Abstract
Microbicide candidates with promising in vitro activity are often advanced for evaluations using human primary tissue explants relevant to the in vivo mucosal transmission of human immunodeficiency virus type 1 (HIV-1), such as tonsil, cervical, or rectal tissue. To compare virus growth or the anti-HIV-1 efficacies of candidate microbicides in tissue explants, a novel soft-endpoint method was evaluated to provide a single, objective measurement of virus growth. The applicability of the soft endpoint is shown across several different ex vivo tissue types, with the method performed in different laboratories, and for a candidate microbicide (PRO 2000). The soft-endpoint method was compared to several other endpoint methods, including (i) the growth of virus on specific days after infection, (ii) the area under the virus growth curve, and (iii) the slope of the virus growth curve. Virus growth at the assay soft endpoint was compared between laboratories, methods, and experimental conditions, using nonparametric statistical analyses. Intra-assay variability determinations using the coefficient of variation demonstrated higher variability for virus growth in rectal explants. Significant virus inhibition by PRO 2000 and significant differences in the growth of certain primary HIV-1 isolates were observed by the majority of laboratories. These studies indicate that different laboratories can provide consistent measurements of anti-HIV-1 microbicide efficacy when (i) the soft endpoint or another standardized endpoint is used, (ii) drugs and/or virus reagents are centrally sourced, and (iii) the same explant tissue type and method are used. Application of the soft-endpoint method reduces the inherent variability in comparisons of preclinical assays used for microbicide development.
Studies using human tissues cultured ex vivo (i.e., tissue explants) are performed for the preclinical evaluation of topical microbicides, compounds that can be applied vaginally or rectally to reduce the sexual transmission of human immunodeficiency virus type 1 (HIV-1) (19). Various tissue explant models and the procedures used for evaluating test compounds for anti-HIV activity have been described (1, 2, 4, 7-9, 13, 14). At present, there is no standard methodology, including calculation of an endpoint, for comparing results between ex vivo experiments.
Although cell-based assays can be standardized (5, 20), tissue explants may represent a more relevant method of testing, since this is where HIV-1 infection occurs in vivo. Since most parameters of cell-based assays (i.e., size of the virus inoculum, number of target cells, and use of a single endpoint measure) can be standardized, the data are often less variable and more easily reproduced than those obtained in assays with tissue explants. For example, the number of target cells in cell-based assays can be controlled, whereas the concentration and distribution of target cells in explant tissues are highly variable. In addition, explant methods vary across laboratories, and as a consequence, a number of parameters may affect the reliability of the results, including (i) tissue type, (ii) HIV-1 strain or isolate, (iii) culture medium formulation, (iv) size of the virus inoculum, (v) length of virus incubation, (vi) frequency of medium change, (vii) concentration of test compound, (viii) drug treatment period prior to or after viral exposure, (ix) use of controls, and (x) endpoint viral growth measurements.
For the reasons described above, a group of microbicide investigators joined a mutual effort to identify an approach for improving the reliability and reproducibility of data from explant studies. To this end, results obtained from different explant models were analyzed using a range of endpoint methods. In general, due to the interdependency of repeated measurements, standard statistical methods (e.g., t test, analysis of variance [ANOVA], Mann-Whitney test, and Kruskal-Wallis test) do not apply in comparing virus growth in the presence of inhibitory compounds. For example, a high level of virus growth on one day in one explant sample is likely to continue to be followed by high-level virus growth throughout the assay period, while a low level of virus growth in a different sample will be followed by low-level growth. In some cases, the relationships between consecutive data points follow certain assumptions, thus allowing statistical modeling techniques to be applied (e.g., repeated-measures ANOVA and generalized linear and mixed models [26]). However, this type of statistical modeling may be outside the expertise of many microbiological research teams, and the inherent assumptions of normal distributions and homogeneity of variance are rarely met for virus growth data. The soft endpoint (SOFT) is presented as a single, summary measure of virus growth to enable direct comparisons between explant experiments.
There is currently no consensus on a method to determine a summary measure of virus growth (25). A number of methods, including the area under the virus growth curve (AUC) (22), the slope of the virus growth curve (33), and virus growth at specific time points (1, 7, 8, 15), have been used. The first aim of this study was to compare a selection of alternative, single measures of virus growth, including SOFT, using data from multisite explant studies, as part of a wider effort to improve the quality of explant studies by standardizing the method used to objectively measure HIV-1 replication in tissues (J. E. Cummins, N. Richardson-Harman, J. Bremer, P. Anton, C. Dezzutti, P. Gupta, N. Lurain, L. Margolis, R. Shattock, and P. Reichelderfer, presented at the 2006 Conference on Retroviruses and Opportunistic Infections, Denver, CO).
The second aim of this study was to apply SOFT to compare treatment conditions relevant to microbicide research, using the explant methods developed by the participating microbiological laboratories. It should be noted that the goal of this work was to identify those key parameters that would improve the overall reliability and reproducibility of explant studies, as opposed to having the investigators standardize every aspect of their protocols. Seven laboratories used their preferred human tissue type and explant method to infect samples with an in-house or commonly sourced HIV-1 stock in the presence or absence of the same commonly sourced drug. The null hypothesis of no effect of the various treatment conditions on virus growth was tested. When the null hypothesis was rejected, similarities in statistically significant treatment effects across explant methods provided evidence of interlaboratory and/or intermethod reproducibility in explant experimental results. Conversely, differences in treatment effects across explant methods indicated a need for either standardization of explant methodology or thorough characterization of the observed differences. A multisite study design was used to evaluate the conditions that could affect virus growth in explants and subsequent interpretation of the efficacy of a candidate microbicide (PRO 2000). These variables included (i) source/type of assay reagents (virus stocks and medium), (ii) tissue type (cervical, rectal, or tonsil tissue), (iii) how the tissue was cultured, (iv) HIV isolate (HIV-1Ba-L and clinical isolates of differing clades), and (v) PRO 2000 concentration.
MATERIALS AND METHODS
Viruses.
Central virus stocks were provided by the Division of AIDS Viral Quality Assurance Laboratory (VQA Lab). Each laboratory was sent central stocks of HIV-1Ba-L (VQA Ba-L [VQAB]) to be compared to the usual laboratory HIV-1Ba-L stock (in-house Ba-L [IHB]). Laboratories were also sent aliquots of four clinical HIV-1 isolate subtypes, namely, UG273 (clade A), DJ263 (clade A/G recombinant), SE364 (clade C), and UG268 (clade C). All viral stocks were kept at −70°C and thawed to room temperature. Immediately after thawing, the contents of each vial were mixed and the vial was placed on wet ice prior to use. Cervical, rectal, and tonsil tissue explants were exposed overnight to VQAB or viral subtypes at 104, 103, and 103 50% tissue culture infective doses (TCID50), respectively. IHB stocks were tested at 104 TCID50 for all laboratories, with the exception of laboratory C, where tissue was exposed to 5 × 104 TCID50. The laboratories were asked to use virus titers in their explant tissues that routinely gave sufficient levels of infection in their virus controls. Thus, tissue explants were inoculated with identical, centrally distributed samples of HIV-1Ba-L at a TCID50 of 103 for tonsil and rectal tissue and 104 for cervical tissue, based on previously established HIV-1 susceptibilities of these tissues.
