Abstract
The vacuolating cytotoxin gene of Helicobacter pylori, vacA, induces cytoplasmic vacuolation in gastric epithelial cells. Recently, the vacA intermediate (i) region, which is located between the signal (s) and middle (m) regions, was identified as a third polymorphic determinant of vacA activity. In vacA, there are approximately 81-bp deletions between the vacA i and m regions (denoted the d region). The aim was to clarify the roles of the vacA d region in relation to H. pylori-related diseases and histopathological gastric mucosal changes. We assessed the vacA signal s-, m-, i-, and d-region genotypes and cagA status in H. pylori isolates recovered from Western countries (n = 266) and East Asian countries (n = 244) by PCR. In East Asian countries, there were no relationships between the vacA genotypes and the clinical outcomes and histopathological changes. In Western countries, strains with the vacA s1, m1, i1, or d1 (no deletion) genotype significantly increased the risk for the development of gastric cancer compared with the risk from strains with the s2, m2, i2, or d2 genotype (adjusted odd ratios, 3.17 [95% confidence interval {CI}, 1.07 to 9.45] for s1, 10.65 [95% CI, 3.36 to 31.35] for m1, 8.57 [95% CI, 2.85 to 25.81] for i1, and 8.04 [95% CI, 2.67 to 24.16] for d1). The highly virulent vacA genotypes significantly enhanced neutrophil infiltration and gastric atrophy in univariant analysis, whereas only the vacA d-region genotype was significantly associated with neutrophil infiltration and gastric atrophy in both the antrum and the corpus by multiple linear regression analysis. The presence of the vacA d1 genotype in H. pylori strains could be an improved predictor of histological inflammation and the potential for atrophy compared with the presence of the vacA s-, m-, and i-region genotypes in Western countries.
Gastric cancer arises through steps related to the presence of a chronic Helicobacter pylori infection, which leads to the precursor lesion, atrophic gastritis. The progression of gastric mucosal atrophy varies within and between different populations; and the differences relate to differences in H. pylori virulence, host genetic factors, and/or environmental factors. The important H. pylori virulence factors and host genetic factors are related to an increased inflammatory response and include the presence of the cag pathogenicity island, polymorphisms of host inflammation-related cytokines (5, 6), drug metabolism-related enzymes (19, 22), and growth factors (5, 6, 12, 19, 22, 30).
The vacuolating cytotoxin (VacA) was one of the first putative virulence factors discovered in H. pylori (4). Virtually all H. pylori strains possess a vacA gene; however, the in vitro vacuolating activities for cell lines vary considerably among strains; and the differences in the vacuolating activities are related to differences in the vacA structures at the signal (s) region (s1 and s2) and the middle (m) region (m1 and m2) (1). The s region encodes part of the signal peptide and the N terminus of the mature protein, whereas the m region encodes part of the 55,000-Da (55K) C-terminal subunit. The amount of toxin produced also varies according to the vacA m-region genotypes: vacA s1-m1 strains induce greater vacuolating activity than vacA s1-m2 strains (1, 11).
Recently, a third polymorphic determinant of vacuolating activity was described as being located between the s and m regions and was termed the intermediate (i) region (16). Two i-region subtypes were described: the i1 and i2 subtypes. Among Western strains, vacA s1-m2 strains were noted to vary in their i-region genotypes; vacA s1-m1 and s2-m2 strains were exclusively i1 and i2, respectively. The vacA s1-i1-m2 strains induced vacuolation in rabbit kidney RK13 cells, whereas s1-i2-m2 strains did not. Clinically, the prevalence of the vacA i1 genotype in patients with gastric cancer was 80%, which was significantly higher than that in patients in an Iranian population with nonulcer dyspepsia (37%) (16). Subsequent studies showed that infection with vacA i1 strains was associated with gastric cancer in patients in Iranian and Italian populations and gastric ulcer in patients in Iraqi and Italian populations (2, 8, 16). This allowed the conclusion that the vacA i-region genotype might be a better predictor of the carcinogenic potential of H. pylori than the previously used vacA s- and m-region genotypes. However, our recent study shows that determination of the vacA genotypes for the combination of three regions (the s, m, and i regions) did not provide any advantage as a disease determinant marker over determination of s- and m-region genotypes in East Asian and Southeast Asian countries (15).
