10 μg/ml). Cellular cholesterol efflux was analyzed by liquid scintillation counting assays, and the results are expressed as means ± standard deviations from three independent experiments, each performed in triplicate. *, P < 0.05. (C) Coimmunoprecipitation of RARα with NFATc2 from ATRA-treated BMDM cells. Whole-cell lysates extracted from untreated or ATRA-treated BMDMs were immunoprecipitated with antibodies against RARα or nonimmune rabbit IgG and separated by SDS-polyacrylamide gel electrophoresis followed by Western analysis using NFATc2-specific antibodies (upper panel). The same blots were probed with RARα antibody as a control for the immunoprecipitation step. The position of the NFATc2-specific band is indicated by an arrowhead.
Previous studies suggested that NFAT is a weak DNA binding factor and usually cooperates with other transcription partners to regulate transcription (33, 34). In order to determine whether NFAT physically associates with RARα, immunoprecipitation assays were carried out using RARα-specific antibodies followed by immunoblotting with anti-NFATc2 antibodies. The results demonstrated that NFATc2 directly interacts with RARα in IRAK-1−/− macrophages (Fig. 5C).
LPS reduces nuclear RARα levels, ABCA1 expression, and macrophage cholesterol efflux in WT but not IRAK-1−/− macrophages.
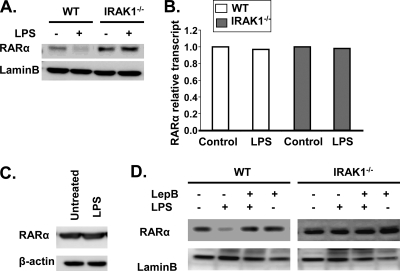
Inflammatory challenges have been shown to dampen ABCA1 expression and macrophage cholesterol efflux, contributing to foam cell formation and atherosclerosis (37). Therefore, we tested whether LPS treatment would differentially modulate nuclear RARα levels and ATRA-mediated ABCA1 expression in WT and IRAK-1−/− macrophages. As shown in Fig. 6A, LPS treatment led to a dramatic decrease in nuclear levels of RARα in WT but not IRAK-1−/− macrophages. RARα levels have been previously shown to be modulated at multiple levels, including expression and nuclear export (4). To determine the mechanism involved in reduced nuclear RARα levels in WT macrophages following LPS challenge, we measured the levels of RARα transcript by RT-PCR (Fig. 6B), as well as the levels of total RARα protein by Western blotting (Fig. 6C), and observed no significant changes. This prompted us to further determine whether LPS selectively triggers the nuclear export of RARα protein, causing decreased nuclear levels of RARα. As shown in Fig. 6D, leptomycin B, an inhibitor of nuclear export, completely blocked LPS-mediated reduction of nuclear RARα. Taken together, our data indicate that LPS treatment causes rapid IRAK-1-dependent nuclear export of RARα.
FIG. 6.
Differential effects of LPS on nuclear RARα protein levels in WT and IRAK-1−/− BMDMs. (A) Effects of LPS on nuclear RARα levels in BMDMs. WT and IRAK-1−/− BMDMs were treated with 100 ng/ml LPS for 2 h followed by nuclear protein extraction. The samples were analyzed by immunoblotting using the indicated antibodies. Lamin B was used as the loading control. (B) The BMDMs derived from WT and IRAK-1−/− mice were treated with LPS for 2 h followed by RNA extraction. The resulting cDNAs were used to detect RARα transcript levels by real-time RT-PCR and standardized against GAPDH levels. (C) WT BMDMs were treated with 100 ng/ml LPS for 2 h followed by whole-cell protein extraction. The samples were resolved by SDS-polyacrylamide gel electrophoresis followed by immunoblotting with anti-RARα antibodies. (D) BMDM cells derived from WT and IRAK-1−/− mice were either untreated or treated with leptomycin B (LepB) alone or in the presence of LPS. After 2 h of incubation, nuclear lysates were prepared and subjected to SDS-polyacrylamide gel electrophoresis followed by Western blot analysis with RARα-specific antibodies. The blots were also probed with lamin B-specific antibodies as a loading control.
