Abstract
We evaluated boronic acid (BA)-based methods for their ability to detect extended-spectrum β-lactamases (ESBLs) among clinical isolates of KPC-producing members of the Enterobacteriaceae family. A total of 155 isolates of Klebsiella pneumoniae (n = 141), Escherichia coli (n = 6), Enterobacter aerogenes (n = 6), and Klebsiella oxytoca (n = 2) genotypically confirmed to be KPC producers were analyzed. As many as 118 isolates harbored ESBLs (103 harbored SHV-type ESBLs, 13 harbored CTX-M-type ESBLs, and 2 harbored both SHV- and CTX-M-type ESBLs); the remaining 37 isolates were genotypically negative for ESBL production. The CLSI ESBL confirmatory test was positive for 79 of the 118 ESBL producers (sensitivity, 66.9%), while all 37 non-ESBL producers were negative (specificity, 100%). When a ≥5-mm increase in the zone diameter of either the cefotaxime (CTX)-clavulanate (CA) or the ceftazidime (CAZ)-CA disks containing BA compared with the zone diameter of the CTX or CAZ disks containing BA was considered to be a positive result for ESBL production, the method detected all 118 ESBL producers (sensitivity, 100%) and showed no false-positive results for non-ESBL producers (specificity, 100%). Double-disk synergy tests, in which disks of CTX, CAZ, aztreonam, or cefepime in combination with BA were placed at distances of 20, 25, and 30 mm (center to center) from a disk containing amoxicillin (amoxicilline)-clavulanate-BA, were able to detect 116 (98.3%), 101 (85.6%), and 28 (23.7%) of the ESBL-positive isolates, respectively; no false-positive results for non-ESBL-producing isolates were detected. Our results demonstrate that the modified CLSI ESBL confirmatory test with antibiotic disks containing BA is the most accurate phenotypic method for the detection of ESBLs in Enterobacteriaceae producing KPC carbapenemases.
During the last decade, carbapenem resistance has emerged among clinical isolates of the Enterobacteriaceae family, and this is increasingly attributed to the production of β-lactamases capable of hydrolyzing carbapenems (23). Among these enzymes, a new type of Ambler class A β-lactamase, the Klebsiella pneumoniae carbapenemase (KPC), has been rapidly spreading among K. pneumoniae isolates and other Enterobacteriaceae in the northeastern regions of the United States and has now spread to several regions of North and South America, as well as in Israel, China, and Greece (2, 13, 16, 21).
The current spread of KPC enzymes makes them a potential threat to currently available antibiotic-based treatments. These enzymes confer various levels of resistance to all β-lactams, including carbapenems, even though cefamycins and ceftazidime are only weakly hydrolyzed (15, 18). KPC-possessing strains frequently carry extended-spectrum β-lactamase (ESBL) genes (1, 3, 8, 13, 24), which could possibly contribute to the expression and dissemination of the β-lactam resistance trait (8, 18, 21). It should be also noted that KPCs and ESBLs are mostly plasmid-encoded determinants that can easily disseminate to other enterobacterial strains (3, 7, 15, 18, 26). Therefore, the phenotypic detection of ESBLs in KPC-producing isolates of the Enterobacteriaceae is of potential interest for epidemiological purposes as well as for limiting the spread of the underlying resistance mechanisms.
The CLSI recommends a phenotypic confirmatory test for ESBL production that consists of measuring the growth-inhibitory zones around both cefotaxime (CTX) and ceftazidime (CAZ) disks with or without clavulanate (CA) for K. pneumoniae, Klebsiella oxytoca, Escherichia coli, and Proteus mirabilis (4). Different double-disk synergy tests (DDSTs) based on the synergy of amoxicillin (amoxicilline)-clavulanate (AMC) with extended-spectrum cephalosporins and aztreonam have also been extensively used for the detection of ESBLs (7). However, strategies for the laboratory identification of ESBLs need to be reviewed and adjusted as additional mechanisms of resistance to β-lactams coexist in enterobacterial strains (7). KPCs hydrolyze several β-lactam antibiotics, and hence, the presence of an ESBL can be masked by the expression of a KPC. Moreover, the weak inhibition of KPCs by the β-lactam inhibitors (15, 18, 30) may interfere with the interpretation of ESBL detection methods and KPC enzymes may be mistaken for ESBLs. Thus, there is a need to accurately detect ESBLs in the presence of coexisting KPC expression.