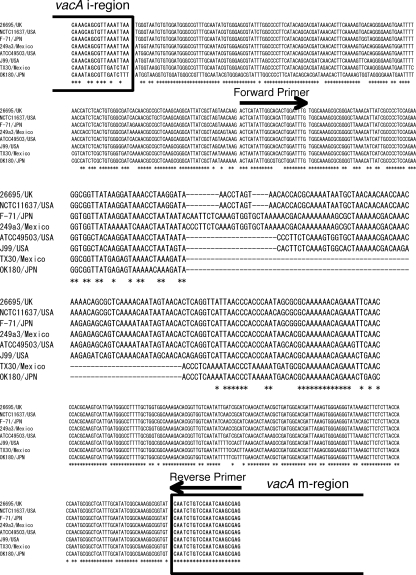
Examination of the information for 49 complete, nonpartial vacA gene sequences covering the vacA s to m regions deposited in the GenBank database showed that 37 strains were of the s1-i1-m1 genotype, 10 were of the s1-i1-m2 genotype, 1 was of the s1-i2-m2 genotype, and 1 was of the s2-i2-m2 genotype. Interestingly, there was a deletion of 81 bp between the vacA i region and the vacA m region in two strains (Fig. 1), both of which were of the i2-m2 genotype. In contrast, the remaining 47 strains had either no deletion (31 strains) or a short deletion ranging from 9 bp (5 strains) to 23 bp (1 strain); and there was no correlation between the presence of a short deletion and the vacA s-, m-, and i-region type. The frequency of the 81-bp deletion and the short deletion in clinical strains is unclear, and it is also unknown whether the presence of the 81-bp deletion and the short deletion in clinical strains is associated with other vacA genotypes (e.g., the i2 and/or m2 genotype) and with the clinical outcome and/or the degree of gastric histopathological mucosal injury. Of the various deletions, we focused on the 81-bp deletion in this study because the 81-bp deletion was expected to have correlations with the vacA genotype. We also examined whether the vacA deletion status and/or a combination of the genotypes of the vacA s, m, and i regions and the deletion is of advantage as a marker of disease that may be used in addition to currently used vacA genotyping systems.
FIG. 1.
Nucleotide sequences of vacA d1 or d2 region from eight strains. Strains Tx30a and OK180 have the vacA d2 genotype; and strains 26695, NATC11637, F-71, 249a3, ATCC 49503, and J99 have the vacA d1 genotype. The vacA d2 genotype had a 69- to 81-bp deletion between the vacA i and m regions.
MATERIALS AND METHODS
Patients.
H. pylori strains were obtained from infected patients from the United States, Colombia, South Korea, and Japan (Table 1). The study population consisted of patients with gastric ulcers, duodenal ulcers, gastric cancer, or gastritis alone. Gastritis was defined as H. pylori gastritis without peptic ulcers or gastric malignancy. None of the patients had received nonsteroidal anti-inflammatory drugs or steroids at least 3 months prior to endoscopy. None had previously received treatment for H. pylori infection. Informed consent was obtained from all patients under protocols approved by the local hospital's ethics committee.
TABLE 1.
Demographic characteristics of Western and East Asian patients enrolled in this study
| Characteristic | Result for patients from: |
P valuea | ||||
|---|---|---|---|---|---|---|
| United States | Colombia | South Korea | Japan | Total | ||
| No. of patients | 170 | 96 | 124 | 120 | 510 | |
| No. of males/no. of females | 142/28 | 55/41 | 94/30 | 75/45 | 366/144 | <0.01 |
| Mean age (yr) ± SD | 49.8 ± 1.1 | 57.1 ± 1.5 | 47.5 ± 1.2 | 56.9 ± 1.3 | 52.3 ± 0.6 | <0.01 |
| No. (%) of patients with the following diseaseb: | ||||||
| Gastritis | 91 (53.5) | 32 (33.3) | 33 (26.6) | 27 (22.5) | 183 (35.9) | <0.01 |
| GU | 30 (17.6) | 0 (0.0) | 27 (21.8) | 33 (27.5) | 90 (17.6) | |
| DU | 40 (23.5) | 25 (26.0) | 24 (19.4) | 28 (23.3) | 117 (22.9) | |
| GC | 9 (5.3) | 39 (40.6) | 40 (32.3) | 32 (26.7) | 120 (23.5) | |
P values were used to analyze the differences in the demographic data among patients from the United States, Colombia, South Korea, and Japan.
Abbreviations: GU, gastric ulcer; DU, duodenal ulcer; GC, gastric cancer.
Genotyping of vacA s, m, and i regions and cagA status by PCR methods.
Chromosomal DNA was isolated from confluent plate cultures expanded from a single colony by using the QIAamp tissue kit (Qiagen Inc., Santa Clarita, CA), according to the manufacturer's instructions. Genotyping of the vacA s, m, and i regions and cagA status were determined by PCR methods, as described previously (1, 10, 16, 28).
Detection of vacA d region between i and m regions.
We designated the region covering the 81-bp deletion between the i and m regions as the deletion (d) region and designed the following PCR primers to cover that region: 5′-ACTAATATTGGCACACTGGATTTG-3′ (forward primer VAS-5F) and 5′-CTCGCTTGATTGGACAGATTG-3′ (reverse primer VAGF-R). The PCR conditions were 95°C for 90 s; then 35 cycles of 95°C for 30 s, 53°C for 60 s, and 72°C for 30 s; and finally, 72°C for 5 min. The fragments classified two types: type d1 without the 81-bp deletion (approximately 367 to 379 bp due to the short deletion) and type d2 with the 81-bp deletion (approximately 298 bp). In this study, a short deletion of 9 to 23 bp was included in the d1 genotype.
Histology.
Gastric biopsy specimens were taken from the antrum (pyloric gland area) and the corpus (fundic gland area). The biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, and cut into sequential 4-μm sections. The H. pylori density, gastritis activity (neutrophil infiltration), and atrophy were graded from 0 (absent/normal) to 5 (maximal), as described previously (7). The score is presented as the mean scores for biopsy samples from the corpus and antral areas.