Media.
All laboratories were asked to test a common medium (VQA Lab medium [VQAM]) alongside their usual in-house medium (IHM). The different media used are described in Table 1 (1, 2, 4, 8, 9, 11, 15-17).
TABLE 1.
Description of explant media and p24 methods used for individual laboratories and VQA Lab
| Lab code (reference) | Tissue type | Polarized | Tissue size (mm)a | Base mediumb | Serum or additivesc | Antibiotic(s)d | p24 assay kit |
|---|---|---|---|---|---|---|---|
| VQA Labe | NA | NA | NA | R | 20% FBS, GL | Gent, Amp | Beckman Coulter |
| A (9) | Cervical | No | 3 × 3 × 2 | R | 10% FBS, GL | PS | Beckman Coulter |
| B (20) | Cervical | No | 3 × 3 × 2 | D | 10% HuAB, GL | PS | Perkin Elmer |
| C (4) | Stimulated cervical | Yes | 5 × 5 × 3 | D | 10% HuAB, 100 U/ml IL-2 | PS | NCIf |
| D (15) | Tonsil | No | 2 × 2 × 2 | R | 15% FBS, GL, N | Gent, F | Beckman Coulter (kinetic) |
| E (11) | Cervical | No | 3 × 3 × 3 | R | 20% FBS, GL | Gent, Amp | Zeptometrix |
| F (8) | Rectal | No | 3 × 3 × 2 | R | 10% FBS, GL | Z, F | NCIf |
| G (2, 16, 17)g | Cervical (PBMC coculture) | Yes | 5 × 5 × 3 | R | 20% FBS, GL | PS | Perkin Elmer |
Presented as length by width by height. NA, not applicable.
R, RPMI 1640; D, Dulbecco's modified Eagle medium.
GL, 2 to 4 mM l-glutamine; HuAB, human AB serum; N, 1× nonessential amino acids (100×); FBS, fetal bovine serum; IL-2, interleukin-2.
Gent, 50 μg/ml gentamicin sulfate; Amp, 50 μg/ml ampicillin; PS, 100 U/ml penicillin plus 100 μg/ml streptomycin; Z, 50 g/ml Zosyn; F, amphotericin B (Fungizone).
VQA Lab analyzed matched culture supernatants for p24 measurements from all laboratories and provided VQAM.
HIV-1 p24 antigen capture assay of AIDS Vaccine Program, NCI-Frederick Cancer Research and Development Center.
Laboratory G followed the published method, with the following modifications: HIV-1 transmission across the mucosa was determined by measuring HIV-1 p24 antigen in supernatants collected from PBMC cultures maintained in the bottom chamber of a transwell system.
Microbicide candidate.
Each laboratory was supplied with an aqueous stock of PRO 2000 (40 mg/ml), an unformulated naphthalene sulfonate polymer, generously provided by Endo Pharmaceuticals Solutions, Inc. (Lexington, MA). PRO 2000 was diluted using culture media and tested at 5, 50, and 500 μg/ml (final concentrations).
Explant tissue.
All human tissue samples were collected under institutional review board-approved protocols at each participating institution. One to three donor samples of cervical (laboratories A, B, C, E, and G), tonsil (laboratory D), or rectal (laboratory F) tissues were used by each laboratory. Explant tissue punch sizes and polarization treatment are listed in Table 1.
Explant assays.
Each of the seven laboratories performed tissue infection and harvesting of culture supernatants by using their chosen tissue explant methods (Table 1). Each condition was tested in duplicate to quadruplicate according to each laboratory's protocol. For all laboratories, supernatants were sampled following viral exposure before washing, at the final wash following virus removal, and then every 2 to 3 days during culture for a maximum of 15 days. Supernatants were stored at −20°C, with the exception of laboratory C, where supernatants were stored at −80°C, prior to evaluation for viral infection by the presence of p24 by enzyme-linked immunosorbent assay (ELISA).
Study design.
Using their usual tissue explant method, all participating laboratories completed three substudies as follows: (i) source of virus/medium evaluated, IHB/IHM, VQAB/IHM, IHB/VQAM, and VQAB/VQAM; (ii) evaluation of a candidate microbicide, where VQAB was compared to VQAB plus PRO 2000 (5, 50, or 500 μg/ml); and (iii) susceptibility of tissue explants to different HIV-1 isolates, where VQAB was compared to UG273 (clade A), DJ263 (clade A/G recombinant), SE364 (clade C), and UG268 (clade C). Each laboratory was asked to submit raw optical density readouts and calculated p24 results for statistical analysis. In addition, for study i, the participating laboratories were requested to dispatch matched aliquots of culture supernatants for independent determination of p24 levels by VQA Lab.
Statistical methods. (i) Definition of SOFT.
SOFT is presented as a cross-sectional index from a growth curve that is reflective of the virus growth achieved in the assay. SOFT was formulated to define the time point at which high, and in some cases exponential, rates of virus growth have been achieved and no further biologically significant increases in virus growth are apparent. SOFT is the last time point when the increase in virus concentration between two consecutive time points is greater than the square root of the sum of sequential changes in virus concentration for the entire assay.
Growth of HIV-1 can be divided into four main phases: lag time (no growth), an exponential growth phase, a stationary phase, and a decline phase (10, 32). SOFT is presented here as an estimation of the start of the stationary phase of virus growth, after the exponential growth phase has been achieved and prior to the onset of the decline phase (10, 32). SOFT is defined as the last time point (k) when the following is true:
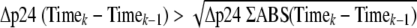 |
SOFT can be described as “one-way,” as it is set only by significant increases in virus growth (see Supplement S1 in the supplemental material).
(ii) Comparison of SOFT to other endpoint methods.
A variety of endpoints were determined for growth curves from a subsample of assays using centrally provided virus and IHM (see Supplement S2 in the supplemental material). The VQAB/IHM assays were run over 12 to 15 days, and endpoints compared virus growth (p24 pg/ml) at three time points, namely, (i) SOFT, (ii) day 12, (iii) and day 15, and for the following two parameters of the growth curve: (iv) the slope of the virus growth curve, calculated using a second-order polynomial equation (PROC NLIN; SAS/STAT, version 9.1); and (v) the AUC, approximated using the trapezoidal rule (34). It has been suggested to limit slope measurements to the linear part of the virus growth curve, where the linear portion is determined by visual inspection of each curve (33). Since the aim of this research was to provide an objective single measure of virus growth, a nonlinear formula was applied to the entire growth curve to calculate the slope value. The trapezoidal rule was used to estimate the AUC of the function f(x) by calculating the total area of adjacent trapezoid shapes. SOFT, day 12, and day 15 endpoints were the p24 concentrations measured on these respective days, the slope endpoint was a measure of the rate of p24 production, and the AUC endpoint was a measure of total p24 production. Linear discriminant analysis (LDA) (PROC STEPDISC; SAS/STAT, version 9.1 [18, 28]), a multivariate statistical procedure, was used to test the ability of each endpoint to predict low (p24 concentration, <1,500 pg/ml), medium (1,500 to 20,000 pg/ml), and high (>20,000 pg/ml) levels of virus growth from the VQAB/IHM assays collected for the study arm examining the source of virus and the medium used. LDA mathematically defines a discriminant function to separate data into distinct groups. In this case, the data were the assay endpoints and the groups were low, medium, or high, depending on the virus growth for each assay. A stepwise LDA was performed to compare the abilities of each of the assay endpoints to predict virus growth. The median p24 levels for the endpoint measures SOFT, day 12, day 15, and AUC were compared for the low-, medium-, and high-level virus growth assays, using Kruskal-Wallis ANOVA with Dunn's multiple comparison test (alpha = 0.05).
