Abstract
Antigenemia in patients with Mediterranean visceral leishmaniasis (MVL) due to Leishmania infantum was retrospectively assessed by sandwich enzyme-linked immunosorbent assay (ELISA). Circulating Leishmania antigens, partially in free form, were in evidence in 53% of serum samples from immunocompetent individuals with MVL. Following successful therapy, antigenemia decline as measured by ELISA was more pronounced than antibody decrease.
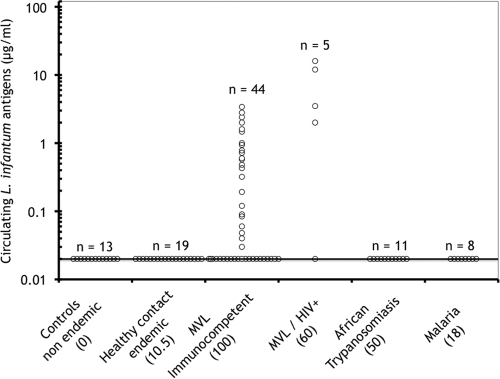
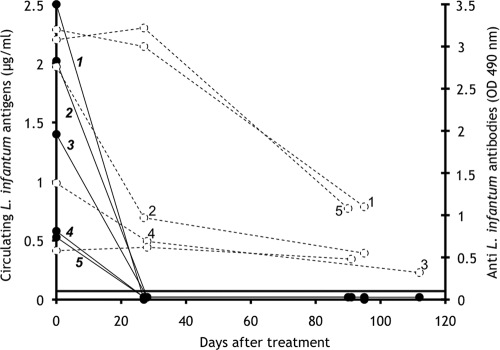
Although the definitive diagnosis of Mediterranean visceral leishmaniasis (MVL) due to Leishmania infantum is established upon the detection of parasites by microscopic examination, culture, or PCR, generally performed with bone marrow aspirates or blood samples, the detection of specific anti-L. infantum antibodies is generally concomitantly performed as an aid for diagnosis. Nevertheless, anti-L. infantum antibodies persist for months in cured patients and occur in some asymptomatic individuals living in areas of endemicity (7), therefore somewhat limiting the diagnostic and prognostic value of antibody detection. In addition, the low antibody responses occurring in some human immunodeficiency virus (HIV)-L. infantum-coinfected patients remain undetectable by enzyme-linked immunosorbent assays (ELISA) (9). A few previous studies (1, 2, 8) using ELISA and Western blot analyses of polyethylene glycol-precipitated immune complexes have been devoted to the detection of circulating Leishmania antigens (CLAs) in patients with MVL or leishmaniasis due to other parasite species. However, these methods are not quantitative and do not detect circulating, nonprecipitable free antigens. Therefore, in this study we assessed the presence of Leishmania antigens in MVL patients by using a sandwich-type ELISA (3) previously developed for measuring parasite burdens in BALB/c mice experimentally infected with L. infantum. The assay was performed as follows. High-binding microtiter plates (Greiner Bio-One) were coated for 4 h with an anti-L. infantum human immunoglobulin G fraction (5 μg in 100 μl of 0.1 M phosphate buffer, pH 7.2) prepared from high-titer MVL human serum (3) by protein A-Sepharose chromatography. The plates were saturated for 30 min with phosphate buffer containing 1% skim milk and 0.12% Triton X-100 (assay buffer). The assay (detection range, 0 to 2 μg/ml Leishmania antigens) was calibrated with a soluble Nonidet P-40 extract of L. infantum promastigotes (a sample of 106 promastigotes corresponds to 4 μg of a bovine serum albumin equivalent of Leishmania proteins) diluted in pooled human sera originating from an area where Leishmania is not endemic (Reims, France). Duplicate 0.1-ml aliquots of standards or undiluted, untested samples were delivered into the wells, and the plates were incubated for 18 h at room temperature. After the plates were repeatedly washed, a peroxidase-labeled anti-L. infantum F(ab)′ fragment (500 ng in 0.1 ml of assay buffer) was dispensed into the wells and the plates were incubated for 2 h. Bound-enzyme activity was revealed with a chromogenic substrate as described previously (3). The threshold assay sensitivity was 0.02 μg/ml of Leishmania antigens, corresponding to 5,000 parasites/ml. The method was validated with a panel of cryoconserved serum samples obtained from the collection of the parasitology-mycology department of the Centre Hospitalier Universitaire de Nice. The analyzed samples included (i) control samples from an area where Leishmania is not endemic (Reims, France), (ii) samples from asymptomatic contacts of infected patients, diagnosed on the basis of positive results from Western blotting against 14- and 18-kDa Leishmania antigens and/or positive skin tests (6, 7), from an area of endemicity (Nice, France), (iii) samples from immunocompetent or HIV-coinfected patients (23 males aged 22 to 75 years and 26 females aged 18 to 81 years) from an area of endemicity (Nice, France) with patent MVL diagnosed on the basis of parasite detection by PCR or direct examination, and (iv) samples from patients with African trypanosomiasis or acute malaria (these samples were a gift from B. Bouteille, Limoges, France). All samples were previously tested at a 1/500 dilution for the presence of anti-L. infantum antibodies by classical ELISA using Leishmania antigen-coated plates (4). CLAs (Fig. 1) were undetectable in 13 control serum samples from an area where Leishmania is not endemic, as well as in samples from 19 healthy contacts from an area of endemicity, 2 (10.5%) in the latter group being antibody positive by ELISA using crude Leishmania antigens. In contrast, at the time of diagnosis, CLAs (range, 0.03 to 4 μg/ml) were detected in 23 (53%) of 44 immunocompetent patients with MVL and higher levels (range, 0.2 to 20 μg/ml) were detected in 4 (80%) of 5 patients coinfected with HIV (Fig. 1). Interestingly, two of these four coinfected patients with detectable CLAs (Fig. 1) were negative by antibody ELISA. In addition (Fig. 1), serum samples from acute malaria patients or individuals with African trypanosomiasis, which showed cross-reacting anti-L. infantum antibodies upon ELISA analysis in 18 and 50% of cases, respectively, gave CLA values close to background levels. Therefore, for the panel of sera studied, direct detection of CLAs by ELISA exhibited overall sensitivity of 55.1% and specificity of 100% for the diagnosis of MVL. Furthermore, monitoring of antigenemia in immunocompetent MVL patients receiving successful liposomal amphotericin B (Ambisome) chemotherapy (Fig. 2) indicated that in all studied cases, CLAs were completely cleared from circulation by day 25 but that antibody levels decreased only slowly during this period. Consequently, antigenemia decline measured by direct ELISA following chemotherapy is a more sensitive indicator of therapeutic efficacy than antibody decrease, at least in immunocompetent MVL patients. Finally, molecular sieving of serum samples from immunocompetent MVL patients by using a Sephacryl S-200 column (data not shown) indicated that a large proportion of assayable circulating L. infantum antigens eluted in the 68- to 46-kDa range and consequently circulated in the free form. The other portion (>150 kDa) is likely to correspond to free high-molecular-mass CLAs and/or immune complexes of various sizes formed in the context of antigen excess.
FIG. 1.
Sensitivity and specificity of CLA level measurement by ELISA for MVL diagnosis. Numbers in parentheses indicate the percentages of samples positive for anti-L. infantum antibodies by classical ELISA using promastigote lysate.
FIG. 2.
Follow-up evaluation showing decreases in CLAs (solid lines with numbers in bold) and the corresponding anti-L. infantum antibodies (dotted lines) in five immunocompetent MVL patients receiving successful liposomal amphotericin B therapy. The horizontal line indicates the optical density cutoff value for antibody positivity. OD 490 nm, optical density at 490 nm.
In this study, using a direct sandwich ELISA, we evaluated the minimal frequency of CLAs in a serum panel from MVL patients at the time of diagnosis and showed for the first time that a great proportion of CLAs occurred in the free form in serum. The method was less sensitive than antibody serology performed with crude promastigote extracts but showed 100% specificity for MVL diagnosis. Nevertheless, the specificity of antibody serology could be improved by selecting serodiagnostic methods based on a single Leishmania antigen such as rK39 or rK26 protein (10, 11). As a portion of CLAs circulate in the form of immune complexes, false-negative results may be reduced by previous dissociation of antigen-antibody complexes, as described elsewhere for improving antibody or antigen detection in samples from patients with various infectious diseases (5, 12, 13). In addition, the detection of parasite antigens in urine samples from patients may increase the assay sensitivity for MVL diagnosis. Finally, the test proved to be a more sensitive indicator than antibody detection to monitor the recovery of MVL patients receiving chemotherapy.