It is well known that atrophic gastritis poses a high risk for the development of gastric ulcer and gastric cancer, and antral dominant gastritis is associated with the development of duodenal ulcer; therefore, the gastritis group contained a mixture of patients, some of whom would be destined to develop severe clinical outcomes. Use of the Operative Link on Gastritis Assessment (OLGA) staging system was recently advocated. By use of that system, gastritis staging combined with H. pylori infection provided clinically relevant information on the overall status of the gastric mucosa and the implications of that status for prognosis, therapy, and management (17, 18). We therefore analyzed the risk of certain clinical outcomes by comparing not only all gastritis patients but also gastritis patients with patients with OLGA stages 0 and I as a control group.
Data analysis.
Statistical differences in demographic characteristics, vacA genotypes, and cagA status among the different geographic groups were determined by one-way analysis of variance or the chi-square test. Differences in the histological scores among the different vacA genotype groups were analyzed by using the Mann-Whitney U test. A multiple linear regression analysis was performed to determine which factor(s) was related to the severity of the histology, with age, sex, bacterial factors, and clinical outcome being explanatory variables. The selection of variables was by backward stepwise deletion in the logistic regression and by the F value out and F value in stepwise method in the linear regression, where both F values were 2.0. A P value of less than 0.05 was accepted as statistically significant. Calculations were carried out by using StatView (version 5.0) statistical software (SAS institute Inc., Cary, NC) or HALBAU statistical software (Gendai-sugaku-sha, Kyoto, Japan).
RESULTS
The vacA genotypes and cagA status were determined for 510 H. pylori isolates, including 266 strains from Western countries (170 strains from the United States and 96 strains from Colombia) and 244 strains from East Asian countries (124 strains from South Korea and 120 strains from Japan) (Table 1). The mean age of the subjects, the ratio of the patients by sex, and the population of individuals with H. pylori-related diseases were significantly different (P < 0.01) among the different countries (Table 1). Therefore, the odds ratio (OR) for the development of peptic ulcer and gastric cancer was adjusted by age and sex in subsequent analyses.
The prevalence of vacA genotypes and cagA status.
We combined the data from the different East Asian and Western countries into the East Asian and the Western groups, respectively. East Asian strains had a higher prevalence of vacA s1, m1, i1, and d1 genotypes than Western strains (Table 2). The prevalence of vacA s-, i-, and d-region genotypes and cagA status were similar between U.S. and Colombian strains and between Japanese and South Korean strains. In contrast, the prevalence of vacA m genotypes differed significantly among East Asian strains but not among strains from Western countries (P < 0.01) (Table 2).
TABLE 2.
Prevalence of virulence factors and different ethnic groups
| Virulence factor | Genotype | No. (%) of patients |
P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Western countries |
East Asian countries |
Total | |||||||
| United States | Colombia | Total | Korea | Japan | Total | ||||
| vacA | |||||||||
| s region | s1 | 141 (83.9) | 74 (77.1) | 215 (80.8) | 124 (100) | 120 (100) | 244 (100) | 459 (90.0) | <0.01 |
| m region | m1 | 106 (62.4) | 65 (67.7) | 171 (64.3) | 109 (87.9) | 117 (97.5) | 226 (92.6) | 397 (77.8) | <0.01 |
| i region | i1 | 122 (71.8) | 69 (71.9) | 191 (71.8) | 120 (96.8) | 118 (98.3) | 238 (97.5) | 429 (84.1) | <0.01 |
| d region | d1 | 126 (74.1) | 71 (74.0) | 197 (74.1) | 121 (97.5) | 118 (98.3) | 239 (98.0) | 436 (85.5) | <0.01 |
| s-m-i combination | s1-m1-i1 | 105 (61.8) | 65 (67.7) | 170 (63.9) | 109 (87.9) | 117 (97.5) | 226 (92.6) | 396 (77.6) | <0.01 |
| s1-m1-i2 | 1 (0.6) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | ||
| s1-m2-i1 | 16 (9.4) | 4 (4.2) | 20 (7.5) | 11 (8.9) | 1 (0.8) | 12 (4.9) | 32 (6.3) | ||
| s1-m2-i2 | 19 (11.2) | 5 (5.2) | 24 (9.0) | 4 (3.2) | 2 (1.7) | 6 (2.5) | 30 (5.9) | ||
| s2-m2-i1 | 1 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | ||
| s2-m2-i2 | 28 (17.1) | 22 (22.9) | 50 (18.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 50 (9.8) | ||
| s-m-i-d combination | s1-m1-i1-d1 | 105 (61.8) | 65 (67.7) | 170 (64.0) | 109 (87.9) | 117 (97.5) | 226 (92.6) | 396 (77.6) | <0.01 |
| s1-m2-i1-d1 | 16 (9.4) | 4 (4.2) | 20 (7.5) | 11 (8.9) | 1 (0.8) | 12 (4.9) | 32 (6.3) | ||
| s1-m2-i2-d2 | 15 (8.8) | 5 (5.2) | 20 (7.5) | 3 (3.2) | 2 (1.7) | 5 (2.0) | 25 (4.9) | ||
| s2-m2-i2-d2 | 28 (16.5) | 20 (20.8) | 48 (18.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 48 (9.4) | ||
| Otherb | 6 (3.5) | 2 (2.1) | 8 (3.0) | 1 (0.8) | 0 (0.0) | 1 (0.4) | 9 (1.8) | ||
| cagA | Pos./neg.c | 143 (84.1) | 78 (81.3) | 221 (83.1) | 121 (97.6) | 120 (100) | 241 (98.8) | 462 (90.6) | <0.01 |
P values were used to analyze differences in the demographic data between patients from Western countries and those from East Asian countries.