Boronic acid (BA) compounds were recently reported to be reversible inhibitors of KPCs (6, 16, 27). In particular, we have shown that BA disk assays are considered positive for the detection of the KPC enzyme when the growth-inhibitory zone diameter around a meropenem, imipenem, or cefepime disk with phenylboronic acid is 5 mm or greater of the growth-inhibitory zone diameter around the disk containing meropenem or cefepime alone (27). The results of this study also showed that BA affected the activity of CAZ in ESBL-negative KPC-producing isolates but not in SHV ESBL-positive KPC-producing isolates, most likely due to the presence of the SHV ESBL, which is not restrained by BA (27). BA-based tests with disks of CAZ and CTX have also been successfully employed for the identification of ESBLs in AmpC producers (11, 25). These observations led us to design a modified CLSI ESBL confirmatory test using antibiotic disks containing BA as well as different DDSTs employing BA for the accurate detection of ESBLs in KPC-producing enterobacterial isolates.
MATERIALS AND METHODS
Clinical isolates.
A total of 155 clinical isolates of K. pneumoniae (n = 141), E. coli (n = 6), Enterobacter aerogenes (n = 6), and Klebsiella oxytoca (n = 2) genotypically confirmed to be KPC producers were included in the study. The isolates were collected from separate patients who were hospitalized in five tertiary-care hospitals located in four distinct Greek regions (two hospitals in the broad region of Athens and one hospital each in Thessaloniki, Larissa, and Serres). The presence of blaKPC was determined by using previously described oligonucleotide primers and cycling conditions (14). The identification of all isolates was confirmed by using the API 20E system (bioMerieux, Marcy l'Etoile, France).
Antimicrobial susceptibility testing and phenotypic screening.
Detailed susceptibility analysis was carried out by the agar dilution method according to the guidelines and interpretative criteria of the CLSI (4). The phenotypic detection of KPC-possessing K. pneumoniae isolates was evaluated by BA disk tests (27). The screening for the possible production of class B carbapenemases was performed with the metallo-β-lactamase (MBL) Etest (AB Biodisk, Solna, Sweden) and the combined disk test with imipenem and EDTA (9).
Molecular testing for β-lactamase genes.
β-Lactamase genes were amplified by using a panel of primers for the detection of all types of MBLs (10); KPCs (14); plasmid-mediated AmpCs in single PCRs for each gene (17); and ESBLs, including the SHV, TEM, CTX-M, and GES/IBC enzymes (29). Total cellular RNA was extracted with the TRI reagent (Ambion, Austin, TX), and reverse transcription of 1 μg of total RNA was performed with a ThermoScript reverse transcription-PCR system (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Derepressed AmpC-hyperproducing E. aerogenes isolates were identified by quantitative real-time PCR with brilliant SYBR green (Qiagen, Hilden, Germany) and primers 5′-TGCGTGTCATAACATTATCCG-3′ and 5′-AACCCGTAGCCCAGGTAAAC-3′ (22). The positive controls used were previously characterized isolates from our collection carrying all types of tested β-lactamases. The PCR products were subjected to direct sequencing. The PCR products were purified with the ExoSAP-IT reagent (USB Corporation, Cleveland, OH) and were used as templates for the sequencing of both strands with an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA).
ESBL detection by the CLSI confirmatory test and two different tests using BA.
The stock solution of BA was prepared as described previously (5) by dissolving phenylboronic acid (benzeneboronic acid; Sigma-Aldrich, Steinheim, Germany) in dimethyl sulfoxide and water at a concentration of 20 mg/ml. From this solution, 20 μl was dispensed onto commercially available disks containing CTX (30 μg) or CAZ (30 μg) with or without CA (10 μg). The final amount of BA on the disks was 400 μg. The disks were then dried and were used within 60 min.
