Abstract
Studies using Drosophila melanogaster have contributed significantly to our understanding of the interaction between stem cells and their protective microenvironments or stem cell niches. During lymph gland hematopoiesis, the Drosophila posterior signaling center functions as a stem cell niche to maintain prohemocyte multipotency through Hedgehog and JAK/STAT signaling. In this study, we provide evidence that the Friend of GATA protein U-shaped is an important regulator of lymph gland prohemocyte potency and differentiation. U-shaped expression was determined to be upregulated in third-instar lymph gland prohemocytes and downregulated in a subpopulation of differentiating blood cells. Genetic analyses indicated that U-shaped maintains the prohemocyte population by blocking differentiation. In addition, activated STAT directly regulated ush expression as evidenced by results from loss- and gain-of-function studies and from analyses of the u-shaped hematopoietic cis-regulatory module. Collectively, these findings identify U-shaped as a downstream effector of the posterior signaling center, establishing a novel link between the stem cell niche and the intrinsic regulation of potency and differentiation. Given the functional conservation of Friend of GATA proteins and the role that GATA factors play during cell fate choice, these factors may regulate essential functions of vertebrate hematopoietic stem cells, including processing signals from the stem cell niche.
Stem cells are essential for tissue regeneration and are defined by their capacity for self-renewal, potency, and differentiation. The choice between the maintenance of stem cell potency and differentiation is controlled by a complex combination of intrinsic factors and a protective stem cell niche (1, 29, 53, 63). Much of our current understanding about the regulation of these developmental processes comes from studies of hematopoietic stem cells (HSCs). In addition, cross-species conservation has shown that the genetically tractable Drosophila system can complement studies of vertebrate hematopoiesis (10, 11, 15, 23, 31, 41, 62).
Studies using Drosophila melanogaster have identified a class of blood cell progenitors (prohemocytes) with characteristics of HSCs, including quiescence, multipotency, and niche dependence (27, 32, 36, 40). The prohemocyte develops within a specialized organ called the lymph gland. The mature third-instar lymph gland consists of one pair of primary lobes and a series of secondary lobes (49). Within the primary lobe, three distinct domains or zones have been characterized based on their roles during hematopoiesis (27, 32, 40). The quiescent, stem cell-like prohemocytes reside within the medullary zone. During the process of differentiation, these cells migrate to the cortical zone. Here they become plasmatocytes and crystal cells, the primary blood cell lineages in the fly (27, 49). The third domain, the posterior signaling center (PSC), functions as a stem cell niche by maintaining prohemocyte quiescence and potency through the Hedgehog and JAK/STAT signal transduction pathways (32, 37, 40). Because signaling pathways function throughout development, the regulation of stem cell potency and differentiation will be determined not only by the signaling molecules but also by the downstream effectors of these pathways. This underscores the importance of identifying the targets of niche-directed signals. Of particular interest are the key regulators and gene networks that control the choice between stem cell quiescence and proliferation and between the maintenance of potency and differentiation.
GATA factors are key regulators of HSC survival, proliferation, and differentiation (6, 59, 60). The Drosophila GATA factor Serpent (Srp) is required for the specification of prohemocytes but also acts later in hematopoiesis to drive plasmatocyte and crystal cell differentiation (18, 48, 52, 61). Given the extensive role of Srp in hematopoiesis, its activity must be regulated to prevent the depletion of the medullary zone prohemocyte pool. A likely candidate is U-shaped (Ush), a Friend of GATA (FOG) family member. These proteins are known to interact with GATA factors to modulate gene expression across taxa ranging from flies to humans (16). During embryonic crystal cell development, Ush converts Srp from an activator to a repressor of lineage commitment and differentiation by downregulating the crystal cell lineage activator, Lozenge (Lz) (18, 45). In addition, Ush and Srp are coexpressed in embryonic prohemocytes (17, 48, 52). In this study, we investigated the role of Ush during lymph gland hematopoiesis and whether ush expression is regulated by the PSC. Here we provide evidence that Ush acts as a key regulator of lymph gland prohemocyte potency. Our analyses indicate that Ush is required to preserve the prohemocyte pool by limiting differentiation and that ush expression requires activated STAT. The upregulation of ush expression by the JAK/STAT pathway positions Ush as a downstream target of the PSC and provides an important link between the stem cell niche and the intrinsic regulation of potency and differentiation. Our studies raise the possibility that these conserved factors interact to regulate vertebrate HSC biology.
MATERIALS AND METHODS
Fly strains.
w1118 flies served as our wild-type stock. y w67c23; UAS-ush, w1118; lz −1236/−737 lacZ (lz-lacZ) and w1118; ush −174/−25 lacZ were described elsewhere previously (17, 44, 45). The following strains were generous gifts from colleagues: y1 w; ushvx22/CyO, y+ and y1 w; ushr24/CyO, y+ (R. A. Schulz and R. P. Sorrentino, University of Notre Dame); domeMESO lacZ strains w; domeMESO/CyO and w p{w+, dome-MESO}BN1 (M. P. Zeidler, University of Sheffield, and J. C. Hombria, Universidad Pablo de Olavide); and FRT 82b, e, ca stat397-6/TM3 (D. J. Montell, Johns Hopkins School of Medicine). We obtained the following strains from the Bloomington stock center: P{Cg-GAL4.A} (collagen-Gal4 [Cg-Gal4]) and y1 v1 hopTum-l/Basc (hopTum-l).
Immunofluorescence.
The dissection and fixation of larval lymph glands were preformed as previously described (45) except that lymph glands were fixed for 8 min at room temperature. Rabbit anti-U-shaped was used at a 1:1,000 dilution (17). The following mouse antibodies directed against specific hemocyte antigens were generous gifts from Istavan Ando, Biological Research Center of the Hungarian Academy of Sciences, and were used at the indicated dilutions: P1, 1:50 (34); L1, 1:200 (35), and anti-Hemese, 1:200 (33). Rabbit anti-prophenoloxidase A1 (anti-ProPO) was used at a 1:100 dilution and was a generous gift from F. C. Kafatos, EMBL (43). The following antibodies were obtained from the Developmental Studies Hybridoma Bank and were used at the indicated dilutions: mouse anti-Antennapedia (anti-Antp), 1:20; mouse anti-Patched (anti-Ptc), 1:60, and rat anti-DE-cadherin (anti-DE-cad), 1:20. Mouse anti-β-galactosidase was used at a 1:2,000 dilution (Promega). Alexafluor-555- or -488-conjugated secondary antibodies directed to rabbit, mouse, or rat (Invitrogen) were used at a 1:2,000 dilution. Ush localizes to the nucleus, and coexpression with cytosolic markers (lz-lacZ and domeMESO lacZ) or membrane-bound proteins (P1, DE-cad, and L1) was determined by observing Ush within the boundary of a single cell using confocal microscopy. In contrast, Antp is a nuclear protein, and the coexpression of Ush was assessed by determining the degree of colocalization between these proteins. Fluorescence was captured, analyzed, and recorded using Zeiss confocal microscopy or Zeiss Axioplan optics. Lymph gland size and densitometric mean values were determined using Zeiss Axiovision 4.6.3 quantitation software. Briefly, lymph glands were photographed using fluorescein isothiocyanate, Cy3, and differential interference contrast filters. At least 12 lymph glands from each genotype were outlined and sized using the outline spline interpolation feature. Axiovision software converted the RGB color image into gray and then calculated the densitometric mean for each color channel across the entire lymph gland. The statistical significances of the size and densitometric mean data were evaluated using the Student t test.