(iii) SOFT used to calculate % virus inhibition.
SOFT was applied to virus growth data for drug-treated and control conditions, where the drug was PRO 2000, a candidate anti-HIV-1 microbicide. Data are presented from a cervical explant experiment from one participating laboratory where three cervical explant donor tissues were infected with HIV-1Ba-L in the presence of three concentrations of PRO 2000 (5, 50, and 500 μg/ml) and in the absence of PRO 2000 (virus control). The SOFT for each donor was set using the averaged virus control results. The % virus inhibition at each level of PRO 2000 was calculated using the formula % virus inhibition = 100 × (treatment p24 level/control p24 level).
The % virus inhibition was compared to 0% (i.e., control p24 level at SOFT) by the Wilcoxon signed-rank test. The variability in the % virus inhibition results using the SOFT, day 10, and day 14 endpoints was compared by the % coefficient of variation (%CV) [100 × (standard deviation/mean)] for replicate measurements. The %CV provided an index of variability, where a higher %CV indicates greater variability in measurements.
(iv) SOFT used to compare drug inhibition.
SOFT was applied to calculate the inhibitory effects of a potential microbicide, PRO 2000. The % virus control for PRO 2000 [(test sample p24 level/Ba-L control p24 level) × 100] was calculated at the HIV-1Ba-L control SOFT. All % virus control values of ≥100% were converted to 100% virus control for the purposes of this study. Nonlinear regression analysis (sigmoidal dose-response curve) of the % virus control measurements onto the log10 PRO 2000 concentration was used to calculate the half-maximal inhibitory concentration (i.e., IC50) of PRO 2000 for each laboratory. The effects of HIV isolate were tested by comparing the log differences in p24 released from each isolate (UG268, SE364, DJ263, and UG273) to that released from HIV-1Ba-L.
The p24 pg/ml or log10 pg/ml results were not normally distributed (Shapiro-Wilk W = 0.26 to 0.95; P < 0.0001), a prerequisite for statistical tests such as ANOVA and the t test. The nonparametric counterparts of the t test (i.e., Mann-Whitney test, Wilcoxon signed-rank test) and ANOVA (Kruskal-Wallis ANOVA with Dunn's multiple comparison test) were used to test the effects of reagent source, explant method, microbicide concentration, and HIV viral isolate on p24 release at the assay SOFT. Nonparametric statistics are routinely used to compare such non-normally distributed data as HIV RNA (21) and HIV-1 p24 (30). All comparisons of treatment effects to the HIV-1Ba-L control were made within donors, and all statistical analyses were performed using SAS (version 9.1; alpha = 0.05).
RESULTS
Seven laboratories, each using the same HIV-1 stock in their respective tissue explant assays, collected supernatants for up to 15 days following virus exposure and performed a p24 ELISA to quantify virus replication. Participating laboratories submitted p24 results for each arm of the study, i.e., (i) source of HIV-1Ba-L and medium used (laboratories A and C to G), (ii) concentration of PRO 2000 (laboratories A to G), and (iii) isolate type (laboratories A to G). In-house calculated p24 values were equivalent to those measurements made by a central laboratory (Pearson r = 0.82), and the standard curve formula used by the laboratories (e.g., linear versus nonlinear) was found to affect only low p24 measurements (<100 pg/ml) (see Supplement S3 in the supplemental material); thus, laboratory-calculated p24 values were used in this analysis. SOFT was applied to all assays using laboratory-calculated p24 values.
Comparison of single measures of virus growth.
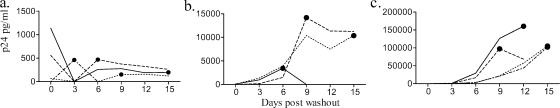
The VQAB/IHM assays with unstimulated cervical tissue (labs A, E, and G), stimulated cervical tissue (lab C), tonsil tissue (lab D), and rectal tissue (lab F) were used to compare SOFT to a selection of growth curve endpoints from the source of HIV-1Ba-L/medium study arm (see Supplement S2 in the supplemental material). VQAB/IHM assays were grouped according to the maximum p24 level achieved during the 15 days of culture, into low (Fig. 1a), medium (Fig. 1b), and high (Fig. 1c) virus growth groups. The SOFT is presented for each VQAB/IHM virus control assay (Fig. 1a to c). The virus growth profiles varied widely in the different tissue explant models, with SOFT ranging from day 3 to day 15 (Fig. 1a to c).
FIG. 1.
SOFT determination of HIV-1Ba-L growth in VQAB/IHM tissue explants. The SOFT (•) is shown for low-yield (maximum p24 level, <1,500 pg/ml) (a), medium-yield (maximum p24 level, 1,500 to 20,000 pg/ml) (b), and high-yield (maximum p24 level, >20,000 pg/ml) (c) assays. Each line represents virus growth for each of 12 donors, where each donor is distinguished by the line pattern within each panel.
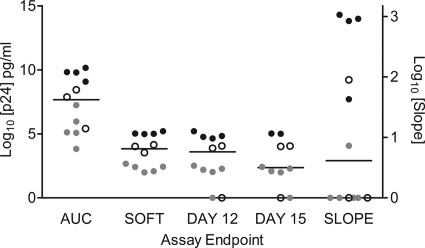
The endpoint measures of SOFT, day 12, day 15, and AUC were compared for the low-, medium-, and high-growth VQAB/IHM assays (Fig. 2). The AUC resulted in larger p24 concentration ranges (median [interquartile range] = 4.63 × 107 [4.97 × 109] pg/ml) than did the SOFT (6.84 × 103 [4.94 × 106] pg/ml), day 12 (3.90 × 104 [5.38 × 105] pg/ml), and day 15 (2.28 × 103 [3.38 × 105] pg/ml) endpoint measures [Kruskal-Wallis H = 25.39, df = 5; P < 0.01] (Fig. 2), where a larger interquartile range reflects higher interassay variability. The high p24 result for the AUC endpoint was expected, as the AUC is an estimation of the total virus growth throughout the duration of the experiment, in contrast to the other endpoint methods, where the growth on specified days (SOFT, day 12, and day 15) was calculated. There were no significant differences between median p24 results for the day 12, day 15, and SOFT endpoints (P > 0.05) (Fig. 2). The utility of slope, AUC, day 12, and day 15 as predictors of low, medium, and high p24-yielding assays was reduced for the medium-yield assays, for which endpoints overlapped with both low- and high-yield assays, while the SOFT resulted in a clear separation between low, medium, and high yields for the subsample of VQAB/IHM assays (Fig. 2).