The CLSI confirmatory test for ESBL production was performed by inoculating Mueller-Hinton agar by the standard diffusion method and placing disks containing CTX or CAZ with or without CA onto the agar (4). The test was considered positive when an increase in the growth-inhibitory zone around either the CTX or the CAZ disk with CA was 5 mm or greater of the growth-inhibitory zone diameter around the disk containing CTX or CAZ alone.
In addition, the following tests were performed for the detection of ESBLs. (i) Similar to the CLSI ESBL confirmatory test, a ≥5-mm increase in the zone diameter of either CTX-CA or CAZ-CA disks tested in combination with BA (CTX-CA-BA and CAZ-CA-BA, respectively) compared with the zone diameter of CTX or CAZ disks containing BA (CTX-BA and CAZ-BA, respectively) was considered a positive result for ESBL production. (ii) DDSTs were performed by placing disks of CTX, CAZ, aztreonam, or cefepime (30 μg each) in combination with BA at distances of 30, 25, and 20 mm (center to center) from a disk containing AMC (10 μg)-BA. (iii) DDSTs were performed by placing disks of CTX, CAZ, aztreonam, or cefepime (30 μg each) at a distance of 20 mm (center to center) from a disk containing AMC (10 μg). For the DDSTs, ESBL production was inferred when the cephalosporin or aztreonam inhibition zone was expanded in the presence of AMC. Interpretation of the results of all tests was performed after incubation of the agar plates at 37°C for 18 h.
Sensitivity and specificity.
The performance of the various tests for the detection of ESBLs in KPC producers was evaluated by using PCR as the “gold standard.” For each test, the sensitivity was calculated from the number of ESBL-possessing organisms that were correctly determined, while the specificity was calculated from the number of non-ESBL-possessing organisms that were correctly determined.
RESULTS
Molecular testing for β-lactamase genes in KPC-producing isolates.
Of the 155 KPC-producing isolates of K. pneumoniae, E. coli, E. aerogenes, and K. oxytoca, 118 harbored ESBLs, while the remaining 37 isolates were genotypically negative for ESBL production. PCR and sequencing analyses showed that 103 (87.3%) of the ESBL producers harbored SHV-type ESBLs and 13 (11.0%) harbored CTX-M-type ESBLs, while the remaining 2 (1.7%) harbored both SHV- and CTX-M-type ESBLs (Table 1). SHV-12 was the predominant SHV-type ESBL, and CTX-M-15 was the predominant CTX-M-type ESBL. None of the isolates contained GES/IBC- or TEM-type ESBLs, plasmid-mediated AmpCs, or MBLs. blaKPC genes were accompanied by blaTEM-1 in as many as 77 of the ESBL producers and 24 of the non-ESBL producers. Moreover, five of the non-ESBL-producing isolates harbored the broad-spectrum β-lactamase SHV-11. In addition, quantitative real-time PCR showed that compared to a control E. aerogenes strain containing inducible chromosomal AmpC, all six E. aerogenes isolates contained stably derepressed AmpCs.
TABLE 1.
Distribution of ESBL genotypes in bacterial isolates used for validation of test methods
| Strain group and genotype(s) | No. of isolates |
||||
|---|---|---|---|---|---|
| Klebsiella pneumoniae (n = 141) | Klebsiella oxytoca (n = 2) | Escherichia coli (n = 6) | Enterobacter aerogenes (n = 6) | Total (n = 155) | |
| ESBL-producing isolatesa | |||||
| blaCTX-M-3 | 3 | 3 | |||
| blaCTX-M-15 | 9 | 1 | 10 | ||
| blaSHV-5 | 5 | 5 | |||
| blaSHV-12 | 94 | 2 | 2 | 98 | |
| blaSHV-12 + blaCTX-M-15 | 2 | 2 | |||
| Subtotal | 113 | 2 | 1 | 2 | 118 |
| Non-ESBL- producing isolatesb | 28 | 0 | 5 | 4 | 37 |
| Total | 141 | 2 | 6 | 6 | 155 |
Of the ESBL-producing isolates, 77 contained blaTEM-1.