Gene expression analyses.
ushvx22/CyO, y+ and stat397-6/TM3 heterozygotes were tested over additional complementing chromosomes to verify that the observed phenotype was independent of the balancer chromosome. y1 w; ushvx22/ushr24 trans-heterozygote larvae were generated by crossing y1 w; ushvx22/CyO, y+ and y1 w; ushr24/CyO, y+ adults and selecting larval progeny with yellow mouth hooks. hopTum-l larvae and wild-type controls were cultured at three different temperatures, 23°C, 25°C, and 27°C, whereas all other larvae were cultured at 23°C. Cg-Gal4 flies were mated to either UAS-ush or w1118 strains, and progeny were evaluated to determine the effect of these transgenes on differentiating hemocytes. Fly strains carrying the wild-type ush −174/−25 cis-regulatory module (CRM) lacZ construct were crossed to either the stat397-6/TM3 or w1118 strain to determine the effect of the reduction in stat activity on ush CRM lacZ expression. All experiments were done in triplicate at a minimum, with at least eight control and eight experimental lymph glands per experiment, unless otherwise indicated. Crystal cell counts were analyzed using the Student t test.
Generation of transgenic fly strains carrying a STAT site mutation in the ush −174/−25 CRM.
The STAT site mutation was introduced into the ush −174/−25 CRM sequence using a method described previously by Barettino et al. (3) except that all of the targets were cloned into the pCRII-TOPO cloning vector (Invitrogen). The ush −420/−25 fragment (44) was used as a template with forward (CACACCCCTTTCTGTTTCTGCGATGTTATCTAAGCGC) and reverse (CGACTTCCTTCGCTCGCCTCGGAATTATTTAAAAC) oligonucleotide primers to produce a fragment with two nucleotide base substitutions (C to T and G to A [underlined]) in the STAT binding site. The fragment was gel purified and used as a forward oligonucleotide primer with the reverse oligonucleotide primer GACGAGACGAGACCTCTTAGCCGAGACTCTCTG to produce the ush −174/−25 lacZ CRM carrying a mutated STAT binding site (ush −175/−25 mSTAT). This fragment was cloned into the pCRII-TOPO cloning vector and subsequently subcloned into the P-element CaSperR-Hsp43-AUG-β-gal germ line transformation vector (58). The sequence of the recombinant vector was verified prior to injection into w1118 embryos by Rainbow Transgenic Flies, Inc. Germ line transformants were established as previously described (20). Twenty independent lines were generated, and six were tested for lacZ activity in the larval lymph gland. In addition, males carrying the wild-type or mSTAT lacZ CRM were crossed to hopTum-l or w1118 females to determine the effect of increased STAT activity on ush CRM lacZ expression.
RESULTS
Ush is differentially expressed in the third-instar larval lymph gland.
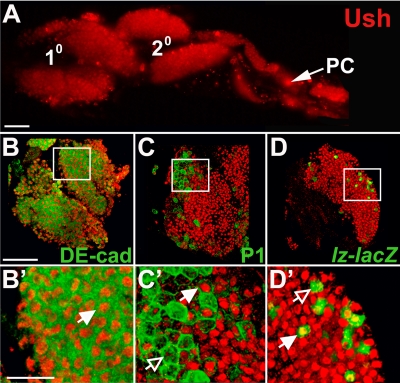
Ush is expressed in third-instar primary and secondary lymph gland lobes (Fig. 1A) (17, 44). As a first step toward identifying the functions of Ush during lymph gland hematopoiesis, we determined the hemocyte-specific expression pattern of this transcriptional regulator. DE-cad is expressed in medullary zone prohemocytes, and this prohemocyte marker is later downregulated as these cells differentiate (27). Ush was coexpressed with DE-cad in these medullary zone cells (Fig. 1B and B′), demonstrating that Ush is expressed in prohemocytes.
FIG. 1.
Differential Ush expression in the third-instar larval lymph gland. (A) Ush expression in primary and secondary lymph gland lobes (fluorescence microscopy) (magnification, ×20). (B to D′) Ush coexpression with hemocyte-specific markers in the primary lymph gland lobe (confocal microscopy) (magnification, ×60). (B and B′) Ush and the medullary zone prohemocyte marker DE-cad. (C and C′) Ush and the plasmatocyte marker P1 (Nimrod). (D and D′) Ush and the crystal cell marker lz-lacZ. Closed arrows indicate coexpression, and open arrows indicate a lack of coexpression. PC, pericardial cells. Scale bars, 50 μm (A and B) and 15 μm (B′).
In contrast, cortical zone cells exhibited a more complex Ush expression pattern. The pattern of expression was determined by measuring Ush coexpression with the plasmatocyte-specific marker, P1 (Nimrod) (34), or the crystal cell-specific marker, lz-lacZ (45). While Ush was detected in both the plasmatocyte and crystal cell lineages, Ush was downregulated in cells from each lineage (Fig. 1C to D′). Previous work showed a similar expression pattern during embryonic crystal cell development (compare with insets in Fig. 1d and e in reference 17). Ush functions in these cells as part of a cross-regulatory subcircuit that blocks lineage commitment and differentiation by downregulating the crystal cell lineage activator, Lz, which in turn downregulates ush expression (45). Based on these studies, we asked if Ush maintains prohemocyte potency by blocking differentiation.
Ush limits prohemocyte differentiation in the lymph gland.
To investigate the role of Ush in the maintenance of prohemocyte potency, we first determined the extent of blood cell differentiation in animals with altered levels of ush expression. ush is an essential gene, and homozygous null animals die during embryogenesis (46). Therefore, we utilized two fly strains with reduced ush expression levels that survive until adulthood: (i) ush trans heterozygotes (ushvx22/ushr24) that carry a null allele and a hypomorphic allele and (ii) ush heterozygotes (ushvx22/+) that carry one null allele (21). We observed that late-third-instar ush heterozygous and trans-heterozygous lymph glands exhibited hyperplasia (Fig. 2A to C ′), which is consistent with data from a previous report (56). In general, the lymph glands of ush trans heterozygotes were larger than those of heterozygotes, and heterozygous lymph glands were larger than those of wild-type controls. We measured the areas of at least 12 lymph gland images for each genotype. The size of trans-heterozygous lymph glands was 68,928 ± 9,827 μm2. On average, this was twofold larger than heterozygous lymph glands (32,782 ± 9,746 μm2 [P < 0.01]) and threefold larger than wild-type lymph glands (21,945 ± 3,133 μm2 [P < 0.01]). These results showed that lymph gland size increased with decreasing levels of ush expression. We next investigated hemocyte differentiation in late-third-instar lymph glands of ush trans heterozygotes, heterozygotes, and wild-type controls.
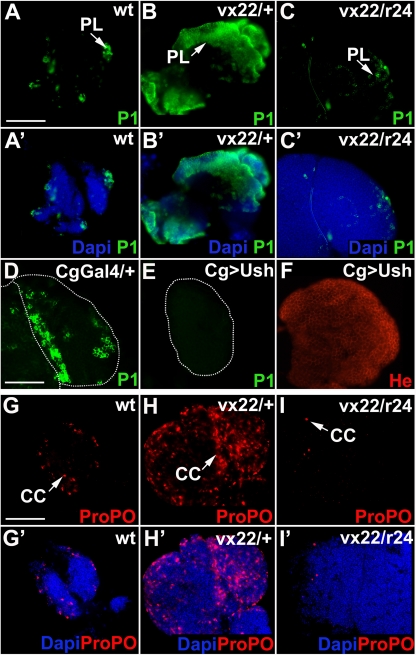
FIG. 2.