The other types of the vacA s-m-i-d combination included s2-m2-i2-d1 (n = 2), s1-m2-i2-d1 (n = 5), s1-m1-i2-d1 (n = 1), and s2-m2-i1-d2 (n = 1).
Pos./neg., positive/negative.
In agreement with the findings of previous studies (15, 16), all vacA s1-m1 strains from East Asian countries were of the i1 genotype and, with the exception of one strain of the i2 genotype, all vacA s1-m1 strains from Western countries were exclusively of the i1 genotype (99.8%) (Table 2). In addition, with the exception of one strain of the i1 genotype, all s2-m2 strains from Western countries were exclusively of the i2 genotype (99.8%). The prevalence of the vacA i1 and i2 genotypes was similar among s1-m2 strains from Western countries (45.5% and 55.5%, respectively), which was in agreement with the findings of previous studies (15, 16); however, only 6 of 18 s1-m2 strains from East Asian countries (33.3%) showed the i2 genotype. When the d-region genotypes were taken into account, i1 and i2 strains exclusively showed the d1 and d2 genotypes, respectively (98.9% and 93.7%, respectively), with the exceptions being one Western strain of the i1-d2 genotype, seven Western strains of the i2-d1 genotype, and one East Asian (South Korean) strain of the i2-d1 genotype (Table 2). Overall, the vacA s-, m-, i-, and d-region genotypes as well as the cagA status were significantly correlated with one another among the Western strains (Table 3). However, nearly all East Asian strains had identical genotypes (vacA s1, m1, i1, and d1 and cagA positive).
TABLE 3.
Relationship among different H. pylori virulence factor genotypes for patients from Western countries
| vacA genotype |
R value |
|||
|---|---|---|---|---|
| vacA m1 | vacA i1 | vacA d1 | Presence of cagA | |
| s1 | 0.410a | 0.462a | 0.506a | 0.535a |
| m1 | 0.617a | 0.591a | 0.385a | |
| i1 | 0.678a | 0.432a | ||
| d1 | 0.475a | |||
P < 0.01.
Influence of vacA genotype and cagA status on clinical outcome.
In East Asia, there was no significant association between the clinical outcome and the vacA genotype or cagA status (Fig. 2). In contrast, the vacA d1 genotype was significantly associated with gastric cancer in Western countries (for all patients versus patients with gastritis, the adjusted OR is 8.04 [95% confidence interval {CI}, 2.67 to 24.16]; for all patients versus patients with OLGA stages 0 and I, the OR is 33.78 [95% CI, 11.26 to 101.39]) (Table 4). Similarly, the vacA s1, m1, and i1 genotypes were significantly associated with gastric cancer (Fig. 2 and Table 4). When all patients were compared with patients with OLGA stages 0 and I, the vacA s1, m1, i1 and d1 genotypes were significantly associated not only with gastric cancer but also with gastric ulcer and duodenal ulcer in Western countries (Table 4). In the multiple linear regression analysis by use of a factor(s) related to the clinical outcome, no single factor was found to be associated with the risk of gastroduodenal disease.
FIG. 2.
Prevalence of strains with vacA s1, m1, i1, and d1 genotypes and cagA status for strains from all patients of East Asian (A) and Western (B) origin. For strains from Western countries, the vacA m1, i1, and d1 genotypes significantly increased the risk of gastric cancer compared with the risk of gastritis alone. *, P < 0.05 versus gastritis alone; #, P < 0.05 versus mild gastritis alone.
TABLE 4.