Of the non-ESBL-producing isolates, 24 contained blaTEM-1 and 5 contained blaSHV-11.
Antimicrobial susceptibility testing of KPC-producing isolates.
The MIC data for the ESBL-producing and non-ESBL-producing isolates are summarized in Table 2. The range of MICs, the MIC50s, and the MIC90s of the β-lactam antibiotics tested were lower for KPC producers that did not harbor ESBLs than for those that harbored ESBLs. These differences were more obvious for CAZ, suggesting the contribution of ESBLs to the CAZ resistance levels.
TABLE 2.
Antimicrobial susceptibilities to β-lactam antibiotics for 118 KPC- and ESBL-producing isolates and 37 KPC-producing non-ESBL-producing isolates
| Strain group and antimicrobial | MIC (μg/ml) |
% Resistanta | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| ESBL-producing isolates | ||||
| Aztreonam | 128->256 | 256 | >256 | 100 |
| Cefepime | 16-128 | 64 | 128 | 85.6 |
| CTX | 64->128 | 64 | >128 | 100 |
| CAZ | 128->256 | 128 | >256 | 100 |
| Ertapenem | 4-64 | 16 | 32 | 90.7 |
| Imipenem | 4-64 | 16 | 32 | 66.1 |
| Meropenem | 2-64 | 16 | 32 | 61.9 |
| Piperacillin-tazobactam | 256->256 | 256 | >256 | 100 |
| Non-ESBL-producing isolates | ||||
| Aztreonam | 64->256 | 128 | 256 | 100 |
| Cefepime | 8-128 | 16 | 128 | 43.2 |
| CTX | 16-128 | 64 | 128 | 73.0 |
| CAZ | 8-128 | 32 | 128 | 70.3 |
| Ertapenem | 4-64 | 8 | 32 | 54.0 |
| Imipenem | 2-32 | 8 | 16 | 43.2 |
| Meropenem | 2-32 | 8 | 16 | 37.8 |
| Piperacillin-tazobactam | 16->256 | 128 | 256 | 81.1 |
The CLSI breakpoints used to define resistance were as follows: aztreonam, ≥32 μg/ml; ertapenem, ≥8 μg/ml; cefepime, ≥32 μg/ml; CTX, ≥64 μg/ml; CAZ, ≥32 μg/ml; imipenem, ≥16 μg/ml; meropenem, ≥16 μg/ml; piperacillin-tazobactam, ≥128/4 μg/ml.
Phenotypic testing for ESBLs.
Table 3 presents the phenotypic test results and the performance characteristics of the tests for the 118 ESBL PCR-positive and the 37 ESBL PCR-negative KPC-producing clinical isolates.
TABLE 3.
Sensitivities, specificities, positive predictive values, and negative predictive values of the various combination tests and DDSTs evaluated for the phenotypic detection of ESBLs in 155 KPC-producing isolatesa
| ESBL screening method | No. (%) of isolates confirmed by PCR to be: |
Test performance (%) |
||||
|---|---|---|---|---|---|---|
| ESBL-producing isolates (n = 118) | Non-ESBL-producing isolates (n = 37) | Sensitivity | Specificity | PPV | NPV | |
| CTX-CA vs CTX and/or CAZ-CA vs CAZ | 79 (66.9) | 0 (0) | 66.9 | 100 | 100 | 48.7 |
| CTX-CA-ΒΑ vs CTX-ΒΑ and/or CAZ-CA-ΒΑ vs CAZ-ΒΑ | 118 (100) | 0 (0) | 100 | 100 | 100 | 100 |
| DDST,b ATM, CAZ, CTX, or FEP 20 mm from AMC | 8 (6.8) | 0 (0) | 6.8 | 100 | 100 | 25.2 |
| DDST,b ATM-BA, CAZ-BA, CTX-BA, or FEP-BA 30 mm from AMC-BA | 28 (23.7) | 0 (0) | 23.7 | 100 | 100 | 29.1 |
| DDST,b ATM-BA, CAZ-BA, CTX-BA, or FEP-BA 25 mm from AMC-BA | 101 (85.6) | 0 (0) | 85.6 | 100 | 100 | 68.5 |
| DDST,b ATM-BA, CAZ-BA, CTX-BA, or FEP-BA 20 mm from AMC-BA | 116 (98.3) | 0 (0) | 98.3 | 100 | 100 | 94.9 |
Abbreviations: ATM, aztreonam; FEP, cefepime; PPV, positive predictive value, NPV, negative predictive value. All other abbreviations are defined in the text.