Ush blocks plasmatocyte and crystal cell differentiation in the lymph gland. Shown is plasmatocyte and crystal cell differentiation in lymph glands with altered levels of ush expression (fluorescence microscopy) (magnification, ×40). (A to F) Plasmatocyte differentiation was monitored using P1 in animals with the following genotypes: wild type (wt) (A and A′), ush heterozygotes (ushvx22/+; vx22/+) (B and B′), ush trans heterozygotes (ushvx22/ushr24; vx22/r24) (C and C′), Cg-Gal4/+ (D), and Cg-Gal4/UAS-ush (Cg>Ush) (E). Arrows mark plasmatocytes. (F) He expression in Cg>Ush lymph glands. (G to I′) Crystal cell differentiation was monitored using ProPO in animals with the following genotypes: wild-type (G and G′), ush heterozygotes (ushvx22/+; vx22/+) (H and H′), and ush trans-heterozygotes (ushvx22/ushr24; vx22/r24) (I and I′). Arrows mark crystal cells. Lymph gland hyperplasia produced by the loss of one (heterozygote) or more (trans-heterozygote) copies of ush often prevented the visualization of the entire lymph gland. Abbreviations: CC, crystal cells; PL, plasmatocytes. Scale bars: 100 μm (A and G) and 170 μm (D).
Plasmatocytes comprise a major portion of the hemocyte population (36, 49, 57). As a result, unregulated plasmatocyte differentiation could dramatically deplete the prohemocyte pool. For this reason, we first asked if Ush blocks plasmatocyte differentiation to maintain the multipotent prohemocyte pool. Plasmatocyte numbers increased substantially in ush heterozygous lymph glands compared to wild-type controls (Fig. 2A to B′). In contrast, plasmatocyte numbers actually decreased in ush trans-heterozygous lymph glands. The number of plasmatocytes was often less than that observed in wild-type controls and considerably less than that in ush heterozygotes (Fig. 2A to C′). The observed increase in plasmatocyte numbers was not due solely to lymph gland size; otherwise, an increase in numbers of plasmatocytes would have been observed for both genotypes. Rather, the data suggest that Ush functions to restrict the number of prohemocytes that become plasmatocytes. The loss of one functional ush copy (heterozygote) appears to release the prohemocyte differentiation block, whereas the loss of more than one functional ush copy (trans heterozygote) limits plasmatocyte production.
The increase in plasmatocyte numbers suggested that ush overexpression would block plasmatocyte production. To test this hypothesis, we used the UAS/Gal4 system (5) with the Cg-Gal4 (2) driver and UAS-ush to overexpress ush (Cg>Ush) in the plasmatocyte lineage. Cg>Ush produced almost a complete loss of plasmatocytes compared to Cg-Gal4/+ and wild-type controls (Fig. 2A, A′, D, and E). In contrast, Cg>Ush did not alter the expression of the panhemocyte marker Hemese and did not alter lymph gland size (Fig. 2F and data not shown). These results indicate that the loss of plasmatocytes in the Cg>Ush lymph gland was due to a differentiation block rather than a reduction in the total number of hemocytes. Collectively, these ush loss- and gain-of-function studies indicate that a reduction in the level of Ush expression is required for differentiation to proceed.
Crystal cells and plasmatocytes constitute the primary blood cell lineages. In the lymph gland, both lineages are derived from medullary zone prohemocytes (27). Therefore, if Ush blocks prohemocyte differentiation, then it should block both crystal cell and plasmatocyte differentiation. Using the crystal cell-specific differentiation marker ProPO (43), we observed that the number of crystal cells, like plasmatocytes, increased in ush heterozygous lymph glands. The number of crystal cells increased by more than twofold in ush heterozygotes compared to wild-type controls (Fig. 2G to H′). The mean crystal cell number was 29.8 ± 9.3 in wild-type lymph glands, whereas in heterozygous lymph gland, the mean cell number was 78.2 ± 13.3 (P < 0.01). When the mean crystal cell number was adjusted to account for lymph gland size, heterozygous lymph glands averaged twice the size of wild-type lymph glands (59.9 ± 19.6 [P < 0.05]). These results showed that the increase in crystal cell numbers was not due solely to the size of the heterozygous lymph gland and indicated that Ush limits the number of prohemocytes that develop into crystal cells during lymph gland hematopoiesis. This again is consistent with the role of Ush during embryonic crystal cell production (18, 45). In contrast, crystal cell numbers decreased in ush trans heterozygotes (Fig. 2G to I′). Together, these results showed that both plasmatocytes and crystal cells respond similarly to changes in the level of Ush expression, increasing in ush heterozygotes and decreasing in ush trans heterozygotes. This demonstrates that the increase in numbers of mature hemocytes is independent of lymph gland size. Increased lymph gland size is most likely a result of overproliferation. However, the mitotic indices of the late-third-instar heterozygous and trans-heterozygous lymph glands are not significantly different than those of the wild type, suggesting that overproliferation occurs at an earlier stage of lymph gland development (56; data not shown). The dynamics of this process have not been fully characterized across the entire third larval instar; however, it is unlikely that the number of these cells increases solely as a result of overproliferation because numbers of mature hemocytes are independent of lymph gland size. Altogether, these data support our hypothesis that Ush limits the differentiation of the prohemocyte pool and regulates hemocyte development.
The lamellocyte, a third class of hemocyte, is not observed in large numbers except during specific immune challenges or in particular genetic backgrounds (2, 9, 11, 13, 22, 36, 39, 42, 47, 54, 55, 59). Like plasmatocytes and crystal cells, lymph gland-derived lamellocytes develop from medullary zone prohemocytes (27, 32, 36). Furthermore, extensive lamellocyte differentiation depletes the prohemocyte pool (32). These observations raised the possibility that Ush would also block lamellocyte differentiation as a means of maintaining prohemocyte potency.
To address the role of Ush in lamellocyte differentiation, we first analyzed Ush expression levels in 16 lamellocytes from wild-type lymph glands. Lamellocytes were identified using the specific marker L1 (35). Fourteen of the 16 lamellocytes showed markedly reduced Ush expression levels compared to those of neighboring cells that lacked L1 expression (Fig. 3A and A ′). However, two of the 16 lamellocytes appeared to have levels of Ush expression that were comparable to those of neighboring hemocytes (data not shown). Overall, these results are consistent with the hypothesis that ush expression is downregulated to permit lamellocyte differentiation and predict that lamellocyte numbers would increase in a ush mutant background. To test this hypothesis, we assayed lymph gland-derived lamellocyte differentiation in ush heterozygotes and trans heterozygotes.
FIG. 3.
Reduction in ush expression levels promotes lamellocyte differentiation. Lamellocytes were identified using the cell-specific marker L1. (A and A′) Ush and L1 expression in cortical zone hemocytes (confocal microscopy) (magnification, ×60). (A) Ush and L1 coexpression. (A′) Same field showing only Ush expression. Closed arrows mark Ush-expressing hemocytes; the open arrow marks a lamellocyte with reduced Ush expression levels. (B to D′) Lamellocyte differentiation in animals with altered levels of ush expression (fluorescence microscopy) (magnification, ×40). Data for L1 expression in animals with the following genotypes are shown: wild type (wt) (B and B′), ush heterozygotes (C and C′), and ush trans heterozygotes (D and D′). Arrows mark lamellocytes and the dorsal vessel, both identified using L1. Abbreviations: DV, dorsal vessel; LM, lamellocyte. Scale bars, 12 μm (A) and 100 μm (B).
As with plasmatocytes and crystal cells, we observed striking differences in the numbers of lamellocytes produced in ush heterozygotes compared to those produced in trans heterozygotes. However, in contrast to plasmatocytes and crystal cells, lamellocyte differentiation was greater in trans heterozygotes than in heterozygotes. Heterozygous lymph glands had only a marginal increase in lamellocyte differentiation. We observed that 19 of 26 ush heterozygous lymph glands tested had lamellocyte numbers that were comparable to those of wild-type controls, which averaged two lamellocytes per lymph gland (Fig. 3B to C′), whereas 5 of 26 lymph glands tested had a 10-fold increase above wild-type numbers (data not shown). In contrast, trans-heterozygous lymph glands showed a dramatic increase in lamellocyte differentiation. Of the 30 trans-heterozygous lymph glands tested, all had at least a 10-fold increase above wild-type numbers. Moreover, 20 of 30 lymph glands were covered with large lamellocyte aggregates that prevented the determination of the precise number of cells (Fig. 3D and D′ and data not shown). Collectively, our results showed that two functional copies of the ush gene are required to limit plasmatocyte and crystal cell production, whereas only one copy is required to limit lamellocyte production.