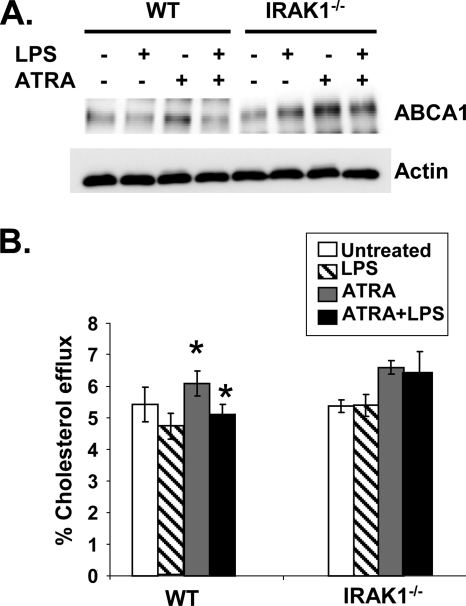
Consequently, we tested the effect of LPS pretreatment on ATRA-induced ABCA1 expression and macrophage cholesterol efflux. As shown in Fig. 7A, LPS treatment significantly decreased ATRA-mediated ABCA1 expression in WT macrophages by reducing ABCA1 to the basal level. In contrast, LPS pretreatment had a minimal effect on ATRA-induced ABCA1 expression in IRAK-1−/− macrophages. We further performed a cholesterol efflux assay and observed that LPS pretreatment completely ablated ATRA-induced cholesterol efflux to apoA1 in WT macrophages. In contrast, LPS had no inhibitory effect on ATRA-induced cholesterol efflux in IRAK-1−/− macrophages (Fig. 7B).
FIG. 7.
LPS reduces ATRA-induced ABCA1 expression and apoA1-dependent cholesterol efflux in WT BMDMs. (A) Reduced levels of ABCA1 in LPS-treated WT BMDMs. WT and IRAK-1−/− BMDM cells were either untreated or treated with LPS for 2 h followed by addition of ATRA alone or in combination. The whole-cell lysates were analyzed by Western blotting using ABCA1-specific antibodies. Antibodies against β-actin were used as the internal control. (B) WT and IRAK-1−/− BMDMs were radiolabeled with [3H]cholesterol in cell culture medium for 16 h and then treated with LPS with or without ATRA (50 nM) in the presence of apoA1 (10 μg/ml). Cellular cholesterol efflux was analyzed by liquid scintillation counting assays, and the results are expressed as means ± standard deviations from three independent experiments, each performed in triplicate. *, P < 0.05.
DISCUSSION
In this report, we demonstrate that the inflammation-promoting kinase IRAK-1 plays a key role in modulating cholesterol efflux in macrophages. Through IRAK-1-mediated signaling processes, inflammatory stimulants such as LPS suppress ATRA-induced expression of ABCA1 and subsequent cholesterol efflux from macrophages. Several lines of evidence collectively support our conclusion. First, ATRA induces significantly higher levels of ABCA1 and enhances cholesterol efflux in IRAK-1−/− macrophages compared to WT. Second, LPS selectively inhibits ATRA-induced ABCA1 expression and cholesterol efflux in WT but not IRAK-1−/− macrophages. Third, LPS reduces nuclear levels of RARα, a key transcription factor required for ABCA1 expression, through an IRAK-1-dependent pathway.
Our study confirms and extends previous findings linking infection, inflammation, and the regulation of cholesterol export in macrophages. Emerging studies indicate that TLR agonists can suppress the expression of macrophage ABCA1 and cholesterol export, leading to foam cell formation (12, 26, 36, 57). Thus, reduced levels of ABCA1 due to infection and subsequent inflammatory signaling processes not only lead to decreased cholesterol export and foam cell formation but also contribute to a reduced ability of these cells for efferocytosis and the clearance of dead cells (16, 18). Furthermore, reduced ABCA1 levels may in turn lead to enhanced TLR signaling and elevated expression of inflammatory cytokines (24, 37). Taken together, the suppression of ABCA1 expression in macrophages by TLR agonists contributes to the pathogenesis of atherosclerosis. These findings are consistent with both clinical observations and basic research studies showing an increased risk of atherosclerosis with infections (9, 14, 37). LPS and related TLR agonists have also been shown to suppress the levels and/or activities of certain nuclear receptors, including RARα and LXRα, which are responsible for the expression of ABCA1 (7, 24, 51). However, the underlying molecular signaling process responsible for LPS-mediated suppression of nuclear receptors is not fully understood.
