Abstract
This study was performed to evaluate the incidence of and risk factors for Enterocytozoon bieneusi carriage in an orphanage in Bangkok, Thailand. E. bieneusi has been identified by PCR every 2 consecutive months since June 2003. The incidence ranged between 0.6 and 4.7/100 person-months. Person-to-person transmission was indicated by risk factor analysis and genotyping information.
Enterocytozoon bieneusi, the most common microsporidial organism infecting humans, causes chronic diarrhea, especially in AIDS patients (4, 12). It can also cause diarrhea in immunocompetent individuals (15, 17). In Thailand, E. bieneusi is one of the most common causes of diarrhea in both adults and children with AIDS (9, 24, 25). It is assumed that E. bieneusi is transmitted by the fecal-oral route; however, the sources of infection and the modes of transmission remain unclear (3). Recently, knowledge about this infection has been increased because of PCR-based detection methods which have higher sensitivity and can identify the organisms' species and genotypes (16, 19). Genotyping of E. bieneusi is determined based on the polymorphic sequences of the internal transcribed spacer (ITS) of the rRNA gene (2, 11, 18). Recent epidemiological studies have indicated the transmission modes of E. bieneusi including person-to-person, zoonotic, waterborne, and food-borne routes (2, 5-7, 10).
We previously reported that ∼4% of human immunodeficiency virus (HIV)-negative children in an orphanage in Bangkok were positive for E. bieneusi (10). To develop effective control strategies, it is essential to understand the epidemiology of this infection. Thus, we conducted a 1-year longitudinal study of E. bieneusi infection in this orphanage. This study was approved by the Ethical Committee, Royal Thai Army Medical Department. A total of 540 orphans and 81 child care workers were enrolled in the study during June 2003 to April 2004. The orphanage consisted of 12 rooms (10 rooms for orphans and 2 rooms for milk and food preparation). Orphans within specific groups were assigned to 10 different rooms (Table 1). Each room accommodated 30 to 40 orphans with 3 child care workers. The child care workers in each room were asked to collect stool samples and complete standardized questionnaires for the orphans for whom they were responsible every 2 months from June 2003 to April 2004. The information, including age, sex, weight, height, HIV status, and present illness, was recorded. The numbers of enrolled subjects during each consecutive round of survey were 338, 337, 321, 286, 340, and 306, respectively. Of 540 orphans, 318 (58.9%) were males. The median age of the orphans was 13 months (0.26 months to 11 years). Seventy-seven orphans (14.3%) were HIV positive (47 males and 30 females). Information on CD4+ T-lymphocyte count was not available. All HIV-positive orphans were prescribed antiretroviral therapy (i.e., zidovudine and didanosine). Child care workers who participated in this study had a median age of 38 years (19 to 55 years).
TABLE 1.
Characteristics of 75 orphans with intestinal microsporidiosis
| Characteristic | No. positive for E. bieneusi | Total (% positive) | P value |
|---|---|---|---|
| Age (mo) | |||
| 0-12 | 18 | 265 (6.8) | |
| 13-24 | 33 | 134 (24.6) | |
| 25-36 | 6 | 56 (10.7) | |
| 37-48 | 4 | 31 (12.9) | |
| 49-60 | 8 | 29 (27.6) | |
| >60 | 6 | 106 (5.7) | <0.001 |
| Room no. (specific group) | |||
| 1 (36-60 mo) | 13 | 54 (24.1) | |
| 2 (newborn to 8 mo) | 3 | 39 (7.7) | |
| 3 (newly enrolled) | 3 | 93 (3.2) | |
| 4 (HIV positive) | 15 | 90 (16.7) | |
| 5 (32-36 mo) | 0 | 45 (0.0) | |
| 6 (24-32 mo) | 14 | 40 (35.0) | |
| 7 (newborn to 8 mo) | 0 | 43 (0.0) | |
| 8 (8-12 mo) | 7 | 47 (14.9) | |
| 9 (12-18 mo) | 6 | 47 (12.8) | |
| 10 (18-24 mo) | 14 | 42 (33.3) | <0.001 |
| Sex | |||
| Male | 52 | 318 (16.4) | |
| Female | 23 | 222 (10.4) | 0.031 |
| HIV infection | |||
| No | 61 | 463 (13.2) | |
| Yes | 14 | 77 (18.2) | 0.159 |
| Diarrhea | |||
| No | 73 | 533 (13.7) | |
| Yes | 2 | 7 (28.6) | 0.252 |
Stool specimens were examined for microsporidial spores under a light microscope using gram-chromotrope staining (13). A sedimentation technique was used to concentrate microsporidial spores as described by van Gool et al. (22). DNA was prepared from concentrated specimens using FTA filter paper (Whatman, Bioscience, United Kingdom) (19). Genomic DNA and primer pairs (MSP3/MSP4B) were used in PCR under the conditions described by Katzwinkel-Wladarsch et al. (8). Genotyping of E. bieneusi was determined by polymorphic sites on the ITS region of the rRNA gene. DNA purification and sequencing were conducted by Macrogen, Inc., Seoul, South Korea. Data analysis was performed using Bioedit for multiple alignments. Chromatograms were manually checked and edited using Sequencher version 4.0.5 (Gene Codes Corporation, Inc., Ann Arbor, MI). The genotype of E. bieneusi from each specimen was confirmed by the homology of the sequenced PCR product to the published sequence in GenBank by multiple alignments in ClustalX version 1.81 for Windows (20).