Ush prevents prohemocyte loss in the medullary zone.
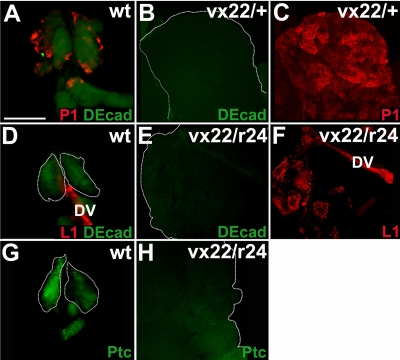
We showed that Ush was expressed in medullary zone prohemocytes and limited the production of all three hemocyte classes. These results suggested that Ush limits prohemocyte differentiation to preserve the multipotent state. To test this hypothesis, we assayed for the presence of prohemocytes in ush mutant lymph glands using the specific markers DE-cad and Ptc (40). We observed a loss of DE-cad expression levels and a concomitant increase in levels of plasmatocyte production in ush heterozygous lymph glands compared to levels in wild-type controls (Fig. 4A to C). DE-cad and Ptc were also downregulated in ush trans-heterozygous lymph glands, which showed a corresponding increase in lamellocyte differentiation (Fig. 4D to H). These results show that in ush mutants, the prohemocyte pool is dramatically reduced, while the mature hemocyte population increases. This shift in the cell population from prohemocytes to mature hemocytes indicates that the loss of Ush function leads to increased prohemocyte differentiation and thereby supports the hypothesis that Ush preserves the multipotent prohemocyte pool by limiting differentiation.
FIG. 4.
Prohemocyte markers are downregulated in ush mutant lymph glands. Prohemocyte differentiation was monitored in lymph glands of animals with altered levels of ush expression (fluorescence microscopy) (magnification, ×40). (A to C) Medullary zone prohemocytes and plasmatocytes were identified using DE-cad and P1, respectively, in the following genotypes: wild type (wt), both prohemocytes and plasmatocytes (A); ush heterozygotes, prohemocytes only (B); and ush heterozygotes, plasmatocytes only (C). (D to F) Medullary zone prohemocytes and lamellocytes were identified using DE-cad and L1, respectively, in the following genotypes: wild type, prohemocytes and lamellocytes (D); ush trans heterozygotes, prohemocytes only (E); and ush trans heterozygotes, lamellocytes only (F). The dorsal vessel also expressed L1. (G and H) Medullary zone prohemocytes were identified using Ptc in the following genotypes: wild type (G) and ush trans heterozygotes (H). Scale bar, 100 μm.
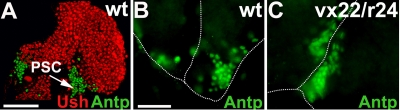
Previous work by others showed that the PSC preserves prohemocyte potency by blocking differentiation (32, 40). Accordingly, we asked if the increase in prohemocyte differentiation resulted from disrupting PSC function in ush loss-of-function lymph glands. Antp is required for the specification of the PSC and serves as a marker for these cells (40). Ush was not detected in the PSC, as determined by its failure to colocalize with Antp (Fig. 5A). This is consistent with data from a previous report (27) and demonstrates that Ush is not an intrinsic regulator of the PSC. Furthermore, we did not detect any change in Antp expression or PSC size in ush trans heterozygotes (Fig. 5B, C). This indicates that a prohemocyte loss in ush hypomorphic backgrounds does not result from a disruption of the PSC. Instead, we concluded that Ush acts either independently of the PSC or as a downstream effector of the PSC to regulate prohemocyte differentiation. In the latter case, signaling from the PSC would upregulate Ush within the prohemocyte population to limit differentiation.
FIG. 5.
The reduction in ush expression levels has no effect on PSC cell number or size. (A) Ush expression in the PSC was monitored by assessing the level of Ush coexpression with the PSC-specific marker Antp (confocal microscopy) (magnification, ×60). (B and C) PSC cell number was monitored using Antp in animals with altered levels of ush expression (fluorescent microscopy) (magnification, ×40). Shown are the wild type (wt) (B) and ush trans heterozygotes (C). Scale bars, 50 μm (A) and 25 μm (B).
JAK/STAT activation maintains Ush expression in the lymph gland.
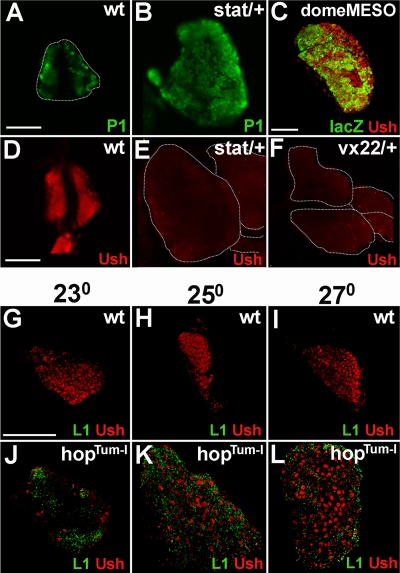
The PSC regulates prohemocyte differentiation through the JAK/STAT and Hedgehog signaling pathways (32, 40). If Ush functions as a downstream effector of the PSC, then at least one of these signal transduction pathways could activate ush expression in prohemocytes. The following observations suggested that ush might be upregulated by JAK/STAT signaling. First, stat and ush heterozygous lymph glands have similar phenotypes. This is supported by a comparison of our data with data reported previously by Krzemień and coworkers that showed both a loss of prohemocytes and an increase in crystal cell differentiation in stat mutants (32). We observed a similar phenotype for ush heterozygous lymph glands (Fig. 2H and 4B). Additional similarities between stat and ush mutants, including lymph gland hyperplasia and increased plasmatocyte differentiation, offered further support for a connection between Ush and JAK/STAT signaling (Fig. 6A and B). Moreover, neither ush nor stat heterozygous lymph glands showed a marked increase in lamellocyte differentiation (Fig. 3C and C′ and data not shown). Furthermore, prohemocytes with JAK/STAT activity also express Ush. This was demonstrated by showing that Ush was coexpressed in the medullary zone with domeMESO lacZ (Fig. 6C), which is a marker for JAK/STAT activity (24, 32). Collectively, these observations pointed to the JAK/STAT pathway as being a regulator of ush expression in the medullary zone, which prompted us to determine the level of ush expression in stat heterozygous lymph glands. The level of Ush was markedly reduced in stat heterozygotes compared to wild-type controls (Fig. 6D and E). We determined the relative levels of Ush expression in wild-type and stat heterozygotes by measuring the densitometric mean of fluorescent lymph gland images. On average, the mean was reduced threefold in stat heterozygotes (1,870 ± 865) compared to wild-type controls (5,902 ± 1,933 [P < 0.01]). The level of Ush was also markedly reduced in ush heterozygotes (Fig. 6D and F). These results indicated that STAT is required for ush expression and predicted that ush expression would increase in STAT gain-of-function mutants.
FIG. 6.