Our present study is the first to determine that a key TLR signaling component IRAK-1 is critically involved in this process. Nuclear levels of RARα were significantly decreased following LPS treatment in WT but not in IRAK-1−/− macrophages. Mechanistically, our data reveal that LPS primarily affects the nuclear export of RARα, instead of its de novo synthesis or degradation (Fig. 6; see also Fig. S2 in the supplemental material). Besides RARα, we also observed that IRAK-1 is required for LPS-mediated suppression of LXRα (see Fig. S3B in the supplemental material), which has also been implicated in the induction of ABCA1 (8, 10, 43, 46, 54). Consequently, we observed that LPS treatment fails to inhibit ATRA-induced ABCA1 expression and cholesterol export in IRAK-1−/− macrophages. Elevated induction of ABCA1 in IRAK-1−/− macrophages also correlates with enhanced macrophage efferocytosis as well as decreased expression of proinflammatory cytokines (unpublished data). Collectively, our current study offers a potential mechanism underlying inflammatory pathway and the pathogenesis of atherosclerosis previously documented in both mice and humans (29, 52). However, the cross talk between inflammatory pathways and nuclear receptor-mediated expression of ABCA1 is highly complex. Multiple issues remain to be addressed at molecular and cellular levels. At the molecular level, the signaling events regulating the levels of nuclear receptors and their activities may be distinct, and both may be required for effective expression of downstream genes. Our data indicate that IRAK-1−/− cells exhibit stimulus (ATRA)-dependent induction of ABCA1 expression, although the basal levels of RARα are already elevated in IRAK-1−/− cells. Thus, the molecular process that leads to elevated levels of nuclear receptors is necessary, yet not sufficient, to yield elevated expression of ABCA1. In addition, RAR agonist ATRA is required to activate RARα, leading to the ligand-dependent expression of ABCA1. At the cellular level, ABCA1 is one of the multiple transporters responsible for the regulation of cholesterol transport. The physiological contribution of ABCA1 to cholesterol transport in macrophages is yet to be fully determined. Besides cholesterol transport, ABCA1 may play equally significant roles in regulating inflammatory signaling processes (59, 60). The diverse functions of ABCA1 warrant caution during the interpretation of related data and literature, to avoid oversimplification of the complex regulatory system involving ABCA1 expression, cholesterol transport, and inflammatory modulations. Future extensive work is highly warranted to fully decipher the complex cross talk between innate immunity signaling processes and nuclear receptor-mediated signaling events, as well as downstream physiological and pathological implications.
Our finding that RAR and NFAT jointly regulate the expression of ABCA1 by binding to its proximal promoter provides a glimpse of the complex signaling networks regulating ABCA1 expression and macrophage physiology. Such complexity is not surprising, as many genes are regulated by the coordinated actions of multiple transcription factors in orchestrating the induction of target genes (20). Presumably, this ensures precision as well as flexibility for a desired cellular response. The novelty, however, lies in that our current study reveals an important target for NFAT in macrophages. The function of NFAT has been widely recognized in T cells, endothelial cells, and muscle cells (3, 21, 22, 33). In contrast, very limited information has been available regarding its function in macrophages, although NFAT is known to be expressed in these cells (41). We have previously reported that IRAK-1 is critically involved in maintaining NFAT in a phosphorylated and inactive state (56). IRAK-1 physically associates with NFAT and contributes to the phosphorylation of NFAT at its Ser-Pro-rich motif (56). Phosphorylated NFAT shuttles from the nucleus to cytoplasm and remains inactive. Consequently, deletion of the IRAK-1 gene leads to elevated nuclear levels of NFAT as well as increased transcriptional activity (56). Our current study reveals that nuclear levels of NFATc2 are highly elevated in IRAK-1−/− macrophages. In addition, NFATc2 associates with RARα to activate the transcription of ABCA1.