Age and sex-adjusted risk for peptic ulcer and gastric cancer in relation to H. pylori virulence factors in Western countries
| Virulence factor | Gastric ulcer (n = 30) |
Duodenal ulcer (n = 65) |
Gastric cancer (n = 48) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| All patients vs patients with gastritis (n = 123) | |||||||||
| vacA s1 vs s2 region | 1.62 | 0.52-5.00 | 0.40 | 2.15 | 0.92-5.01 | 0.08 | 3.17a | 1.07-9.45 | 0.04 |
| vacA m1 vs m2 region | 1.77 | 0.71-4.40 | 0.22 | 1.31 | 0.69-2.49 | 0.42 | 10.65 | 3.36-31.35 | <0.01 |
| vacA i1 vs i2 region | 2.65 | 0.95-7.41 | 0.06 | 1.55 | 0.78-3.01 | 0.21 | 8.57 | 2.85-25.81 | <0.01 |
| vacA d1 vs d2 region | 2.54 | 0.91-7.11 | 0.08 | 1.87 | 0.91-3.87 | 0.09 | 8.04 | 2.67-24.16 | <0.01 |
| cagA presence vs absence | 3.25 | 0.85-12.41 | 0.09 | 3.52 | 1.16-10.64 | 0.03 | 3.52 | 1.16-10.64 | 0.03 |
| All patients vs patients with OLGA stages 0 and I (n = 93) | |||||||||
| vacA s1 vs s2 region | 8.36 | 2.78-25.16 | <0.01 | 5.25 | 2.21-12.37 | <0.01 | 16.55 | 5.28-51.83 | <0.01 |
| vacA m1 vs m2 region | 7.25 | 3.30-15.68 | <0.01 | 2.61 | 1.41-4.82 | <0.01 | 16.04 | 7.11-36.24 | <0.01 |
| vacA i1 vs i2 region | 12.54 | 4.80-31.80 | <0.01 | 3.30 | 1.70-6.41 | <0.01 | 42.73 | 14.27-127.99 | <0.01 |
| vacA d1 vs d2 region | 9.99 | 3.88-25.74 | <0.01 | 3.43 | 1.71-6.89 | <0.01 | 33.78 | 11.26-101.39 | <0.01 |
| cagA presence vs absence | 14.91 | 4.02-55.33 | <0.01 | 6.20 | 2.54-15.16 | <0.01 | 13.81 | 4.62-41.32 | <0.01 |
Boldface data indicate statistically significant results.
Histology.
The gastric mucosa in patients with gastric cancer and gastric ulcer is generally atrophic, and the gastric mucosa in patients with duodenal ulcer generally has enhanced inflammation; thus, the inclusion of those patients in histological analyses might result in a potential bias. We therefore limited the evaluation of the histological analyses by using only patients with gastritis (for the Western population, n = 123; for the East Asian population, n = 60).
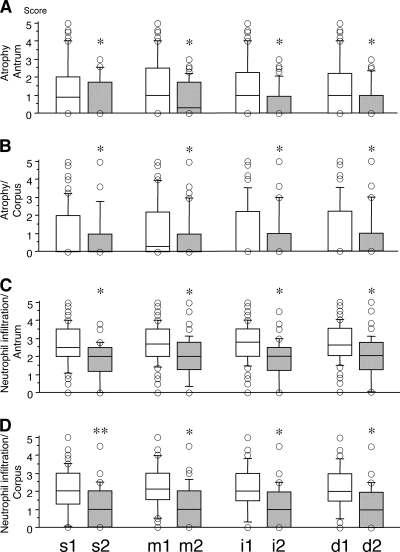
For the patients from East Asian countries, there was no significant association between histology and H. pylori factors (data not shown). For the patients from Western countries, the vacA s1, m1, i1, and d1 genotypes were significantly associated with increased scores for gastric mucosal atrophy and neutrophil infiltration in both the antrum and the corpus compared with the scores for patients with the vacA s2, m2, i2, and d2 genotype (Fig. 3).
FIG. 3.
Gastric mucosal atrophy score (A and B) and inflammation activity (neutrophil infiltration) (C and D) in gastric antrum and corpus among the different vacA genotype groups in Western countries. Patients harboring strains with the vacA s1, m1, i1, and d1 genotypes have a significantly increased atrophy score and significantly increased inflammation activity in both the gastric antrum and the corpus than patients harboring strains with the vacA s2, m2, i2, and d2 genotypes. The bottoms and tops of the columns indicate the 25th and 75th percentiles of the data, respectively; the horizontal lines in the columns indicate the 50th percentile (median); and the bottoms and tops of the bars of the columns indicate the 10th and 90th percentiles, respectively; circles represent data outside the 10th and 90th percentiles. *, P < 0.01; **, P < 0.05.
Multiple linear regression analysis was performed to determine which factor(s) was related to the severity of the histology. The vacA d1 genotype was significantly associated with severe neutrophil infiltration (partial regression coefficients, 1.12 ± 0.17 in the antrum and 0.94 ± 0.17 in the corpus; P < 0.001 for both comparisons) and mucosal atrophy (partial regression coefficient, 0.60 ± 0.30 in the antrum and 0.63 ± 0.35 in the corpus; P = 0.04 for both comparisons) in Western countries (Table 5). The vacA m1 genotype also remained in the final model for antral and corpus mucosal atrophy, although the differences did not reach statistical significance (Table 5). Interestingly, no other variables (i.e., the vacA s1 or i1 genotype or cagA status) remained in the final model.
TABLE 5.
Final model of H. pylori factors associated with histology obtained by multiple linear regression analysis
| Specimen | Pathology | Factor | Partial regression coefficienta | P value |
|---|---|---|---|---|
| Antrum | H. pylori density | None | ||
| PMNb infiltration | vacA d1 vs d2 | 1.12 ± 0.17 | <0.01 | |
| Atrophy | vacA d1 vs d2 | 0.60 ± 0.30 | 0.04 | |
| Atrophy | vacA m1 vs m2 | 0.48 ± 0.27 | 0.08 | |
| Corpus | H. pylori density | None | ||
| PMN infiltration | vacA d1 vs d2 | 0.94 ± 0.17 | <0.01 | |
| Atrophy | vacA d1 vs d2 | 0.63 ± 0.35 | 0.04 | |
| Atrophy | vacA m1 vs m2 | 0.52 ± 0.27 | 0.06 |
The partial regression coefficients are presented as the means ± standard errors.