Activated STAT is required for Ush expression in the lymph gland. (A and B) Plasmatocyte differentiation was monitored using P1 in animals with altered levels of stat expression (fluorescence microscopy) (magnification, ×40). Shown are wild-type (wt) (A) and stat (stat397-6/+) (B) heterozygotes. (C) pSTAT activity in Ush-expressing prohemocytes was assessed by monitoring the coexpression of DomeMESO lacZ and Ush (confocal microscopy) (magnification, ×60). (D to F) Ush expression was monitored in animals with altered levels of stat and ush expression (fluorescence microscopy) (magnification, ×40). Shown are data for the wild type (D), stat heterozygotes (E), and ush heterozygotes (F). (G to L) Ush expression and lamellocyte differentiation were monitored in wild-type and hopTum-l second-instar larval lymph glands of animals cultured at three different temperatures (confocal microscopy) (magnification, ×60). (G to I) Wild-type lymph glands cultured at the following temperatures: 23°C (G), 25°C (H), and 27°C (I). (J to L) hopTum-l lymph glands cultured at the following temperatures: 23°C (J), 25°C (K), and 27°C (L). Scale bars, 100 μm (A and D) and 50 μm (C and G).
A temperature-sensitive mutation of Drosophila JAK (HopscotchTum-l [HopTum-l]) hyperphosphorylates STAT, producing increased STAT activity with increasing temperatures (39). We tested ush expression in wild-type and hopTum-l lymph glands cultured at three different temperatures, 23°C, 25°C, and 27°C (Fig. 6G to L). Because hopTum-l lymph glands disperse prior to the beginning of the third larval instar (56), we tested ush expression in second-instar hopTum-l and wild-type lymph glands.
We noted a difference in both hemocyte composition and Ush expression between wild-type and hopTum-l lymph glands. Wild-type second-instar lymph glands were previously reported to contain primarily prohemocytes and plasmatocyte precursors (27). In addition, we did not detect any lamellocytes in these lymph glands (Fig. 6G to I). In contrast, hopTum-l lymph glands had considerable numbers of lamellocytes at all three culture temperatures (Fig. 6J to L), consistent with data from a previously reported study (56). Our analyses of Ush expression showed that Ush was detected throughout wild-type lymph glands and that the level of Ush was not affected by temperature (Fig. 6G to I). In contrast, hopTum-l lymph glands cultured at 23°C contained a small number of Ush-expressing lamellocytes (Fig. 6J). Similarly, we observed that Ush was downregulated in the majority of wild-type lamellocytes from late-third-instar larval lymph glands (Fig. 3A and A′). However, when the culture temperature was increased to 25°C and 27°C, we observed a progressive increase in the numbers of Ush-expressing lamellocytes (Fig. 6K and L). Because the increase in culture temperature was coupled with the upregulation of both STAT activity and Ush expression (39) (Fig. 6J to L), we propose that activated STAT upregulates Ush expression. However, the increased level of Ush was not sufficient to block hopTum-l-induced lamellocyte production, which is consistent with data from a previous report (56).
Ush is a downstream effector of the JAK/STAT pathway.
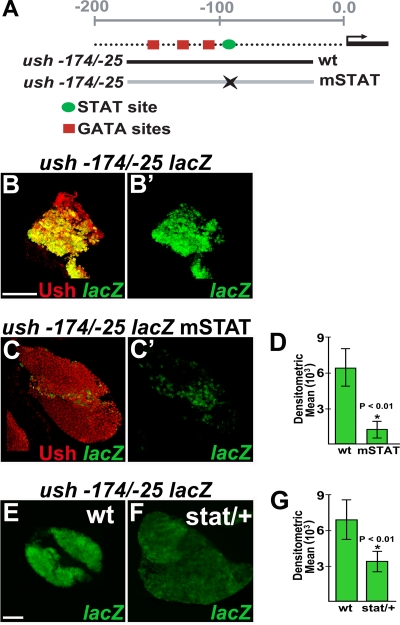
The results of stat loss- and gain-of-function studies prompted us to ask if activated STAT directly regulates ush gene expression. To address this question, we surveyed the ush hematopoietic CRM for STAT binding sites. Ush expression in embryonic and larval lymph gland hemocytes is upregulated by a minimal CRM located between positions −174 and −25 (44) (Fig. 7A). The ability of this CRM to drive reporter gene (lacZ) expression in the majority of cells that express endogenous Ush strongly indicates that it contains cis-regulatory elements required to drive ush expression in the lymph gland (44) (Fig. 7B and B′). The activity of the ush −174/−25 CRM is dependent on three GATA sites located at positions −156/−153, −122/−119, and −111/−108 (44). Our survey of this CRM identified a putative STAT binding site 9 bp downstream of the 3′ GATA site at position −98/−90 (Fig. 7A). This site has a single mismatch in the 3′ half-palindromic sequence (G-to-T substitution), which is a high-affinity variant used by vertebrate STAT1 to regulate a variety of target genes (14).
FIG. 7.
The STAT binding site in the minimal ush hematopoietic CRM is required for activity. (A) Schematic showing the relative positions of the GATA and STAT sites within the ush −174/−25 CRM. The wild-type (wt) and STAT site mutant (mSTAT) CRMs differ by 2 bp within the STAT core sequence. (B to C′) ush −174/−25 lacZ activity and Ush expression in the primary lobe of the lymph gland (confocal microscopy) (magnification, ×60). (B and B′) ush −174/−25 lacZ wild-type sequence. (C and C′) ush −174/−25 lacZ STAT site mutant. (D) Densitometric means for lymph glands carrying the ush −174/−25 lacZ wild-type (wt) and ush −174/−25 lacZ STAT site mutant (mSTAT) constructs were 6,526 ± 1,579 and 1,285 ± 842, respectively (P < 0.01). (E and F) ush −174/−25 lacZ activity in wild-type (E) and stat heterozygous (F) lymph glands (fluorescence microscopy) (magnification, ×40). (G) Densitometric means of ush −174/−25 lacZ activity in wild-type and stat heterozygous (stat/+) lymph glands were 7,043 ± 1,700 and 3,463 ± 840, respectively (P < 0.01). Scale bars, 50 μm.
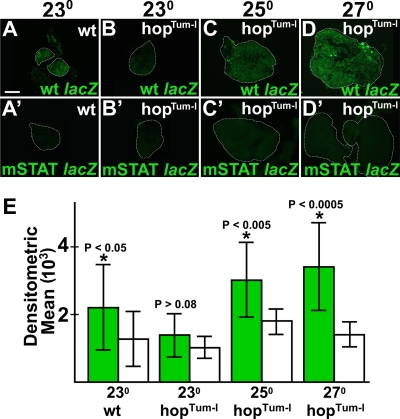
To determine if the STAT site was required for CRM activity, we introduced two single-base-pair substitutions into this site. We then compared lacZ expression levels in the lymph glands of animals harboring the STAT site mutant version of the ush −174/−25 CRM (mSTAT) with those in the lymph glands of animals carrying the wild-type version (Fig. 7A). Ten different strains were analyzed, four harboring wild-type and six harboring mSTAT CRMs. For each strain, a minimum of six lymph glands were assayed. In all cases, animals carrying the mSTAT CRM had markedly reduced lacZ expression levels compared to those of animals carrying wild-type CRM (Fig. 7B to C′ and data not shown). The activity of the wild-type CRM was on average fivefold greater than that of the mSTAT CRM as determined by measuring the densitometric mean from the fluorescence images of lymph glands carrying wild-type and mSTAT CRMs (Fig. 7D). Thus, the STAT site at position −98/−90 is required for ush −174/−25 lacZ activity. To confirm these results, we assayed the activity of wild-type CRM in stat heterozygous mutant lymph glands and observed a twofold reduction in the densitometric mean compared to that of the wild-type controls (Fig. 7E to G). These results predicted that increased levels of STAT activity in hopTum-l mutants would upregulate the wild-type but not the mSTAT CRM. To test this hypothesis, we assayed wild-type and mSTAT CRM activities in hopTum-l lymph glands from animals cultured at three different temperatures (Fig. 8). In wild-type lymph glands, the wild-type CRM was significantly more active than the mSTAT CRM regardless of the culture temperature (Fig. 8A, A′, and E and data not shown). In contrast, we observed no difference between wild-type and mSTAT CRM activities in hopTum-l lymph glands from animals cultured at 23°C (Fig. 8B, B′, and E). However, when the culture temperature was increased to 25°C and 27°C, we observed a statistically significant increase in levels in wild-type CRM activity compared to that of the mSTAT CRM (Fig. 8C to E). Based on these results, we propose that activated STAT upregulates wild-type ush CRM activity through the consensus STAT site. Altogether, these results indicate that the JAK/STAT pathway upregulates ush expression to maintain prohemocyte potency.