E. bieneusi-infected cases are defined as patients with PCR-positive stool specimens. Of 1,930 stool specimens from 621 individuals, 37 samples from the orphans (1.9%) were positive for microsporidial spores by gram-chromotrope staining, while 84 samples (4.4%) were positive by PCR amplification. All PCR-negative specimens were negative by microscopy. None of the stool samples from child care workers was positive by PCR. These findings confirm that PCR is suitable for epidemiological study of E. bieneusi infection because of its higher sensitivity. ITS sequencing showed that all 84 E. bieneusi samples had 100% identity to E. bieneusi genotype A (accession no. AF101197).
To determine the incidence and risk factors of E. bieneusi infection, standardized questionnaires was used in this study. Incidence was defined as the number of new cases occurring during the observation period. The estimated date of infection for the incident cases was taken as the midpoint between the last test negative result and first positive result for E. bieneusi PCR amplification. Possible risk factors were analyzed using incidence rate ratios and their 95% confidence intervals. The chi-square test was used to compare proportions. Poisson regression using STATA 9.2 was performed for multivariate analysis to assess the independent association of the risk factors and E. bieneusi infection.
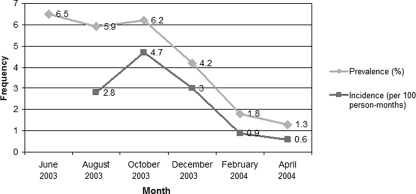
Figure 1 shows the prevalence and incidence of E. bieneusi carriage in orphans at each time point. The patterns of the incidence were similar to those observed for the prevalence. This finding suggests that E. bieneusi infection is a self-limited, short-course disease, which is also supported by our previous study showing that the number of excreted spores tended to decrease and disappear after a period of time (14). A few studies demonstrated that E. bieneusi infection was significantly prevalent in children between 1 and 3 years of age (10, 21). Lower incidence in older age groups may reflect the development of protective immunity. Spore shedding of E. bieneusi in some asymptomatic children could last nearly 2 months (14), so we defined 4-month intervals between two positive PCRs as reinfection. Nine orphans were reinfected in the study. Of these, three orphans had HIV infection. Thus, protective immunity might not be fully developed after an infection in some children since reinfection occurred.
FIG. 1.
Prevalence and incidence of Enterocytozoon bieneusi infection among orphans from June 2003 to April 2004.
The incidence of E. bieneusi carriage in this orphanage was higher during the rainy to early winter season. This seasonal variation was similar to that found among children with diarrhea in Uganda (21). However, the gradually decreased prevalence and incidence of E. bieneusi carriage in this orphanage might be due to the interventions that were introduced during early 2004: i.e., cleaning of clothes and accessories using autoclave heat treatment and health education of child care workers. Although no study has directly supported the effect of autoclave heat treatment against E. bieneusi spores, boiling for 5 min or autoclaving at 120°C for 10 min can kill spores of Encephalitozoon cuniculi, another species of microsporidian (23). Thus, this approach might reduce the viability or infectivity of E. bieneusi spores.