RARα belongs to the nuclear receptor superfamily that regulates the expression of specific genes in a ligand-dependent manner (35). The basic mechanism for transcriptional activation involves direct binding to the response elements within the promoters of target genes. RARs have also been shown to be modulated by phosphorylation, ubiquitination, and proteasomal degradation (2, 4). Protein kinase C and mitogen-activated protein kinase have been shown to regulate the nuclear localization of RARα in Sertoli cells (5). The biochemical basis for IRAK-1-mediated RARα regulation is not known. However, it is interesting that RARα also contains multiple Ser-Pro motifs, a likely target for IRAK-1-directed phosphorylation. As demonstrated previously, the kinase domain of IRAK-1 may adopt a tertiary structure that resembles the cyclin-dependent kinase. (28, 58). Consequently, IRAK-1 preferentially phosphorylates substrate containing Ser/Thr-Pro-rich motifs. To date, all of the well-defined IRAK-1 substrates, including IRAK-1 itself, STAT3, NFATc2/c4, and IRF5/7, contain such motifs (23, 45, 56). IRAK-1 exists in both the cytoplasm and nucleus (23) and is involved in phosphorylating NFAT and exporting NFAT out of the nucleus (56). Further detailed biochemical analyses are warranted to study whether IRAK-1 directly contributes to the phosphorylation of RARα and its nuclear export.
In summary, we have identified a key signaling component within the TLR inflammatory pathway responsible for attenuating ATRA-induced expression of ABCA1, whose downregulation hinders cholesterol efflux from macrophages. ABCA1 plays divergent functions, including prevention of foam cell formation, suppression of inflammation, and augmentation of efferocytosis. Therefore, therapies that are targeted at boosting ABCA1 expression in macrophages by inhibiting IRAK-1 activity may hold promise in the prevention and intervention of the pathogenesis of atherosclerosis.
Supplementary Material
Acknowledgments
This work was supported in part by research grants from the National Institutes of Health (AI64414 to L.L. and HL049373 and HL094525 to J.S.P.).
We thank My-Ngan Duong and Xuewei Zhu for their help with cholesterol efflux assays, Sarah Davis for technical assistance, and Samantha Baglin for critical reading of the manuscript.
Footnotes
Published ahead of print on 14 September 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allenby, G., M. T. Bocquel, M. Saunders, S. Kazmer, J. Speck, M. Rosenberger, A. Lovey, P. Kastner, J. F. Grippo, P. Chambon, et al. 1993. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 90:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andela, V. B., and R. N. Rosier. 2004. The proteosome inhibitor MG132 attenuates retinoic acid receptor trans-activation and enhances trans-repression of nuclear factor κB. Potential relevance to chemo-preventive interventions with retinoids. Mol. Cancer 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armesilla, A. L., E. Lorenzo, P. Gomez del Arco, S. Martinez-Martinez, A. Alfranca, and J. M. Redondo. 1999. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol. Cell. Biol. 19:2032-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bour, G., S. Lalevee, and C. Rochette-Egly. 2007. Protein kinases and the proteasome join in the combinatorial control of transcription by nuclear retinoic acid receptors. Trends Cell Biol. 17:302-309. [DOI] [PubMed] [Google Scholar]
- 5.Braun, K. W., M. N. Vo, and K. H. Kim. 2002. Positive regulation of retinoic acid receptor alpha by protein kinase C and mitogen-activated protein kinase in Sertoli cells. Biol. Reprod. 67:29-37. [DOI] [PubMed] [Google Scholar]
- 6.Brooks-Wilson, A., M. Marcil, S. M. Clee, L. H. Zhang, K. Roomp, M. van Dam, L. Yu, C. Brewer, J. A. Collins, H. O. Molhuizen, O. Loubser, B. F. Ouelette, K. Fichter, K. J. Ashbourne-Excoffon, C. W. Sensen, S. Scherer, S. Mott, M. Denis, D. Martindale, J. Frohlich, K. Morgan, B. Koop, S. Pimstone, J. J. Kastelein, J. Genest, Jr., and M. R. Hayden. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22:336-345. [DOI] [PubMed] [Google Scholar]