FIG. 8.
ush hematopoietic CRM activity in hopTum-l mutant lymph glands. Shown are data for wild-type (wt)and mutant STAT binding site (mSTAT) ush hematopoietic CRM activity in wild-type or hopTum-l second-instar larval lymph glands (fluorescence microscopy) (magnification, ×40). (A and B′) Wild-type and mSTAT ush hematopoietic CRM activity in wild-type second-instar larval lymph glands of animals cultured at 23°C. (B, C, and D) Wild-type hematopoietic CRM activity in hopTum-l lymph glands cultured at the following temperatures: 23°C (B), 25°C (C), and 27°C (D). (B′, C′, and D′) mSTAT hematopoietic CRM activity in hopTum-l lymph glands cultured at the following temperatures: 23°C (B′), 25°C (C′), and 27°C (D′). (E) Densitometric means of wild-type (green bars) and mSTAT (white bars) ush hematopoietic CRM activity in wild-type and hopTum-l lymph glands. Densitometric means of wild-type lymph glands carrying the wild-type and mSTAT ush hematopoietic CRM were 2,216 ± 1,258 and 1,272 ± 810, respectively (P < 0.05). Densitometric means of hopTum-l lymph glands carrying the wild-type or mSTAT ush hematopoietic CRM were as follows: 1,392 ± 636 (wild type) and 1,017 ± 323 (mSTAT) for lymph glands cultured at 23°C (P > 0.08), 3,046 ± 1,126 (wild type) and 1,787 ± 369 (mSTAT) for lymph glands cultured at 25°C (P < 0.005), and 3,391 ± 1,298 (wild type) and 1,435 + 369 (mSTAT) for lymph glands cultured at 27°C (P < 0.0005). Asterisks indicate a significant difference (Student t test) between wild-type and mSTAT CRMs. Scale bar, 50 μm.
DISCUSSION
In this study, we showed that a decrease in ush expression levels resulted in a loss of medullary zone prohemocytes and a concomitant increase in hemocyte differentiation. In addition, we provide evidence that ush expression is regulated directly by activated STAT. Collectively, these observations indicate that ush is a target of the JAK/STAT signaling pathway and that Ush functions to restrict the forward momentum of prohemocyte differentiation. Considering that the PSC maintains prohemocyte potency through the JAK/STAT pathway (32), our findings provide an important link between the stem cell niche and the intrinsic regulation of prohemocyte differentiation. Furthermore, these results may have implications for the regulation of mammalian HSCs because FOG-1 is expressed in mouse HSCs (8). The role of FOG-1 in these cells is unknown; however, like Ush, FOG-1 may block stem cell differentiation and maintain the multipotent state. In addition, FOG-1 expression may be regulated by the stem cell niche. Finally, the modulation of FOG expression by the JAK/STAT pathway may be part of a conserved strategy for the regulation of HSCs and other stem cell types. In this regard, STAT5 regulates mammalian HSC self-renewal (28). Perhaps, similar to their counterparts in the fly, STAT5 activates FOG-1 expression to maintain mammalian HSC potency.
Ush not only regulates the choice between the maintenance of potency and differentiation in prohemocytes but also regulates the choice between lineage-specific developmental pathways. In this regard, we observed that a change in the level of ush expression dramatically altered the composition of the lymph gland hemocyte population. Plasmatocyte and crystal cell differentiation increased with the loss of one functional ush gene (heterozygote), whereas the loss of more than one functional ush gene (trans heterozygote) produced fewer crystal cells and plasmatocytes and a corresponding increase in numbers of lamellocytes. These results indicate that a threshold level of Ush is required to promote the crystal cell and plasmatocyte developmental programs and are consistent with the observation that Ush is expressed in a subpopulation of these cells. Maintaining Ush at this threshold level may also limit lamellocyte differentiation to prevent the depletion of the prohemocyte population. In this regard, we observed that an increase in numbers of lamellocytes was accompanied by a decrease in numbers of prohemocytes, which is consistent with data from a previous report (32). ush trans-heterozygous lymph glands contain large aggregates of lamellocytes and a substantial number of cells that are not terminally differentiated lamellocytes (L1-negative cells). In addition, we observed few plasmatocytes and crystal cells and no prohemocytes in ush trans-heterozygous lymph glands. From these observations, we conclude that most of the L1-negative cells are lamellocyte precursors.
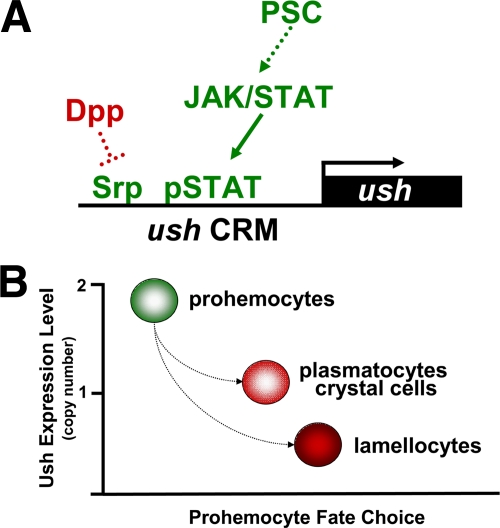
The ability of Ush to control prohemocyte fate choice is achieved through a complex gene regulatory strategy. Srp is an intrinsic regulator of ush expression in the lymph gland and, like activated STAT, is part of a larger regulatory cascade. Srp directly activates ush expression through clustered GATA sites within the hematopoietic CRM (44). Recently, srp expression was shown to be negatively regulated by the transforming growth factor β homolog Decapentaplegic (Dpp). Dpp blocks zinc finger homeodomain protein 1 expression, which is required for the expression of srp. As a result, dpp mutant larvae have elevated Ush levels and are refractory to Salmonella-induced lamellocyte differentiation (19). Together, these observations suggest that the ush hematopoietic CRM acts as a nexus between the Dpp and JAK/STAT pathways, and antagonism between these pathways modulates ush expression (Fig. 9A). One key element of this gene regulatory network is the architecture of the ush hematopoietic CRM, in which the STAT binding site is located 9 bp from the clustered GATA sites. The proximity of the STAT and GATA binding sites may facilitate a cooperative interaction between Srp and STAT in much that same way as adjacent GATA and RUNX sites promote a cooperative interaction between Srp and Lz (45). Thus, this network may operate as a graded rheostat mechanism to regulate the level of ush expression through combinatorial interactions involving Srp and STAT and thereby control prohemocyte potency and lineage choice (Fig. 9).
FIG. 9.