Acknowledgments
This work was supported by the GACL (Groupe d'Action contre la Leishmaniose).
Footnotes
Published ahead of print on 16 September 2009.
REFERENCES
- 1.Azazy, A. A., E. Devaney, and M. L. Chance. 1994. A PEG-ELISA for the detection of Leishmania donovani antigen in circulating immune complexes. Trans. R. Soc. Trop. Med. Hyg. 88:62-66. [DOI] [PubMed] [Google Scholar]
- 2.Evans, T. G., and R. D. Pearson. 1988. Identification of leishmanial antigens in the sera of patients with American visceral leishmaniasis. Infect. Immun. 56:3139-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrua, B., Y. Le Fichoux, I. Suffia, D. Rousseau, C. Roptin, and J. Kubar. 2001. Quantitation of Leishmania infantum in tissues of infected BALB/c mouse by sandwich ELISA. J. Immunoassay Immunochem. 22:165-181. [DOI] [PubMed] [Google Scholar]
- 4.Ferrua, B., C. Luci, Y. Le Fichoux, A. Paul, and P. Marty. 2006. Imprinting of BALB/c mice with low Leishmania infantum parasite dose markedly protects spleen against high-dose challenge. Vaccine 24:589-596. [DOI] [PubMed] [Google Scholar]
- 5.Harrington, J., Jr., J. L. Ho, J. R. Lapa e Silva, M. B. Conde, A. L. Kritski, L. S. Fonseca, and M. H. Saad. 2000. Mycobacterium tuberculosis lipid antigens: use of multi-antigen based enzyme immunoassay for free and complex dissociated antibodies. Int. J. Tuberc. Lung Dis. 4:161-167. [PubMed] [Google Scholar]
- 6.le Fichoux, Y., J. F. Quaranta, J. P. Aufeuvre, A. Lelievre, P. Marty, I. Suffia, D. Rousseau, and J. Kubar. 1999. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J. Clin. Microbiol. 37:1953-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marty, P., A. Lelievre, J. F. Quaranta, A. Rahal, M. Gari-Toussaint, and Y. Le Fichoux. 1994. Use of the leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France). Trans. R. Soc. Trop. Med. Hyg. 88:658-659. [DOI] [PubMed] [Google Scholar]
- 8.Mary, C., G. Ange, S. Dunan, D. Lamouroux, and M. Quilici. 1993. Characterization of a circulating antigen involved in immune complexes in visceral leishmaniasis patients. Am. J. Trop. Med. Hyg. 49:492-501. [DOI] [PubMed] [Google Scholar]
- 9.Medrano, F. J., C. Canavate, M. Leal, C. Rey, E. Lissen, and J. Alvar. 1998. The role of serology in the diagnosis and prognosis of visceral leishmaniasis in patients coinfected with human immunodeficiency virus type-1. Am. J. Trop. Med. Hyg. 59:155-162. [DOI] [PubMed] [Google Scholar]
- 10.Pedras, M. J., L. de Gouvea Viana, E. J. de Oliveira, and A. Rabello. 2008. Comparative evaluation of direct agglutination test, rK39 and soluble antigen ELISA and IFAT for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 102:172-178. [DOI] [PubMed] [Google Scholar]
- 11.Rosario, E. Y., O. Genaro, J. C. Franca-Silva, R. T. da Costa, W. Mayrink, A. B. Reis, and M. Carneiro. 2005. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 100:197-203. [DOI] [PubMed] [Google Scholar]
- 12.Schupbach, J., M. Flepp, D. Pontelli, Z. Tomasik, R. Luthy, and J. Boni. 1996. Heat-mediated immune complex dissociation and enzyme-linked immunosorbent assay signal amplification render p24 antigen detection in plasma as sensitive as HIV-1 RNA detection by polymerase chain reaction. AIDS 10:1085-1090. [PubMed] [Google Scholar]
- 13.Steindl, F., C. Armbruster, K. Pierer, M. Purtscher, and H. W. Katinger. 1998. A simple and robust method for the complete dissociation of HIV-1 p24 and other antigens from immune complexes in serum and plasma samples. J. Immunol. Methods 217:143-151. [DOI] [PubMed] [Google Scholar]