PMN, polymorphonuclear leukocyte.
When patients with gastroduodenal diseases were added, the vacA d1 genotype was also observed to be significantly associated with severe neutrophil infiltration and mucosal atrophy, which is the same result obtained when patients with gastroduodenal diseases were not included (data not shown).
vacA genotypes in other Asian countries.
Because most of the strains in East Asia were of the vacA d1 and i1 genotypes and there was no significant association between clinical outcomes and the vacA d1 and i1 genotypes, we evaluated the prevalence of the vacA s, m, i, and d genotypes and cagA status among strains from other Asian countries in a preliminary analysis (Table 6). In China, Vietnam, and Thailand, all strains had the vacA d1 and i1 genotypes and were cagA positive; these were the same vacA genotypes and cagA status as strains from East Asian countries (Table 6).
TABLE 6.
Demographic characteristics of patients from other Asian countries
| Characteristic | Result for patients from: |
||
|---|---|---|---|
| China | Vietnam | Thailand | |
| No. of patients | 48 | 7 | 31 |
| No. of males/no. of females | 36/12 | 5/2 | 19/12 |
| Mean age (yr) ± SD | 59.7 ± 1.2 | 36.7 ± 1.9 | 52.7 ± 1.7 |
| No. (%) of patients with the following diseasea: | |||
| Gastritis | 29 (60.4) | 7 (100) | 16 (51.6) |
| GU | 0 (0) | 0 (0) | 5 (16.1) |
| DU | 0 (0) | 0 (0) | 10 (32.3) |
| GC | 19 (39.6) | 0 (0) | 0 (0) |
| No. (%) of patients harboring isolates with the following vacA genotypes: | |||
| s region, s1 | 48 (100) | 7 (100) | 31 (100) |
| m region, m1 | 31 (64.6) | 6 (85.7) | 17 (54.8) |
| i region, i1 | 48 (100) | 7 (100) | 31 (100) |
| d region, d1 | 48 (100) | 7 (100) | 31 (100) |
| No. (%) of patients with the following s-m-i combination: | |||
| s1-m1-i1 | 32 (66.7) | 6 (85.7) | 17 (54.8) |
| s1-m1-i2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| s1-m2-i1 | 16 (33.3) | 1 (14.3) | 14 (45.2) |
| s1-m2-i2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| s2-m2-i1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| s2-m2-i2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No. (%) of patients with the following s-m-i-d combination: | |||
| s1-m1-i1-d1 | 32 (66.7) | 6 (85.7) | 17 (54.8) |
| s1-m2-i1-d1 | 16 (33.3) | 1 (14.3) | 14 (45.2) |
| s1-m2-i2-d2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| s2-m2-i2-d2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No. (%) of patients positive/negative for cagA | 48 (100) | 7 (100) | 31 (100) |
Abbreviations: GU, gastric ulcer; DU, duodenal ulcer; GC, gastric cancer.
DISCUSSION
The role of VacA in the pathogenesis of H. pylori disease remains unclear (13). VacA is expressed as a 140K protoxin that undergoes amino- and carboxyl-terminal processing, yielding a mature 88K secreted product called the VacA toxin (3). Partial proteolytic digestion of the mature 88K toxin yields two fragments, one of 33K (p33) and one of p55K (p55). Amino acid sequences located within a hydrophobic region near the amino terminus of the p33 domain are required for the formation of anion-selective membrane channels (9), and the p55 domain is responsible for VacA binding to host cells (25). The expression of the p33 domain, as well as the expression of about 111 amino acids from the N terminus of the p55 domain, is required for vacuolating activity (24, 25). Both recombinant p33 and p55 domains have been shown to associate with lipid bilayers (14) and to bind to HeLa cells (23); and an interaction between them is required for binding, internalization, and vacuolating activity (23). Although Wang and Wang (26) reported that the deletion of a region of about 100 amino acids from the C-terminal portion of the p55 domain significantly decreased the binding activity of the toxin to cells, the role of the N-terminal portion of the p55 domain remained unclear. The d regions of the vacA gene are located in the N-terminal side of the p55 domain, and the d regions might be considered to be related to VacA binding to host gastric cells and vacuolating activity. In our preliminary experiments, we tried to investigate the biological role of the vacA d region by immunoblot analysis for VacA production and enzyme-linked immunosorbent assay methods for cytokine (i.e., interleukin-8) production. However, since there were few H. pylori strains with vacA d1 and d2 mosaic patterns and these strains had no effect on the vacA s, m, and i regions (e.g., s1-i1-d2-m1), we could not demonstrate a significant difference in the biological roles of the vacA d1 and d2 regions (unpublished data).