Ush is a key component of a gene regulatory network that controls prohemocyte fate choice. (A) Model showing regulation of ush expression levels by the convergence of the Dpp and JAK/STAT pathways. The positive regulatory pathway (green) begins with a signal from the PSC, which maintains JAK/STAT activity in the lymph gland medullary zone. ush gene expression is coactivated by Srp and activated STAT. Ush expression is negatively regulated through the inhibition of srp expression by Dpp (red). The solid arrow indicates a direct link between pathway components. The dotted arrow and blocked line indicate additional unspecified components that comprise the pathway. (B) Model showing that prohemocyte fate choice is determined by the level of ush expression. Two copies of Ush maintain prohemocyte potency. The loss of one copy favors plasmatocyte and crystal cell differentiation. The loss of more than one copy favors lamellocyte differentiation. Together, these models describe key components of a larger integrated genetic network that adjusts hemocyte composition in response to the needs of the organism.
FOG proteins bind the N-terminal zinc finger of their respective GATA partners to modify GATA-activated gene expression (16). During embryonic crystal cell development, Ush converts SrpNC from an activator to a repressor, which limits commitment and maturation by blocking lz expression (18, 45). Similarly, Ush may interact with SrpNC during lymph gland hematopoiesis to maintain prohemocyte potency and control lineage choice. In order to perform these diverse functions during the different stages of hematopoiesis, the SrpNC-Ush repressor complex may interact with additional protein binding partners to confer gene regulatory specificity, as is the case with the vertebrate GATA-FOG complex (4, 7, 12, 25, 26, 30, 38, 50, 51). Conversely, a reduction in the level of Ush may free SrpNC to act with lineage-specific factors to drive commitment and differentiation, much as the downregulation of Ush in embryonic crystal cell precursors allows SrpNC to act with Lz to drive crystal cell maturation (18, 45). In this regard, we observed that Ush was downregulated in crystal cells and plasmatocytes. Furthermore, this loss of ush expression is most likely due to decreased STAT activity in these cells (32).
In conclusion, Ush is a key component of the overall gene regulatory network that controls the blood organ's response to the changing needs of the organism. We show that ush expression is regulated directly by activated STAT and that changes in ush expression alter prohemocyte fate. Current models of Drosophila lymph gland hematopoiesis describe a stem cell niche that regulates prohemocyte maintenance through the Hedgehog and JAK/STAT signaling pathways (32, 40). Most importantly, our findings identify a downstream target of the niche and provide a mechanism for the processing of these signals to control prohemocyte potency and differentiation.
Acknowledgments
This work was supported by Public Health Service grant DK072229 from the National Institutes of Health.
We gratefully acknowledge our colleagues for providing fly strains and antibodies. R. A. Schulz and R. P. Sorrentino provided y1 w; ushvx22/CyO, y+ and y1 w; ushr24/CyO, y+ stocks. M. P. Zeidler and J. C. Hombria provided domeMESO strains w; domeMESO/CyO and w p{w+, dome-MESO}BN1. D. J. Montell provided the FRT 82b, e, ca stat397-6/TM3 stock. Istavan Ando provided the P1, L1, and He antibodies. F. C. Kafatos provided the ProPO antibody. We also thank Noam Broder for excellent technical assistance. We are grateful to T. Antalis, S. DasSharma, and A. Keegan for critical comments.
Footnotes
Published ahead of print on 8 September 2009.
REFERENCES
- 1.Akala, O. O., and M. F. Clarke. 2006. Hematopoietic stem cell self-renewal. Curr. Opin. Genet. Dev. 165:496-501. [DOI] [PubMed] [Google Scholar]
- 2.Asha, H., I. Nagy, G. Kovacs, D. Stetson, I. Ando, and C. R. Dearolf. 2003. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163:203-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barettino, D., M. Feigenbutz, R. Valcarcel, and H. G. Stunnenberg. 1994. Improved method for PCR-mediated site-directed mutagenesis. Nucleic Acids Res. 22:541-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottardi, S., A. G. Ghiam, F. Bergeron, and E. Milot. 2007. Lineage-specific transcription factors in multipotent hematopoietic progenitors: a little bit goes a long way. Cell Cycle 6:1035-1039. [DOI] [PubMed] [Google Scholar]
- 5.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 6.Cantor, A. B., and S. H. Orkin. 2002. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 21:3368-3376. [DOI] [PubMed] [Google Scholar]
- 7.Cantor, A. B., S. G. Katz, and S. H. Orkin. 2002. Distinct domains of the GATA-1 cofactor FOG-1 differentially influence erythroid versus megakaryocytic maturation. Mol. Cell. Biol. 22:4268-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantor, A. B., H. Iwasaki, Y. Arinobu, T. B. Moran, H. Shigematsu, M. R. Sullivan, K. Akashi, and S. H. Orkin. 2008. Antagonism of FOG-1 and GATA factors in fate choice for the mast cell lineage. J. Exp. Med. 205:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crozatier, M., J. M. Ubeda, A. Vincent, and M. Meister. 2004. Cellular immune response to parasitization in Drosophila requires the EBF orthologue Collier. PLoS Biol. 2:1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crozatier, M., J. Krzemien, and A. Vincent. 2007. The hematopoietic niche: a Drosophila model, at last. Cell Cycle 6:1443-1444. [PubMed] [Google Scholar]
- 11.Crozatier, M., and M. Meister. 2007. Drosophila haematopoiesis. Cell. Microbiol. 9:1117-1126. [DOI] [PubMed] [Google Scholar]
- 12.Dale, R. M., B. F. Remo, and E. C. Svensson. 2007. An alternative transcript of the FOG-2 gene encodes a FOG-2 isoform lacking the FOG repression motif. Biochem. Biophys. Res. Commun. 357:683-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dearolf, C. R. 1998. Fruit fly “leukemia”. Biochim. Biophys. Acta 1377:M13-M23. [DOI] [PubMed] [Google Scholar]
- 14.Ehret, G. B., P. Reichenbach, U. Schindleri, C. M. Horvath, S. Fritz, M. Nabholz, and P. Bucher. 2001. DNA binding specificity of different STAT proteins. J. Biol. Chem. 276:6675-6688. [DOI] [PubMed] [Google Scholar]
- 15.Evans, C. J., V. Hartenstein, and U. Banerjee. 2003. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5:673-690. [DOI] [PubMed] [Google Scholar]
- 16.Fossett, N., and R. A. Schulz. 2001. Functional conservation of hematopoietic factors in Drosophila and vertebrates. Differentiation 69:83-90. [DOI] [PubMed] [Google Scholar]
- 17.Fossett, N., S. G. Tevosian, K. Gajewski, Q. Zhang, S. H. Orkin, and R. A. Schulz. 2001. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. USA 98:7342-7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fossett, N., K. Hyman, K. Gajewski, S. H. Orkin, and R. A. Schulz. 2003. Combinatorial interactions of Serpent, Lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc. Natl. Acad. Sci. USA 100:11451-11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frandsen, J. L., B. Gunn, S. Muratoglu, N. Fossett, and S. J. Newfeld. 2008. Salmonella pathogenesis reveals that BMP signaling regulates a transcription factor cascade that controls blood cell homeostasis and immune responses in Drosophila. Proc. Natl. Acad. Sci. USA 105:14952-14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajewski, K., Y. Kim, Y. M. Lee, E. N. Olson, and R. A. Schulz. 1997. D-mef2 is a target for Tinman activation during Drosophila heart development. EMBO J. 16:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haenlin, M., Y. Cubadda, F. Blondeau, P. Heitzler, Y. Lutz, P. Simpson, and P. Ramain. 1997. Transcriptional activity of pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 11:3096-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison, D. A., R. Binari, T. S. Nahreini, M. Gilman, and N. Perrimon. 1995. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14:2857-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartenstein, V. 2006. Blood cells and blood cell development in the animal kingdom. Annu. Rev. Cell Dev. Biol. 22:677-712. [DOI] [PubMed] [Google Scholar]