FIG. 2.
Comparison of five endpoint measures to determine HIV-1Ba-L growth in explant tissues. Viral growth determined at the endpoints SOFT, day 12, day 15, and AUC are plotted on the left y axis (log10 p24 pg/ml), while the slope was plotted on the right y axis for low ( )-, medium (○)-, and high (•)-yielding assays. Horizontal lines indicate the median of each endpoint. Note that for two of the high-level growth assays (laboratory D, donors 1 and 2), growth was measured for only 12 days.
)-, medium (○)-, and high (•)-yielding assays. Horizontal lines indicate the median of each endpoint. Note that for two of the high-level growth assays (laboratory D, donors 1 and 2), growth was measured for only 12 days.
LDA was used to test the ability of each endpoint to predict low, medium, and high levels of growth for the subsample of VQAB/IHM assays, where a high, significant R2 value describes greater accuracy in prediction. The LDA procedure assumed a multivariate normal distribution of endpoint measures within each assay yield group (18). Since it has been recommended to log transform certain types of biological data prior to parametric analyses (31), p24 (pg/ml) at SOFT was entered into the LDA as both a linear and a log-transformed variable. The results of the LDA are presented in Table 2, where the significance level (P value) of an F test from an analysis of covariance indicated the ability of each endpoint to predict assay virus growth. The endpoints that were not the focus of the analysis were entered into the analysis of covariance as covariates. The squared partial correlations for each endpoint are given (R2) (Table 2), controlling for the effects of the other endpoints in the model. The SOFT and log-transformed SOFT metrics were the most effective predictors of low, medium, and high p24-yielding assay group assignment, while day 15 was the least effective predictor of p24 yield (Table 2).
TABLE 2.
LDA of various endpoint measuresa
| Endpoint measure of virus growth | R2 | F value | Probability (F) |
|---|---|---|---|
| SOFT | 0.93 | 57.24 | <0.0001 |
| Log SOFTb | 0.96 | 140.02 | <0.0001 |
| Day 12 | 0.67 | 9.12 | 0.007 |
| Day 15 | 0.38 | 2.71 | 0.120 |
| AUC | 0.60 | 6.76 | 0.016 |
| Slope | 0.68 | 9.51 | 0.006 |
Results of LDA in which virus endpoint measures of viral growth (pg/ml p24) in explant tissues were differentiated between low (five assays), medium (three assays), and high (four assays).
Due to multicolinearity between the linear and logarithmic SOFT results, the log10 p24 at the SOFT (log SOFT) was entered into a separate LDA.
SOFT used to compare the effects of tissue type and culture model on HIV-1Ba-L infection.
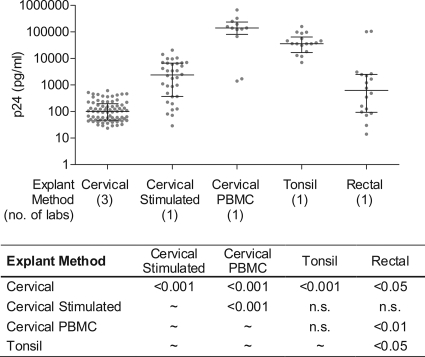
The p24 level at the assay SOFT, across tissue types and methods, is presented in Fig. 3. Virus production was found to vary widely according to tissue type and explant culture method (Kruskal-Wallis ANOVA; P < 0.0001) (Fig. 3). Using all measurements of HIV-1Ba-L growth (in the absence of PRO 2000), irrespective of virus/medium source, release of p24 at SOFT was lowest for the laboratories using (unstimulated) cervical tissue and highest for laboratories using either cervical tissue with peripheral blood mononuclear cell (PBMC) coculture or tonsil tissue. There were no statistical differences in virus yield between stimulated cervical explants and rectal or tonsil explants. Comparison of the three model systems used for cervical tissue demonstrated that unstimulated cervical tissue resulted in the lowest viral growth, while the use of a PBMC coculture resulted in the most p24 release. Stimulated cervical tissue resulted in lower growth than the use of a PBMC coculture but in higher growth than unstimulated cervical tissue. A similar trend was observed if only the best growth condition (VQAB/IHM) was evaluated, except that the difference between stimulated cervical tissue and cervical tissue with PBMC coculture was not statistically significant, and the difference in viral growth between rectal tissue and cervical tissue with PBMC coculture or tonsil tissue increased in statistical significance (P < 0.001) (data not shown).
FIG. 3.
Effect of explant method type on virus yield. Virus growth (p24) was compared across explant methods by Kruskal-Wallis ANOVA (P < 0.0001). The p24 level at the assay SOFT is presented for HIV-1Ba-L grown in cervical (14 donors), stimulated cervical (6 donors), cervical tissue-PBMC coculture (2 donors), tonsil (4 donors), and rectal (4 donors) explant experiments, sourced from IHB, IHM, VQAB, and VQAM conditions. The median and interquartile range are indicated for each method. The table shows the probability values for significantly different pairwise comparisons (Dunn's multiple comparison test). n.s., not significant.
SOFT used to measure virus inhibition.
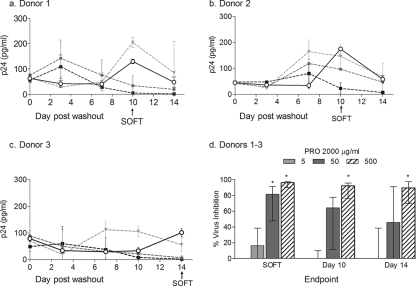
An example of viral inhibition by PRO 2000 on HIV-1Ba-L growth is presented using unstimulated cervical tissue explants (n = 3) to demonstrate the utility of SOFT in a compound screening program (Fig. 4). Unstimulated cervical tissue explants at SOFT were found to have less p24 production (median, 101.1 pg/ml) than rectal (626.9 pg/ml), tonsil (35,800 pg/ml), stimulated cervical (2,381 pg/ml), and cervical tissue-PBMC coculture (139,950 pg/ml) explant models (P < 0.0001; Kruskal-Wallis ANOVA and Dunn's multiple comparison test) (Fig. 3). Since less viral replication was likely to result in a reduced ability to detect inhibition of virus, SOFT was used to calculate virus inhibition in the unstimulated cervical tissue. For two donors (donors 1 and 2), HIV-1Ba-L growth in the absence of drug increased through days 3 to 10 but then decreased by day 14 (Fig. 4a and b), while in the third donor (donor 3), viral growth increased through days 7 to 14 (Fig. 4c). SOFT accommodated these interdonor variations in virus growth by setting the time point for comparison for donors 1 and 2 at day 10, prior to the decline in virus growth, and at day 14 for donor 3, where delayed onset of virus replication was exhibited. Using SOFT, the % virus inhibition ranged from 80.6 to 99.0% for the highest concentration of drug (500 μg/ml) across replicate measurements and donors (Fig. 4d), while a wider range in results was found using either the day 0 (61.2 to 96.9%) or day 14 (41.3 to 99.0%) endpoint measure. The % virus inhibition results using all endpoints were more variable for the 5- and 50-μg/ml PRO-2000 conditions, which may be due to these concentrations being at the threshold of efficacy for this compound (Fig. 4d). Although a dose-response effect was found for % virus inhibition using all three endpoint measures (Wilcoxon signed-rank test; P < 0.05), less variability was observed with SOFT (%CV = 54.9%) than with day 10 (100.2%) and day 14 (95.5%) (Fig. 4d). Based on these results, SOFT was applied for the remaining comparisons.