The gastritis group contained a mixture of patients, some of whom would be destined to develop severe clinical outcomes. It is well known that atrophic gastritis poses a high risk for the development of gastric ulcer and gastric cancer and that antrum-dominant gastritis is associated with the development of duodenal ulcer. In fact, in the present study, the grade of gastric atrophy among gastric cancer patients and gastric ulcer patients was significantly higher than that among patients with gastritis alone (data not shown). We therefore used patients with OLGA staging system stages 0 and I as the control group. When we used all gastritis patients as the control group, only the vacA m1, i1, and d1 genotypes were significantly related to gastric cancer. In contrast, these genotypes were significantly related not only to gastric cancer but also to gastric and duodenal ulcers when we used patients with mild gastritis as the control group. Most previous studies did not take the heterogeneity of gastritis into account, and this might be one reason why some previous studies, even those performed in Western countries, could not find a relationship between the vacA genotype and the clinical outcome. However, these relationships could not be detected in Asian countries, where most strains exclusively possessed the vacA s1, m1, i1, and d1 genotypes and were cagA positive. In Asian countries, other virulence factors (e.g., the presence of the duodenal ulcer-promoting [dupA] gene [12]) and/or host genetic factors (e.g., inflammation-related cytokines [20]) should have influences on gastric injury. Moreover, the evaluation of populations from Africa and Southeast Asia, where, enigmatically, the frequency of infection is high but the incidence of gastroduodenal disease is low, might help us obtain more evidence on the influence of the vacA d region.
We previously reported that although the prevalence of the vacA s- and m-region genotypes differed significantly among strains from different countries, there were no relationships between the vacA genotype and the risks of disease, probably due to the small sample size (27, 29). We therefore randomly selected strains from large stocks in our university strain bank for which histology information for the patients was available to avoid sample bias. In univariate analyses, not only the vacA d-region genotype but also the s-, m-, and i-region genotypes were associated with the histological grade of atrophy and inflammatory cell infiltration; importantly, however, multivariate analysis revealed that only the vacA d1 genotype was significantly associated with atrophy and inflammatory cell infiltration. Although there was a significant association between the i-region genotype and the clinical outcome in Western countries, as reported in previous studies (8, 16), the increased risk of disease associated with the i-region genotype was the same as that associated with the vacA s- and m-region genotypes. In addition, in combination analysis of the vacA s-, m- and i-region genotypes, carriage of the vacA i1 genotype had no significant additive effect on the risk for the development of peptic ulcer or gastric cancer in either geographically defined group. Moreover, multivariate analysis revealed no significantly increased risk of H. pylori-related disease in association with carriage of a strain of the vacA i1 genotype and is thus inconsistent with the hypothesis of Rhead et al. (16), who suggested that the vacA i-region genotype might be a better predictor of gastric cancer than the s- and m-region genotype in an Iranian population. We were also unable to confirm their observation that only strains with the naturally occurring vacA s1-m2 genotype had variations in the vacA i region, that strains of the s1-i1-m2 genotype are more virulent, and that the s1-i1-m2 genotype is more likely associated with gastric cancer than the s1-i2-m2 genotype (16). In contrast, the current study showed that there was also no significant additive effect of infection with a strain of the vacA s1-i1-m2 subtype compared with infection with a strain of the s1-i2-m2 subtype on the risk of development of peptic ulcer and gastric cancer in both Western and Asian countries.
In conclusion, we consider the genotype of the vacA d region to be a histologically more sensitive marker of severe inflammation and gastric mucosal atrophy than the genotype of the vacA s or m region and the genotype of the vacA i region, as recently reported, and therefore, the d-region genotype might be one of risk factors for gastric cancer and peptic ulcer. However, the biological role of the d region is still unclear; and combination analysis of the genotype of this region and the host genetic genotype, which plays a significant role in the pathogenesis of H. pylori, may also be required. Therefore, future studies with isogenic strains with mutations in the d region will be necessary. This study might be limited due to the insufficient sample size. This limitation might cause a discrepancy in the differences in histology and disease risk determined on the basis of the vacA genotype. Future studies with a sufficient sample size will thus also be required.
Acknowledgments
The material presented here is based upon work supported in part by the Office of Research and Development Medical Research Service of the U.S. Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center. Y. Yamaoka is supported in part by NIH grant DK62813.