Characteristics of E. bieneusi carriers are shown in Table 1. Significant differences in the prevalence of E. bieneusi carriage were found among children by different age groups, sexes, and rooms. Seven (1.3%) orphans experienced episodes of diarrhea; only two HIV-negative orphans were positive for E. bieneusi. Most cases of symptomatic intestinal microsporidiosis were among HIV-positive patients with low CD4+ T-cell counts (1). Thus, asymptomatic infection in these children could be due to their intact immunity. These asymptomatic carriages were the unexpected sources of E. bieneusi infection. Univariate and multivariate analyses of risk factors associated with E. bieneusi carriage are shown in Table 2. Multivariate analysis showed that the only significant risk of E. bieneusi carriage was for orphans living in room 10. Since this room was occupied by children 18 to 24 months of age, the high incidence was probably related to their behavior favoring the spread of this infection: i.e., active movement with independent eating habits but poor toilet training and poor hygienic food-handling habits. In addition, the crowded condition of each room could favor the spread of infection. This might also explain the high prevalence in the lower age group compared to that found in the study by Nkinin et al. (15), together with the finding that all E. bieneusi isolates from these orphans were the human-specific genotype, A. Thus, person-to-person transmission plays the most important role in E. bieneusi infection in this setting. Based on this information, universal precautions should be performed since most infected children were asymptomatic. Careful handling of contaminated materials and regular hand-washing should be effective preventative measures.
TABLE 2.
Univariate and multivariate analysis of risk factors associated with E. bieneusi infection
| Characteristic | No. positive for E. bieneusi | Person-mo of follow-up | Incidence rate ratio (95% confidence interval) |
|
|---|---|---|---|---|
| Crude | Adjusted | |||
| Age (mo) | ||||
| 0-12 | 15 | 643.0 | 1 | 1 |
| 13-24 | 21 | 282.3 | 3.2 (1.6-6.7) | 1.8 (0.8-4.1) |
| >24 | 13 | 774.9 | 0.7 (0.3-1.6) | 0.7 (0.3-1.5) |
| Sex | ||||
| Female | 17 | 772.3 | 1 | 1 |
| Male | 32 | 927.8 | 1.6 (0.8-3.0) | 1.4 (0.8-2.5) |
| Room | ||||
| Others | 36 | 1,603.8 | 1 | 1 |
| No. 10 | 13 | 96.3 | 6.0 (2.9-11.6) | 3.5 (1.6-7.6) |
| HIV status | ||||
| Negative | 39 | 1,257.5 | 1 | |
| Positive | 10 | 310.6 | 1.0 (0.5-2.1) | |
Acknowledgments
We thank the director and child care workers of the orphanage for their cooperation.
This study was financially supported by the Thailand-Tropical Diseases Research Programme (T-2) (grant no. ID 02-2-ARI-24-007).
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Asmuth, D. M., P. C. DeGirolami, M. Federman, C. R. Ezratty, D. K. Pleskow, G. Desai, and C. A. Wanke. 1994. Clinical features of microsporidiosis in patients with AIDS. Clin. Infect. Dis. 18:819-825. [DOI] [PubMed] [Google Scholar]
- 2.Dengjel, B., M. Zahler, W. Hermanns, K. Heinritzi, T. Spillmann, A. Thomschke, T. Löscher, R. Gothe, and H. Rinder. 2001. Zoonotic potential of Enterocytozoon bieneusi. J. Clin. Microbiol. 39:4495-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Didier, E. S. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 94:61-76. [DOI] [PubMed] [Google Scholar]
- 4.Didier, E. S., and L. M. Weiss. 2006. Microsporidiosis: current status. Curr. Opin. Infect. Dis. 19:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowd, S. E., C. P. Gerba, and I. L. Pepper. 1998. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 64:3332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fournier, S., O. Liguory, M. Santillana-Hayat, E. Guillot, C. Sarfati, N. Dumoutier, J. Molina, and F. Derouin. 2000. Detection of microsporidia in surface water: a one-year follow-up study. FEMS Immunol. Med. Microbiol. 29:95-100. [DOI] [PubMed] [Google Scholar]
- 7.Hutin, Y. J. F., M. N. Sombardier, O. Liguory, C. Sarfati, F. Derouin, J. Modaï, and J. Molina. 1998. Risk factors for intestinal microsporidiosis in patients with human immunodeficiency virus infection: a case-control study. J. Infect. Dis. 178:904-907. [DOI] [PubMed] [Google Scholar]