FIG. 4.
Application of SOFT analyses to “low” viral growth in unstimulated cervical tissue for donors 1 (a), 2 (b), and 3 (c) and % virus inhibition across donors (d). Data shown represent the viral growth in the absence (○; solid lines) or presence (dotted lines) of the candidate microbicide PRO 2000 (5 μg/ml [ ], 50 μg/ml [
], 50 μg/ml [ ], and 500 μg/ml [▪]). Medians and interquartile ranges for replicate measurements are shown. The SOFT (↑) for donors 1 and 2 occurred at day 10, while that for donor 3 occurred at day 14. (d) Inhibition of viral infection determined using different endpoint analyses. The % virus inhibition results were compared for PRO 2000 (5, 50, and 500 μg/ml) for three donor cervical tissue explant tissue samples from one laboratory. Virus inhibition (%) was calculated using p24 measurements for (i) SOFT, (ii) day 10, and (iii) day 14 endpoint analyses. The median and interquartile ranges across replicates and donors are shown. Virus inhibition for each treatment was compared to that for the Ba-L control at SOFT, day 12, and day 14 (Wilcoxon signed-rank test; *, P < 0.05; **, P < 0.01).
], and 500 μg/ml [▪]). Medians and interquartile ranges for replicate measurements are shown. The SOFT (↑) for donors 1 and 2 occurred at day 10, while that for donor 3 occurred at day 14. (d) Inhibition of viral infection determined using different endpoint analyses. The % virus inhibition results were compared for PRO 2000 (5, 50, and 500 μg/ml) for three donor cervical tissue explant tissue samples from one laboratory. Virus inhibition (%) was calculated using p24 measurements for (i) SOFT, (ii) day 10, and (iii) day 14 endpoint analyses. The median and interquartile ranges across replicates and donors are shown. Virus inhibition for each treatment was compared to that for the Ba-L control at SOFT, day 12, and day 14 (Wilcoxon signed-rank test; *, P < 0.05; **, P < 0.01).
SOFT used for evaluation of a candidate microbicide.
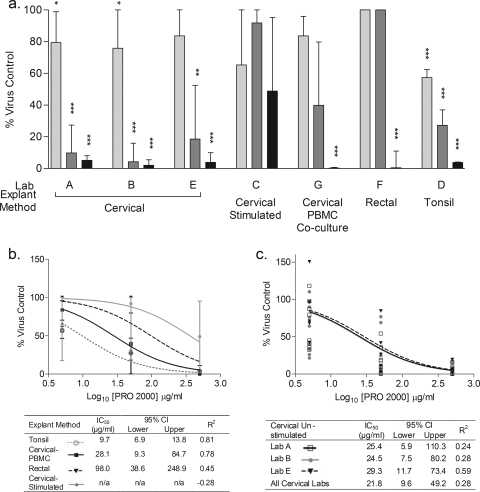
The % virus inhibition of PRO 2000, a candidate microbicide, was calculated using SOFT for donor-matched, untreated HIV-1Ba-L control results for each laboratory and explant tissue type (Fig. 5). With the exception of stimulated cervical tissue explants, all tissue types demonstrated a dose-dependent effect of virus inhibition by PRO 2000 (Fig. 5a). Significant viral inhibition was observed at (i) 500 μg/ml PRO 2000 (cervical, cervical tissue-PBMC coculture, rectal, and tonsil samples) (P < 0.001; Wilcoxon signed-rank test), (ii) 50 μg/ml PRO 2000 (unstimulated cervical and tonsil tissue in labs A, B, and D [P < 0.001] and unstimulated cervical tissue in lab E [P < 0.01]), and (iii) 5 μg/ml PRO 2000 (unstimulated cervical tissue in labs A and B [P < 0.05] and tonsil tissue [P < 0.001]). The sigmoidal dose-response curve explained 24 to 81% of the variance across all explants, with the exception of the results from the stimulated cervical tissue experiment, where the data did not conform to a dose-response curve (Fig. 5b and c, tables). The IC50 of PRO 2000 varied considerably across tonsil, cervical tissue-PBMC coculture, rectal, and stimulated cervical explants (9.7 to 98.0 μg/ml) (Fig. 5b); greater consistency in IC50 measurements was found for the three laboratories testing unstimulated cervical explants (24.5 to 29.3 μg/ml) (Fig. 5c).
FIG. 5.
Inhibition of HIV-1Ba-L infection by PRO 2000. Inhibition of viral infection by PRO 2000 was determined for five explant tissue types (cervical, stimulated cervical, cervical tissue-PBMC coculture, tonsil, and rectal methods) in seven laboratories (A to G). (a) Median % virus control values were compared across three concentrations of PRO 2000 (5 μg/ml [ ], 50 μg/ml [
], 50 μg/ml [ ], and 500 μg/ml [
], and 500 μg/ml [ ]) to 100% virus control, where significant effects of PRO 2000 are indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (Wilcoxon signed-rank test). Data represent the median and interquartile range of % virus control for each tissue type. (b) The IC50 for PRO 2000 was calculated using the amount of p24 released at the assay SOFT and derived by nonlinear regression analysis for each tissue type (stimulated cervical, cervical tissue-PBMC coculture, rectal, and tonsil tissue). (c) The IC50 for PRO 2000 was calculated using the amount of p24 released at the assay SOFT and derived by nonlinear regression analysis for each laboratory using unstimulated cervical tissue (labs A, B, and E). Data represent the replicate % virus control for each lab. The embedded tables in panels b and c outline the individual IC50 (μg/ml) values, upper and lower 95% confidence intervals, and the goodness of fit (R2) of each curve. n/a, values could not be estimated with confidence from the nonlinear regression analysis.
]) to 100% virus control, where significant effects of PRO 2000 are indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (Wilcoxon signed-rank test). Data represent the median and interquartile range of % virus control for each tissue type. (b) The IC50 for PRO 2000 was calculated using the amount of p24 released at the assay SOFT and derived by nonlinear regression analysis for each tissue type (stimulated cervical, cervical tissue-PBMC coculture, rectal, and tonsil tissue). (c) The IC50 for PRO 2000 was calculated using the amount of p24 released at the assay SOFT and derived by nonlinear regression analysis for each laboratory using unstimulated cervical tissue (labs A, B, and E). Data represent the replicate % virus control for each lab. The embedded tables in panels b and c outline the individual IC50 (μg/ml) values, upper and lower 95% confidence intervals, and the goodness of fit (R2) of each curve. n/a, values could not be estimated with confidence from the nonlinear regression analysis.