We thank Oscar Gutierrez (Universidad Nacional de Colombia, Bogota, Colombia) for providing clinical samples from Colombia.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 2.Basso, D., C. F. Zambon, D. P. Letley, A. Stranges, A. Marchet, J. L. Rhead, et al. 2008. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 135:91-99. [DOI] [PubMed] [Google Scholar]
- 3.Cover, T. L., and M. J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570-10575. [PubMed] [Google Scholar]
- 4.Cover, T. L., M. K. Tummuru, P. Cao, S. A. Thompson, and M. J. Blaser. 1994. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 269:10566-10573. [PubMed] [Google Scholar]
- 5.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, et al. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 6.El-Omar, E. M., C. S. Rabkin, M. D. Gammon, T. L. Vaughan, H. A. Risch, J. B. Schoenberg, et al. 2003. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124:1193-1201. [DOI] [PubMed] [Google Scholar]
- 7.el-Zimaity, H. M., D. Y. Graham, M. T. al-Assi, H. Malaty, T. J. Karttunen, D. P. Graham, et al. 1996. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum. Pathol. 27:35-41. [DOI] [PubMed] [Google Scholar]
- 8.Hussein, N. R., M. Mohammadi, Y. Talebkhan, M. Doraghi, D. P. Letley, M. K. Muhammad, et al. 2008. Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H. pylori-associated disease. J. Clin. Microbiol. 46:1774-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwamoto, H., D. M. Czajkowsky, T. L. Cover, G. Szabo, and Z. Shao. 1999. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 450:101-104. [DOI] [PubMed] [Google Scholar]
- 10.Kersulyte, D., A. K. Mukhopadhyay, B. Velapatino, W. Su, Z. Pan, C. Garcia, et al. 2000. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 182:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letley, D. P., and J. C. Atherton. 2000. Natural diversity in the N terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J. Bacteriol. 182:3278-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu, H., P. I. Hsu, D. Y. Graham, and Y. Yamaoka. 2005. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology 128:833-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu, H., Y. Yamaoka, and D. Y. Graham. 2005. Helicobacter pylori virulence factors: facts and fantasies. Curr. Opin. Gastroenterol. 21:653-659. [DOI] [PubMed] [Google Scholar]
- 14.Moll, G., E. Papini, R. Colonna, D. Burroni, J. Telford, R. Rappuoli, et al. 1995. Lipid interaction of the 37-kDa and 58-kDa fragments of the Helicobacter pylori cytotoxin. Eur. J. Biochem. 234:947-952. [DOI] [PubMed] [Google Scholar]
- 15.Ogiwara, H., D. Y. Graham, and Y. Yamaoka. 2008. vacA i-region subtyping. Gastroenterology 134:1267. [DOI] [PubMed] [Google Scholar]
- 16.Rhead, J. L., D. P. Letley, M. Mohammadi, N. Hussein, M. A. Mohagheghi, M. Eshagh Hosseini, et al. 2007. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133:926-936. [DOI] [PubMed] [Google Scholar]
- 17.Rugge, M., P. Correa, F. Di Mario, E. El-Omar, R. Fiocca, K. Geboes, et al. 2008. OLGA staging for gastritis: a tutorial. Dig. Liver Dis. 40:650-658. [DOI] [PubMed] [Google Scholar]
- 18.Rugge, M., A. Meggio, G. Pennelli, F. Piscioli, L. Giacomelli, G. De Pretis, et al. 2007. Gastritis staging in clinical practice: the OLGA staging system. Gut 56:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto, M., T. Furuta, N. Shirai, A. Nakamura, M. Kajimura, H. Sugimura, et al. 2005. Poor metabolizer genotype status of CYP2C19 is a risk factor for developing gastric cancer in Japanese patients with Helicobacter pylori infection. Aliment. Pharmacol. Ther. 22:1033-1040. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto, M., T. Furuta, N. Shirai, A. Nakamura, F. Xiao, M. Kajimura, et al. 2007. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J. Gastroenterol. Hepatol. 22:51-59. [DOI] [PubMed] [Google Scholar]
- 21.Reference deleted.
- 22.Suzuki, S., Y. Muroishi, I. Nakanishi, and Y. Oda. 2004. Relationship between genetic polymorphisms of drug-metabolizing enzymes (CYP1A1, CYP2E1, GSTM1, and NAT2), drinking habits, histological subtypes, and p53 gene point mutations in Japanese patients with gastric cancer. J. Gastroenterol. 39:220-230. [DOI] [PubMed] [Google Scholar]
- 23.Torres, V. J., S. E. Ivie, M. S. McClain, and T. L. Cover. 2005. Functional properties of the p33 and p55 domains of the Helicobacter pylori vacuolating cytotoxin. J. Biol. Chem. 280:21107-21114. [DOI] [PubMed] [Google Scholar]
- 24.Torres, V. J., M. S. McClain, and T. L. Cover. 2004. Interactions between p-33 and p-55 domains of the Helicobacter pylori vacuolating cytotoxin (VacA). J. Biol. Chem. 279:2324-2331. [DOI] [PubMed] [Google Scholar]
- 25.Vinion-Dubiel, A. D., M. S. McClain, D. M. Czajkowsky, H. Iwamoto, D. Ye, P. Cao, et al. 1999. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J. Biol. Chem. 274:37736-37742. [DOI] [PubMed] [Google Scholar]
- 26.Wang, H. J., and W. C. Wang. 2000. Expression and binding analysis of GST-VacA fusions reveals that the C-terminal approximately 100-residue segment of exotoxin is crucial for binding in HeLa cells. Biochem. Biophys. Res. Commun. 278:449-454. [DOI] [PubMed] [Google Scholar]
- 27.Yamaoka, Y., T. Kodama, O. Gutierrez, J. G. Kim, K. Kashima, and D. Y. Graham. 1999. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J. Clin. Microbiol. 37:2274-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaoka, Y., T. Kodama, K. Kashima, D. Y. Graham, and A. R. Sepulveda. 1998. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J. Clin. Microbiol. 36:2258-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaoka, Y., T. Kodama, M. Kita, J. Imanishi, K. Kashima, and D. Y. Graham. 1998. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter 3:241-253. [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka, Y., O. Ojo, S. Fujimoto, S. Odenbreit, R. Haas, O. Gutierrez, et al. 2006. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut 55:775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]