- 24.Hombría, J. C., S. Brown, S. Häder, and M. P. Zeidler. 2005. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288:420-433. [DOI] [PubMed] [Google Scholar]
- 25.Hong, W., M. Nakazawa, Y. Y. Chen, R. Kori, C. R. Vakoc, C. Rakowski, and G. A. Blobel. 2005. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 24:2367-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, K. D., S. I. Kim, and E. H. Bresnick. 2006. Differential sensitivities of transcription factor target genes underlie cell type-specific gene expression patterns. Proc. Natl. Acad. Sci. USA 103:15939-15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung, S. H., C. J. Evans, C. Uemura, and U. Banerjee. 2005. The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132:2521-2533. [DOI] [PubMed] [Google Scholar]
- 28.Kato, Y., A. Iwama, Y. Tadokoro, K. Shimoda, M. Minoguchi, S. Akira, M. Tanaka, A. Miyajima, T. Kitamura, and H. Nakauchi. 2005. Selective activation of STAT5 unveils its role in stem cell self-renewal in normal and leukemic hematopoiesis. J. Exp. Med. 202:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiel, M. J., and S. J. Morrison. 2008. Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol. 8:290-301. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. I., and E. H. Bresnick. 2007. Transcriptional control of erythropoiesis: emerging mechanisms and principles. Oncogene 26:6777-6794. [DOI] [PubMed] [Google Scholar]
- 31.Koch, U., and F. Radtke. 2007. Haematopoietic stem cell niche in Drosophila. Bioessays 8:713-716. [DOI] [PubMed] [Google Scholar]
- 32.Krzemień, J., L. Dubois, R. Makki, M. Meister, A. Vincent, and M. Crozatier. 2007. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446:325-328. [DOI] [PubMed] [Google Scholar]
- 33.Kurucz, E., C. J. Zettervall, R. Sinka, P. Vilmos, A. Pivarcsi, S. Ekengren, Z. Hegedüs, I. Ando, and D. Hultmark. 2003. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. USA 100:2622-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurucz, E., R. Márkus, J. Zsámboki, K. Folkl-Medzihradszky, Z. Darula, P. Vilmos, A. Udvardy, I. Krausz, T. Lukacsovich, E. Gateff, C. J. Zettervall, D. Hultmark, and I. Andó. 2007. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17:649-654. [DOI] [PubMed] [Google Scholar]
- 35.Kurucz, E., B. Váczi, R. Márkus, B. Laurinyecz, P. Vilmos, J. Zsámboki, K. Csorba, E. Gateff, D. Hultmark, and I. Andó. 2007. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol. Hung. 58(Suppl.):95-111. [DOI] [PubMed] [Google Scholar]
- 36.Lanot, R., D. Zachary, F. Holder, and M. Meister. 2001. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 230:243-257. [DOI] [PubMed] [Google Scholar]
- 37.Lebestky, T., S. H. Jung, and U. Banerjee. 2003. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 17:348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin, A. C., A. E. Roche, J. Wilk, and E. C. Svensson. 2004. The N termini of Friend of GATA (FOG) proteins define a novel transcriptional repression motif and a superfamily of transcriptional repressors. J. Biol. Chem. 279:55017-55023. [DOI] [PubMed] [Google Scholar]
- 39.Luo, H., P. Rose, D. Barber, W. P. Hanratty, S. Lee, T. M. Roberts, A. D. D'Andrea, and C. R. Dearolf. 1997. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol. Cell. Biol. 3:1562-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandal, L., J. A. Martinez-Agosto, C. J. Evans, V. Hartenstein, and U. Banerjee. 2007. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446:320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Agosto, J. A., H. K. Mikkola, V. Hartenstein, and U. Banerjee. 2007. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 21:3044-3060. [DOI] [PubMed] [Google Scholar]
- 42.Minakhina, S., and R. Steward. 2006. Melanotic mutants in Drosophila: pathways and phenotypes. Genetics 174:253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller, H. M., G. Dimopoulos, C. Blass, and F. C. Kafatos. 1999. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 274:11727-11735. [DOI] [PubMed] [Google Scholar]
- 44.Muratoglu, S., B. Garratt, K. Hyman, K. Gajewski, R. A. Schulz, and N. Fossett. 2006. Regulation of Drosophila Friend of GATA gene, u-shaped, during hematopoiesis: a direct role for Serpent and Lozenge. Dev. Biol. 296:561-579. [DOI] [PubMed] [Google Scholar]
- 45.Muratoglu, S., B. Hough, S. T. Mon, and N. Fossett. 2007. The GATA factor Serpent cross-regulates lozenge and u-shaped expression during Drosophila blood cell development. Dev. Biol. 311:636-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nusslein-Volhard, C., E. Wieschaus, and H. Kluding. 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux Arch. Dev. Biol. 193:267-282. [DOI] [PubMed] [Google Scholar]
- 47.Qiu, P., P. Pan, and S. Govind. 1998. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 125:1909-1920. [DOI] [PubMed] [Google Scholar]
- 48.Rehorn, K. P., H. Thelen, A. M. Michelson, and R. Reuter. 1996. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development 122:4023-4031. [DOI] [PubMed] [Google Scholar]
- 49.Rizki, T. M. 1978. The circulatory system and associated cells and tissues, p. 397-452. In M. Ashburner and T. R. F. Wright (ed.), The genetics and biology of Drosophila. Academic Press, New York, NY.
- 50.Roche, A. E., B. J. Bassett, S. A. Samant, W. Hong, G. A. Blobel, and E. C. Svensson. 2008. The zinc finger and C-terminal domains of MTA proteins are required for FOG-2-mediated transcriptional repression via the NuRD complex. J. Mol. Cell. Cardiol. 44:352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez, P., E. Bonte, J. Krijgsveld, K. E. Kolodziej, B. Guyot, A. J. Heck, P. Vyas, E. de Boer, F. Grosveld, and J. Strouboulis. 2005. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 24:2354-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sam, S., W. Leise, and D. K. Hoshizaki. 1996. The serpent gene is necessary for progression through the early stages of fat-body development. Mech. Dev. 60:197-205. [DOI] [PubMed] [Google Scholar]
- 53.Scadden, D. T. 2006. The stem-cell niche as an entity of action. Nature 441:1075-1079. [DOI] [PubMed] [Google Scholar]
- 54.Sorrentino, R. P., Y. Carton, and S. Govind. 2002. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243:65-80. [DOI] [PubMed] [Google Scholar]
- 55.Sorrentino, R. P., J. P. Melk, and S. Govind. 2004. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and egg encapsulation response in Drosophila. Genetics 166:1343-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorrentino, R. P., T. Tokusumi, and R. A. Schulz. 2007. The Friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in Drosophila. Dev. Biol. 311:311-323. [DOI] [PubMed] [Google Scholar]
- 57.Tepass, U., L. I. Fessler, A. Aziz, and V. Hartenstein. 1994. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120:1829-1837. [DOI] [PubMed] [Google Scholar]
- 58.Thummel, C. S., A. M. Boulet, and H. D. Lipshitz. 1988. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene 74:445-456. [DOI] [PubMed] [Google Scholar]
- 59.Tsai, F. Y., G. Keller, F. C. Kuo, M. Weiss, J. Chen, M. Rosenblatt, F. W. Alt, and S. H. Orkin. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221-226. [DOI] [PubMed] [Google Scholar]
- 60.Tsai, F. Y., and S. H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89:3636-3643. [PubMed] [Google Scholar]
- 61.Waltzer, L., G. Ferjoux, L. Bataille, and M. Haenlin. 2003. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J. 22:6516-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zettervall, C.-J., I. Ander, M. J. Williams, R. Palmer, E. Kurucz, I. Ando, and D. Hultmark. 2004. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101:14192-14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zon, L. I. 2008. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature 453:306-313. [DOI] [PubMed] [Google Scholar]