Tissue responses vary according to HIV-1 isolate.
The rates of viral growth of four primary isolates of HIV-1 (i.e., UG268, SE364, DJ263, and UG273) were compared to the growth of HIV-1Ba-L in each of the tissue explant models. Release of p24 was found to be lower for clade C isolates, UG268 (−0.73 log10 p24; P < 0.001) and SE364 (−1.05 log10 p24; P < 0.001), than for HIV-1Ba-L across all explant assays. The clade A isolate (UG273) resulted in less p24 production than that by HIV-1Ba-L for cervical (−0.24 log10 p24 pg/ml; P < 0.05) and tonsil (−0.42 log10 p24 pg/ml; P < 0.001) tissues. There was no difference in p24 production between A/G recombinant isolate DJ263 and HIV-1Ba-L (P is nonsignificant) for any of the tissue types tested.
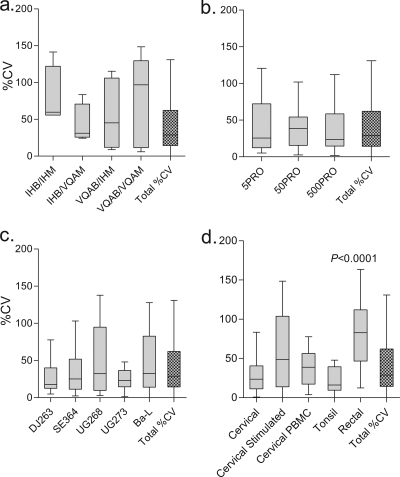
Intra-assay variability across experimental conditions.
Intra-assay variability was measured using the %CV for all replicate p24 measurements, where a higher %CV indicates greater variability for replicate measurements. The %CVs were compared across assays (Fig. 6a to d). There was no difference in %CV across experimental conditions (virus/medium source, use of PRO 2000, or isolate type) (Kruskal-Wallis ANOVA; P = 0.26 to 0.98) (Fig. 6a to c), with a median %CV across all experimental conditions of 28.8%. Intra-assay reproducibility did vary across explant tissues and model types, where greater variability was found using the rectal tissue explant method than the total %CV for the study (Mann-Whitney test; P < 0.0001) (Fig. 6d). Tonsil and unstimulated cervical tissue explant cultures resulted in the most reproducible explant virus growth (i.e., median %CV) (Fig. 6d).
FIG. 6.
Intra-assay variability across experimental conditions. Intra-assay variability was measured using the %CV for replicate p24 measurements. Data shown represent the median %CV and interquartile range for p24 release determined at the assay SOFT for seven laboratories using their preferred explant method under each experimental condition ( ), alongside the total %CV for the study (
), alongside the total %CV for the study ( ). (a) Source of virus/medium; (b) effect of microbicide PRO 2000; (c) growth of different HIV-1 isolates; (d) tissue type. Significant differences were determined using the Mann-Whitney test.
). (a) Source of virus/medium; (b) effect of microbicide PRO 2000; (c) growth of different HIV-1 isolates; (d) tissue type. Significant differences were determined using the Mann-Whitney test.
DISCUSSION
While explant cultures are currently used to study HIV pathogenesis and transmission in tissues relevant to in vivo sites of infection, they are also being used to evaluate the antiviral activity of candidate topical microbicides prior to subsequent studies in animals and humans. Given the variety of protocols currently used in tissue explant studies (e.g., see references 1, 2, 4, 6, 8, and 12), the first aim of this study was to identify a uniform method for analyzing, comparing, and presenting virus growth curve data. To this end, the SOFT metric provides such a method. SOFT was developed to enable interlaboratory comparisons of HIV-1 replication across explant methods (with cervical, rectal, and tonsil tissues) by determining the endpoint to compare virus growth between control and microbicide-treated tissues. Comparison of virus growth between control and treated tissues at time points prior to the accelerated growth phase or after virus growth has tapered off could underestimate the level of virus inhibition exhibited by a microbicide candidate. Comparison of virus growth at SOFT provides an “ideal” endpoint comparison for each growth curve by excluding the variability in measurements at time points prior to SOFT, when growth in the virus control may be at low or nondetectable levels, or after SOFT, when the system may no longer be able to support viral growth. SOFT aims to provide a sensitive indicator of drug efficacy to be used early in a drug screening program, prior to additional confirmatory testing, when the risks of making a type II error (i.e., false-negative result when a potentially efficacious compound is excluded) outweigh the risks of a type I error (i.e., false-positive result when a potentially ineffective compound is included).
An evaluation of calculation time, ease of performance, availability of resources, and other factors that may be important to a laboratory should be considered prior to the adoption of any endpoint method. Although the AUC and slope calculations proved to be effective predictors of virus growth, the complexity of these calculations may reduce their utility for research teams without access to statistical software necessary to compute nonlinear regressions and trapezoidal functions. In contrast, SOFT can readily be calculated and thus allows direct comparison of growth curves by standard statistical tests (e.g., ANOVA, t test, Kruskal-Wallis test, and Mann-Whitney test). A multivariate comparison (LDA) of various endpoint approaches further supported the application of SOFT as an objective method for evaluating virus replication. In addition, SOFT neither over- nor underestimated virus growth compared to other endpoints and provided a measure of growth that reliably reflected the virus yield of the assay.
An added advantage to the identification of a robust assay endpoint is the ability to use it for identifying key parameters that, once standardized, can lead to more reproducible data between laboratories. As a second aim of this study, SOFT was used to compare conditions that could affect virus growth in explant assays. The variables found to affect virus growth in the explants studied here included (i) source/type of assay reagents, (ii) tissue type, (iii) how the tissue was cultured (i.e., tissue stimulation), (iv) HIV-1 isolate used, and (v) microbicide concentration. In contrast, laboratory measurement of p24 protein in culture supernatants was found to be generally consistent, regardless of assay-specific factors such as ELISA manufacturer, standard curve range, method of ELISA analysis, or the laboratory running the analysis.
Since HIV-1 replication can be monitored in tissue culture by a variety of methods (including measurement of p24 protein), different patterns of virus growth can be observed depending on whether virus is measured in the cell-free or cell-associated compartments of cultured cells and tissues (3, 23). In the microbicide field, researchers rely primarily on p24 protein levels in culture supernatants as a measure of virus replication over the course of the explant study. Using this approach for the current study, greater viral replication was observed in all explant models by use of the centrally provided VQAB stock and the laboratory's own IHM formulations. Use of a common virus stock provided by a central repository increased p24 production for all tissue types and methods, but medium formulations routinely used by each laboratory were found to be better suited to the laboratory's preferred explant method.
Perhaps the largest effect on p24 production was the tissue type and model used. For example, the least viral replication was observed using unstimulated cervical tissue. When cervical tissue was immune stimulated or cocultured with PBMC, viral replication was noticeably increased, by 10- or 1,000-fold, respectively. Use of either rectal or tonsil tissue also significantly increased viral replication, suggesting that viral replication can be significantly affected by endogenous stimulation or the concentration and distribution of target cells present in each tissue type or model system.