- 7.Castrillo, A., S. B. Joseph, S. A. Vaidya, M. Haberland, A. M. Fogelman, G. Cheng, and P. Tontonoz. 2003. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 12:805-816. [DOI] [PubMed] [Google Scholar]
- 8.Chawla, A., W. A. Boisvert, C. H. Lee, B. A. Laffitte, Y. Barak, S. B. Joseph, D. Liao, L. Nagy, P. A. Edwards, L. K. Curtiss, R. M. Evans, and P. Tontonoz. 2001. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7:161-171. [DOI] [PubMed] [Google Scholar]
- 9.Chen, S., R. Sorrentino, K. Shimada, Y. Bulut, T. M. Doherty, T. R. Crother, and M. Arditi. 2008. Chlamydia pneumoniae-induced foam cell formation requires MyD88-dependent and -independent signaling and is reciprocally modulated by liver X receptor activation. J. Immunol. 181:7186-7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costet, P., F. Lalanne, M. C. Gerbod-Giannone, J. R. Molina, X. Fu, E. G. Lund, L. J. Gudas, and A. R. Tall. 2003. Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol. Cell. Biol. 23:7756-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costet, P., Y. Luo, N. Wang, and A. R. Tall. 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275:28240-28245. [DOI] [PubMed] [Google Scholar]
- 12.Curtiss, L. K., and P. S. Tobias. 2008. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. 50(Suppl.):S340-S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng, C., C. Radu, A. Diab, M. F. Tsen, R. Hussain, J. S. Cowdery, M. K. Racke, and J. A. Thomas. 2003. IL-1 receptor-associated kinase 1 regulates susceptibility to organ-specific autoimmunity. J. Immunol. 170:2833-2842. [DOI] [PubMed] [Google Scholar]
- 14.Epstein, S. E. 2002. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ. Res. 90:2-4. [PubMed] [Google Scholar]
- 15.Gan, L., and L. Li. 2006. Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol. Res. 35:295-302. [DOI] [PubMed] [Google Scholar]
- 16.Gerbod-Giannone, M. C., Y. Li, A. Holleboom, S. Han, L. C. Hsu, I. Tabas, and A. R. Tall. 2006. TNFα induces ABCA1 through NF-κB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl. Acad. Sci. USA 103:3112-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottipati, S., N. L. Rao, and W. P. Fung-Leung. 2008. IRAK-1: a critical signaling mediator of innate immunity. Cell Signal. 20:269-276. [DOI] [PubMed] [Google Scholar]
- 18.Hamon, Y., O. Chambenoit, and G. Chimini. 2002. ABCA1 and the engulfment of apoptotic cells. Biochim. Biophys. Acta 1585:64-71. [DOI] [PubMed] [Google Scholar]
- 19.Hayden, M. R., S. M. Clee, A. Brooks-Wilson, J. Genest, Jr., A. Attie, and J. J. Kastelein. 2000. Cholesterol efflux regulatory protein, Tangier disease and familial high-density lipoprotein deficiency. Curr. Opin. Lipidol. 11:117-122. [DOI] [PubMed] [Google Scholar]
- 20.Hershko, D. D., B. W. Robb, G. Luo, and P. O. Hasselgren. 2002. Multiple transcription factors regulating the IL-6 gene are activated by cAMP in cultured Caco-2 cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R1140-R1148. [DOI] [PubMed] [Google Scholar]
- 21.Horsley, V., B. B. Friday, S. Matteson, K. M. Kegley, J. Gephart, and G. K. Pavlath. 2001. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J. Cell Biol. 153:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horsley, V., and G. K. Pavlath. 2002. NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156:771-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, Y., T. Li, D. C. Sane, and L. Li. 2004. IRAK-1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J. Biol. Chem. 279:51697-51703. [DOI] [PubMed] [Google Scholar]
- 24.Joseph, S. B., A. Castrillo, B. A. Laffitte, D. J. Mangelsdorf, and P. Tontonoz. 2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9:213-219. [DOI] [PubMed] [Google Scholar]
- 25.Joseph, S. B., E. McKilligin, L. Pei, M. A. Watson, A. R. Collins, B. A. Laffitte, M. Chen, G. Noh, J. Goodman, G. N. Hagger, J. Tran, T. K. Tippin, X. Wang, A. J. Lusis, W. A. Hsueh, R. E. Law, J. L. Collins, T. M. Willson, and P. Tontonoz. 2002. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 99:7604-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khovidhunkit, W., A. H. Moser, J. K. Shigenaga, C. Grunfeld, and K. R. Feingold. 2003. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J. Lipid Res. 44:1728-1736. [DOI] [PubMed] [Google Scholar]