- 8.Katzwinkel-Wladarsch, S., M. Lieb, W. Helse, T. Löscher, and H. Rinder. 1996. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1:373-378. [DOI] [PubMed] [Google Scholar]
- 9.Leelayoova, S., N. Vithayasai, V. Watanaveeradej, T. Chotpitayasunondh, V. Therapong, T. Naaglor, and M. Mungthin. 2001. Intestinal microsporidiosis in HIV-infected children with acute and chronic diarrhea. Southeast Asian J. Trop. Med. Public Health 32:33-37. [PubMed] [Google Scholar]
- 10.Leelayoova, S., I. Subrungruang, R. Rangsin, P. Chavalitshewinkoon-Petmitr, J. Worapong, T. Naaglor, and M. Mungthin. 2005. Transmission of Enterocytozoon bieneusi genotype A in Thai orphanage. Am. J. Trop. Med. Hyg. 73:104-107. [PubMed] [Google Scholar]
- 11.Liguory, O., F. David, C. Sarfati, F. Derouin, and J.-M. Molina. 1998. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. J. Clin. Microbiol. 36:1882-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathis, A., R. Weber, and P. Deplazes. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18:423-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moura, H., J. L. Da Silva, F. C. Sodré, P. Brasil, K. Wallmo, S. Wahlquist, S. Wallace, G. P. Croppo, and G. S. Visvesvara. 1996. Gram-chromotrope: a new technique that enhances detection of microsporidial spores in clinical samples. J. Eukaryot. Microbiol. 43:94S-95S. [DOI] [PubMed] [Google Scholar]
- 14.Mungthin, M., I. Subrungruang, T. Naaglor, P. Aimpun, W. Areekul, and S. Leelayoova. 2005. Spore shedding pattern of Enterocytozoon bieneusi in asymptomatic children. J. Med. Microbiol. 54:473-476. [DOI] [PubMed] [Google Scholar]
- 15.Nkinin, S. W., T. Asonganyi, E.S. Didier, and E. S. Kaneshiro. 2007. Microsporidian infection is prevalent in healthy people in Cameroon. J. Clin. Microbiol. 45:2841-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinder, H., K. Janitschke, H. Aspöck, A. J. Da Silva, P. Deplazes, D.P. Fedorko, C. Franzen, U. Futh, F. Hünger, A. Lehmacher, C.G. Meyer, J.-M. Molina, J. Sandfort, R. Weber, T. Löscher, and the Diagnostic Multicenter Study Group on Microsporidia. 1998. Blinded, externally controlled multicenter evaluation of light microscopy and PCR for detection of microsporidia in stool specimens. J. Clin. Microbiol. 36:1814-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandfort, J., A. Hannemann, H. Gelderblom, K. Stark, R. L. Owen, and B. Ruf. 1994. Enterocytozoon bieneusi infection in an immunocompetent patient who had acute diarrhea and who was not infected with the human immunodeficiency virus. Clin. Infect. Dis. 19:514-516. [DOI] [PubMed] [Google Scholar]
- 18.Santín, M., and R. Fayer. 2009. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J. Eukaryot. Microbiol. 56:34-38. [DOI] [PubMed] [Google Scholar]
- 19.Subrungruang, I., M. Mungthin, P. Chavalitshewinkoon-Petmitr, R. Rangsin, T. Naaglor, and S. Leelayoova. 2004. Evaluation of DNA extraction and PCR methods for detection of Enterocytozoon bieneusi in stool specimens. J. Clin. Microbiol. 42:3490-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, M. A. Buckholt, and S. Tzipori. 2002. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am. J. Trop. Med. Hyg. 67:299-303. [DOI] [PubMed] [Google Scholar]
- 22.van Gool, T., F. Snijders, P. Reiss, J. K. Eeftinck Schattenkerk, M. A. van den Bergh Weerman, J. F. Bartelsman, J. J. Bruins, E. U. Canning, and J. Dankert. 1993. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J. Clin. Pathol. 46:694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waller, T. 1979. Sensitivity of Encephalitozoon cuniculi to various temperatures, disinfectants and drugs. Lab. Anim. 13:227-230. [DOI] [PubMed] [Google Scholar]
- 24.Wanachiwanawin, D., S. Manatsathit, P. Lertlaituan, K. Thakerngpol, and P. Suwanagool. 1998. Intestinal microsporidiosis in HIV infected patients with chronic diarrhea in Thailand. Southeast Asian J. Trop. Med. Public Health 29:767-771. [PubMed] [Google Scholar]
- 25.Wanachiwanawin, D., K. Chokephaibulkit, P. Lertlaituan, J. Ongrotchanakun, P. Chinabut, and K. Thakerngpol. 2002. Intestinal microsporidiosis in HIV-infected children with diarrhea. Southeast Asian J. Trop. Med. Public Health 33:241-245. [PubMed] [Google Scholar]