The absolute quantity of p24 protein produced by tissue explant cultures did not appear to correlate with the ability to detect a drug effect. The candidate microbicide PRO 2000 was found to inhibit virus growth for the majority (four of five methods) of explant methods used by the seven laboratories (i.e., cervical, tonsil, rectal, and cervical tissue-PBMC coculture explants), where p24 production at SOFT ranged from 14 to 670,300 pg/ml. Cultures releasing both high (tonsil) and low (cervical) p24 levels produced reliable drug effects, while another high-p24-release explant model (stimulated cervical tissue) failed to demonstrate any drug effect in this study.
Given the lack of a standard endpoint for virus growth in explant studies, few laboratories to date have reported microbicide activity in terms of an IC50. In fact, only one laboratory has published an IC50 for PRO 2000 in cervical tissue, at ∼80 μg/ml (9). In the current study, the IC50 for PRO 2000 varied widely among the tissue types evaluated (9.7 to 98.0 μg/ml) but remained fairly consistent within an explant method, as demonstrated by the three laboratories working with cervical tissue (24.5 to 29.3 μg/ml). These data indicate that inhibitory concentrations of PRO 2000 in explant tissues are higher than those in cell lines and primary T cells or macrophages (IC50s ranging from 0.7 to 12.8 μg/ml) (20, 27, 29), suggesting that more drug is required to penetrate tissue than to penetrate monolayers or cell suspensions. Comparing the same tissue type used in different model systems, such as cervical culture and cervical tissue-PBMC coculture, PRO 2000 was also consistently active (24.5 to 29.3 μg/ml compared to 28.1 μg/ml, respectively), but not compared with the stimulated cervical (IC50 could not be determined) or rectal (9.7 μg/ml) model. This suggests that drug IC50s could be compared between laboratories using the same tissue type under the same model conditions, but not necessarily between laboratories using either different explant tissues or different assay methods.
Although the HIV-1 isolate used had a significant impact on virus growth (clade C strains resulted in less virus growth and clades A and A/G showed comparable growth to that of HIV-1Ba-L), it is important to note that the explant models examined in this study supported growth of viruses that represent only a subsample of isolates available to microbicide researchers (https://www.aidsreagent.org/Index.cfm). These results support the inclusion of non-HIV-1Ba-L isolates in future explant studies by determining microbicide activity against more clinically relevant, non-laboratory-adapted strains of HIV-1.
While an intra-assay variability of <20% is generally considered acceptable for in vitro assays (24), this study found a wide range for explant intra-assay %CVs (0.8 to 163.4%), where rectal tissue measurements were the most variable. High variability in rectal explant tissues may be due to such factors as susceptibility of different tissue areas to infection (immune cell pockets, areas with large M-cell populations, etc.) or friability of tissue that is of a less structured type (1, 8). However, even though the high intra-assay variability reduced the statistical power of treatment comparisons, consistent drug and isolate effects were found. Additionally, statistical power was not consistent across explant methods; cervical explants used by three laboratories provided greater statistical power for drug and isolate effects than did other explant methods tested by only a single laboratory (i.e., stimulated cervical, cervical tissue-PBMC coculture, tonsil, and rectal methods).
In summary, in order to improve the quality of data from laboratories using tissue explants to evaluate topical microbicides, this study addressed the following two aims: (i) identification of a uniform method for analyzing, comparing, and presenting virus growth curve data; and (ii) identification of those key parameters that, once standardized, will lead to more reproducible data between laboratories. A comparison of various endpoint approaches demonstrated that the SOFT can provide a measure of virus growth that is easily calculated, reliably indicates the virus yield of the assay, and allows direct comparison of growth curves by standard statistical tests. In terms of key assay parameters, use of a common virus stock was shown to be most important for improving virus growth among the tested explant models. In addition, as the data with primary strains indicated, identification of clinically relevant HIV-1 clades that are able to replicate well in tissue explants will be important for future microbicide studies. Although the tissue type and model used had the greatest impact on virus growth, this study also demonstrated that a reliable drug effect can be observed in tissues with low or high p24 protein levels and that IC50s can be compared between laboratories using the same tissue type. Finally, wide intra-assay variability may be inherent to tissue explant studies, where the extent of measurement variability found here depended upon the tissue type used. Further studies using a variety of tissue types are recommended to determine the number of donors and replicates necessary for adequate statistical power in comparisons of HIV inhibition between compounds.
Reproducible tissue explant research has the potential to provide an early indicator of drug efficacy, thus accelerating the best microbicide candidates into subsequent animal studies and, ultimately, human clinical trials. It is hoped that the identification of a reliable endpoint and other key methodological parameters will increase future standardization and comparability of preclinical explant assays.
Supplementary Material
Acknowledgments
This work was part of the Microbicide Quality Assurance Program (MQAP), supported by the following contracts from the U.S. National Institutes of Health: NICHD N01-HD-3-3350 (September 2003 to January 2007) and NIAID N01-AI-33350 (February 2007 to August 2008).
The views of the authors do not necessarily reflect those of the funding agencies. The authors have no conflicts of interest or financial interests regarding the work presented.
We thank the following: A. Profy, Endo Pharmaceuticals Solutions, Inc. (Lexington, MA), for providing PRO 2000; Brigitte E. Sanders-Beer (Bioqual, Inc., Rockville, MD) for her leadership of the MQAP from 2003 thru 2005; Donald Brambilla (New England Research Institutes, Watertown, MA) for helpful discussions at the initial stages of this research; and Timothy A. Green (Branch Chief of the Quantitative Sciences and Data Management Branch, Centers for Disease Control and Prevention, Atlanta, GA) for helpful comments on the manuscript. We also thank the following NIH program staff for their scientific expertise and guidance in relation to the MQAP studies: Kailash Gupta (NIAID), Fulvia Veronese (Office for AIDS Research to NIAID), and James Turpin (NIAID).
Investigators using explant assays in their respective microbicide-related research were invited to participate in the design of the studies described in this paper. Technical staff participated in regular conference calls and discussions of the standardized procedures as the studies were implemented and data were acquired. The author list includes the principal investigators from the participating laboratories and their respective technical representatives. Since participation in the MQAP was voluntary, it should be noted that the explant studies described in this paper were facilitated by the infrastructure, resources, and microbicide-related grant support available at each of the following participating laboratories (this order does not correspond to the randomly assigned lab identification letters): National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (NIH Intramural Program); Rush University Medical Center, Department of Immunology/Microbiology, Chicago, IL (Virology Quality Assurance [VQA] Program supported by NIAID grant N01-AI-50044); St. George's, University of London, London, United Kingdom (DFID/MRD to R.J.S. as part of the Microbicide Development Programme); Magee-Womens Research Institute, University of Pittsburgh, School of Medicine, Department of Obstetrics, Gynecology, and Reproductive Sciences, Pittsburgh, PA (NIH grant U01 AI068633-07 to C.S.D.); and Center for Prevention Research at David Geffen School of Medicine, University of California-Los Angeles AIDS Institute (NIH CFAR Mucosal Immunology Core grant AI 28997 and NIH IP/CP U19 grant AI 060614 to P.A.A.).