- 27.Klucken, J., C. Buchler, E. Orso, W. E. Kaminski, M. Porsch-Ozcurumez, G. Liebisch, M. Kapinsky, W. Diederich, W. Drobnik, M. Dean, R. Allikmets, and G. Schmitz. 2000. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. USA 97:817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuglstatter, A., A. G. Villasenor, D. Shaw, S. W. Lee, S. Tsing, L. Niu, K. W. Song, J. W. Barnett, and M. F. Browner. 2007. Cutting edge: IL-1 receptor-associated kinase 4 structures reveal novel features and multiple conformations. J. Immunol. 178:2641-2645. [DOI] [PubMed] [Google Scholar]
- 29.Lakoski, S. G., L. Li, C. D. Langefeld, Y. Liu, T. D. Howard, K. B. Brosnihan, J. Xu, D. W. Bowden, and D. M. Herrington. 2007. The association between innate immunity gene (IRAK-1) and C-reactive protein in the Diabetes Heart Study. Exp. Mol. Pathol. 82:280-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, M., and J. Park. 2006. Regulation of NFAT activation: a potential therapeutic target for immunosuppression. Mol. Cell 22:1-7. [PubMed] [Google Scholar]
- 31.Li, L. 2004. Regulation of innate immunity signaling and its connection with human diseases. Curr. Drug Targets Inflamm. Allergy 3:81-86. [DOI] [PubMed] [Google Scholar]
- 32.Luciani, M. F., F. Denizot, S. Savary, M. G. Mattei, and G. Chimini. 1994. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics 21:150-159. [DOI] [PubMed] [Google Scholar]
- 33.Macian, F. 2005. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5:472-484. [DOI] [PubMed] [Google Scholar]
- 34.Macian, F., C. Lopez-Rodriguez, and A. Rao. 2001. Partners in transcription: NFAT and AP-1. Oncogene 20:2476-2489. [DOI] [PubMed] [Google Scholar]
- 35.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullick, A. E., P. S. Tobias, and L. K. Curtiss. 2005. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Investig. 115:3149-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naiki, Y., R. Sorrentino, M. H. Wong, K. S. Michelsen, K. Shimada, S. Chen, A. Yilmaz, A. Slepenkin, N. W. Schroder, T. R. Crother, Y. Bulut, T. M. Doherty, M. Bradley, Z. Shaposhnik, E. M. Peterson, P. Tontonoz, P. K. Shah, and M. Arditi. 2008. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J. Immunol. 181:7176-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohashi, R., H. Mu, X. Wang, Q. Yao, and C. Chen. 2005. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 98:845-856. [DOI] [PubMed] [Google Scholar]
- 39.Oram, J. F. 2000. Tangier disease and ABCA1. Biochim. Biophys. Acta 1529:321-330. [DOI] [PubMed] [Google Scholar]
- 40.Orso, E., C. Broccardo, W. E. Kaminski, A. Bottcher, G. Liebisch, W. Drobnik, A. Gotz, O. Chambenoit, W. Diederich, T. Langmann, T. Spruss, M. F. Luciani, G. Rothe, K. J. Lackner, G. Chimini, and G. Schmitz. 2000. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 24:192-196. [DOI] [PubMed] [Google Scholar]
- 41.Pessler, F., L. Dai, R. Q. Cron, and H. R. Schumacher. 2006. NFAT transcription factors: new players in the pathogenesis of inflammatory arthropathies? Autoimmun. Rev. 5:106-110. [DOI] [PubMed] [Google Scholar]
- 42.Repa, J. J., S. D. Turley, J. A. Lobaccaro, J. Medina, L. Li, K. Lustig, B. Shan, R. A. Heyman, J. M. Dietschy, and D. J. Mangelsdorf. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524-1529. [DOI] [PubMed] [Google Scholar]
- 43.Ruan, X. Z., J. F. Moorhead, R. Fernando, D. C. Wheeler, S. H. Powis, and Z. Varghese. 2003. PPAR agonists protect mesangial cells from interleukin 1β-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J. Am. Soc. Nephrol. 14:593-600. [DOI] [PubMed] [Google Scholar]