Footnotes
Published ahead of print on 2 September 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Abner, S. R., P. C. Guenthner, J. Guarner, K. A. Hancock, J. E. Cummins, Jr., A. Fink, G. T. Gilmore, C. Staley, A. Ward, O. Ali, S. Binderow, S. Cohen, L. A. Grohskopf, L. Paxton, C. E. Hart, and C. S. Dezzutti. 2005. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J. Infect. Dis. 192:1545-1556. [DOI] [PubMed] [Google Scholar]
- 2.Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475-479. [DOI] [PubMed] [Google Scholar]
- 3.Collman, R., N. F. Hassan, R. Walker, B. Godfrey, J. Cutilli, J. C. Hastings, H. Friedman, S. D. Douglas, and N. Nathanson. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 170:1149-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummins, J. E., Jr., J. Guarner, L. Flowers, P. C. Guenthner, J. Bartlett, T. Morken, L. A. Grohskopf, L. Paxton, and C. S. Dezzutti. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother. 51:1770-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischetti, L., S. M. Barry, T. J. Hope, and R. J. Shattock. 2009. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 28:319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher, P., Y. Kiselyeva, G. Wallace, J. Romano, G. Griffin, L. Margolis, and R. Shattock. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 79:11179-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher, P. S., J. Elliott, J. C. Grivel, L. Margolis, P. Anton, I. McGowan, and R. J. Shattock. 2006. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 20:1237-1245. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher, P. S., G. S. Wallace, P. M. Mesquita, and R. J. Shattock. 2006. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology 3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flint, S. J., L. W. Enquist, V. R. Racaniello, and A. M. Skalka (ed.). 2004. Principles of virology: molecular biology, pathogenesis, and control of animal viruses, 2nd ed. ASM Press, Washington, DC.
- 11.Fox-Canale, A. M., T. J. Hope, J. Martinson, J. R. Lurain, A. W. Rademaker, J. W. Bremer, A. Landay, G. T. Spear, and N. S. Lurain. 2007. Human cytomegalovirus and human immunodeficiency virus type-1 co-infection in human cervical tissue. Virology 369:55-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 13.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grivel, J. C., J. Elliott, A. Lisco, A. Biancotto, C. Condack, R. J. Shattock, I. McGowan, L. Margolis, and P. Anton. 2007. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. AIDS 21:1263-1272. [DOI] [PubMed] [Google Scholar]
- 15.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:344-346. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, P., K. B. Collins, D. Ratner, S. Watkins, G. J. Naus, D. V. Landers, and B. K. Patterson. 2002. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J. Virol. 76:9868-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, P., D. Ratner, B. K. Patterson, K. Kulka, L. C. Rohan, M. A. Parniak, C. E. Isaacs, and S. Hillier. 2006. Use of frozen-thawed cervical tissues in the organ culture system to measure anti-HIV activities of candidate microbicides. AIDS Res. Hum. Retrovir. 22:419-424. [DOI] [PubMed] [Google Scholar]
- 18.Khattree, R. 2000. Multivariate data reduction and discrimination with SAS® software. SAS Institute Inc., Cary, NC.
- 19.Klasse, P. J., R. J. Shattock, and J. P. Moore. 2006. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 3:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lackman-Smith, C., C. Osterling, K. Luckenbaugh, M. Mankowski, B. Snyder, G. Lewis, J. Paull, A. Profy, R. G. Ptak, R. W. Buckheit, Jr., K. M. Watson, J. E. Cummins, Jr., and B. E. Sanders-Beer. 2008. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob. Agents Chemother. 52:1768-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz-Fernandez, M. A., J. Navarro, M. G. Montes, J. Cosin, J. M. Zabay, and E. Fernandez-Cruz. 1996. Quantification of low levels of human immunodeficiency virus (HIV) type 1 RNA in P24 antigen-negative, asymptomatic, HIV-positive patients by PCR. J. Clin. Microbiol. 34:404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niioka, T., T. Uno, N. Yasui-Furukori, M. Shimizu, K. Sugawara, and T. Tateishi. 2006. Identification of the time-point which gives a plasma rabeprazole concentration that adequately reflects the area under the concentration-time curve. Eur. J. Clin. Pharmacol. 62:855-861. [DOI] [PubMed] [Google Scholar]
- 23.Patterson, B. K., A. Landay, J. N. Siegel, Z. Flener, D. Pessis, A. Chaviano, and R. C. Bailey. 2002. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am. J. Pathol. 161:867-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, G. F., F. Lynn, and B. D. Meade. 2002. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 9:1235-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson, B. A., and J. Overbaugh. 2005. Basic statistical considerations in virological experiments. J. Virol. 79:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth, S., M. Monsour, A. Dowland, P. C. Guenthner, K. Hancock, C. Y. Ou, and C. S. Dezzutti. 2007. Effect of topical microbicides on infectious human immunodeficiency virus type 1 binding to epithelial cells. Antimicrob. Agents Chemother. 51:1972-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusconi, S., M. Moonis, D. P. Merrill, P. V. Pallai, E. A. Neidhardt, S. K. Singh, K. J. Willis, M. S. Osburne, A. T. Profy, J. C. Jenson, and M. S. Hirsch. 1996. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob. Agents Chemother. 40:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAS Institute, Inc. 1990. SAS/STAT users guide, version 6, 4th ed. SAS Institute Inc., Cary, NC.
- 29.Scordi-Bello, I. A., A. Mosoian, C. He, Y. Chen, Y. Cheng, G. A. Jarvis, M. J. Keller, K. Hogarty, D. P. Waller, A. T. Profy, B. C. Herold, and M. E. Klotman. 2005. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob. Agents Chemother. 49:3607-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutthent, R., N. Gaudart, K. Chokpaibulkit, N. Tanliang, C. Kanoksinsombath, and P. Chaisilwatana. 2003. p24 antigen detection assay modified with a booster step for diagnosis and monitoring of human immunodeficiency virus type 1 infection. J. Clin. Microbiol. 41:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor, R. N., K. M. Fulford, and A. Y. Huong. 1978. Comparison of kinetic and end-point diffusion methods for quantitating human serum immunoglobulins. J. Clin. Microbiol. 8:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai, W. P., S. R. Conley, H. F. Kung, R. R. Garrity, and P. L. Nara. 1996. Preliminary in vitro growth cycle and transmission studies of HIV-1 in an autologous primary cell assay of blood-derived macrophages and peripheral blood mononuclear cells. Virology 226:205-216. [DOI] [PubMed] [Google Scholar]
- 33.Wang, G. P., and F. D. Bushman. 2006. A statistical method for comparing viral growth curves. J. Virol. Methods 135:118-123. [DOI] [PubMed] [Google Scholar]
- 34.Yeh, S. H. 1991. Using the trapezoidal rule for the area under the curve calculation. SAS Users Group Int. 27:227-229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.