- 44.Santamarina-Fojo, S., K. Peterson, C. Knapper, Y. Qiu, L. Freeman, J. F. Cheng, J. Osorio, A. Remaley, X. P. Yang, C. Haudenschild, C. Prades, G. Chimini, E. Blackmon, T. Francois, N. Duverger, E. M. Rubin, M. Rosier, P. Denefle, D. S. Fredrickson, and H. B. Brewer, Jr. 2000. Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc. Natl. Acad. Sci. USA 97:7987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoenemeyer, A., B. J. Barnes, M. E. Mancl, E. Latz, N. Goutagny, P. M. Pitha, K. A. Fitzgerald, and D. T. Golenbock. 2005. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 280:17005-17012. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz, K., R. M. Lawn, and D. P. Wade. 2000. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun. 274:794-802. [DOI] [PubMed] [Google Scholar]
- 47.Shachter, N. S. 2005. ABCG1: how critical for cholesterol transport? Cell. Metab. 1:87-88. [DOI] [PubMed] [Google Scholar]
- 48.Sparrow, C. P., J. Baffic, M. H. Lam, E. G. Lund, A. D. Adams, X. Fu, N. Hayes, A. B. Jones, K. L. Macnaul, J. Ondeyka, S. Singh, J. Wang, G. Zhou, D. E. Moller, S. D. Wright, and J. G. Menke. 2002. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J. Biol. Chem. 277:10021-10027. [DOI] [PubMed] [Google Scholar]
- 49.Swantek, J. L., M. F. Tsen, M. H. Cobb, and J. A. Thomas. 2000. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 164:4301-4306. [DOI] [PubMed] [Google Scholar]
- 50.Tall, A. R. 2003. Role of ABCA1 in cellular cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 23:710-711. [DOI] [PubMed] [Google Scholar]
- 51.Tangirala, R. K., E. D. Bischoff, S. B. Joseph, B. L. Wagner, R. Walczak, B. A. Laffitte, C. L. Daige, D. Thomas, R. A. Heyman, D. J. Mangelsdorf, X. Wang, A. J. Lusis, P. Tontonoz, and I. G. Schulman. 2002. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA 99:11896-118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas, J. A., S. B. Haudek, T. Koroglu, M. F. Tsen, D. D. Bryant, D. J. White, D. F. Kusewitt, J. W. Horton, and B. P. Giroir. 2003. IRAK-1 deletion disrupts cardiac Toll/IL-1 signaling and protects against contractile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 285:H597-H606. [DOI] [PubMed] [Google Scholar]
- 53.Vaisman, B. L., G. Lambert, M. Amar, C. Joyce, T. Ito, R. D. Shamburek, W. J. Cain, J. Fruchart-Najib, E. D. Neufeld, A. T. Remaley, H. B. Brewer, Jr., and S. Santamarina-Fojo. 2001. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J. Clin. Investig. 108:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkateswaran, A., B. A. Laffitte, S. B. Joseph, P. A. Mak, D. C. Wilpitz, P. A. Edwards, and P. Tontonoz. 2000. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. USA 97:12097-12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagsater, D., J. Dimberg, and A. Sirsjo. 2003. Induction of ATP-binding cassette A1 by all-trans retinoic acid: possible role of liver X receptor-alpha. Int. J. Mol. Med. 11:419-423. [PubMed] [Google Scholar]
- 56.Wang, D., S. Fasciano, and L. Li. 2008. The interleukin-1 receptor associated kinase 1 contributes to the regulation of NFAT. Mol. Immunol. 45:3902-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, Y., A. H. Moser, J. K. Shigenaga, C. Grunfeld, and K. R. Feingold. 2005. Downregulation of liver X receptor-alpha in mouse kidney and HK-2 proximal tubular cells by LPS and cytokines. J. Lipid Res. 46:2377-2387. [DOI] [PubMed] [Google Scholar]
- 58.Wang, Z., J. Liu, A. Sudom, M. Ayres, S. Li, H. Wesche, J. P. Powers, and N. P. Walker. 2006. Crystal structures of IRAK-4 kinase in complex with inhibitors: a serine/threonine kinase with tyrosine as a gatekeeper. Structure 14:1835-1844. [DOI] [PubMed] [Google Scholar]
- 59.Yvan-Charvet, L., C. Welch, T. A. Pagler, M. Ranalletta, M. Lamkanfi, S. Han, M. Ishibashi, R. Li, N. Wang, and A. R. Tall. 2008. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118:1837-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, X., J. Y. Lee, J. M. Timmins, J. M. Brown, E. Boudyguina, A. Mulya, A. K. Gebre, M. C. Willingham, E. M. Hiltbold, N. Mishra, N. Maeda, and J. S. Parks. 2008. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances proinflammatory response of macrophages. J. Biol. Chem. 283:22930-22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.