At least one combination.
(i) CLSI ESBL confirmatory test.
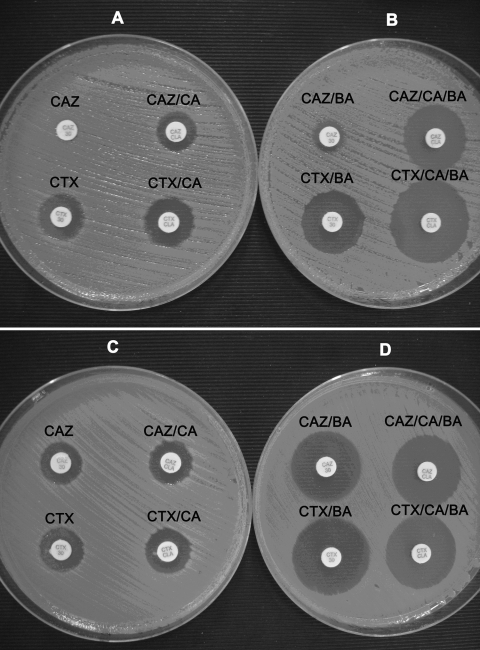
The CLSI confirmatory test for ESBL production, performed with CTX and CAZ disks with and without CA, yielded positive results for 13 (11.0%) and 79 (66.9%) of the 118 ESBL-producing isolates, respectively (Fig. 1). In total, by the CLSI confirmatory test, 79 of the ESBL producers showed a ≥5-mm increase in the zone diameters around either the CTX-CA or the CAZ-CA disks (sensitivity, 66.9%; Table 3). It is worth mentioning that the test was not able to detect any one of the 15 KPC producers that harbored CTX-M enzymes. None of the 37 non-ESBL-producing isolates showed a ≥5-mm increase in the zone diameters of either the CTX-CA or the CAZ-CA disks (specificity, 100%; Table 3).
FIG. 1.
Representative results of the CLSI ESBL confirmatory test (A and C) and its modification using antibiotic disks containing BA (B and D) for ESBL PCR-positive (A and B) and ESBL PCR-negative (C and D) KPC-possessing isolates.
(ii) Modified CLSI ESBL confirmatory test with both CA and BA.
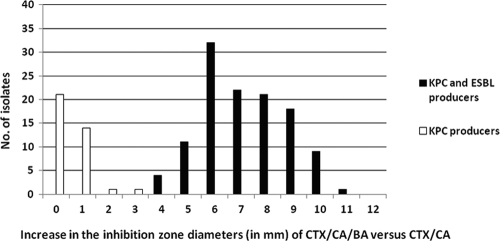
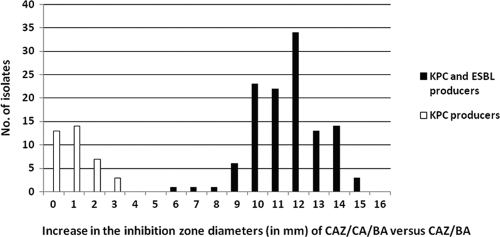
When the modified CLSI ESBL confirmatory test, which uses both CA and BA, was applied, the CTX and CAZ disks yielded positive results for 114 (96.6%) and 118 (100%) of the ESBL-producing isolates, respectively (Fig. 1). In total, the combined phenotypic test in the presence of BA showed for all 118 ESBL producers a ≥5-mm increase in the zone diameters of either the CTX-CA-BA or the CAZ-CA-BA disks (sensitivity, 100%; Table 3). The increases in the zone diameters were higher for CAZ-CA-BA disks than for CTX-CA-BA disks, possibly because the majority of isolates produced SHV-type ESBLs (Fig. 2 and 3). Compared with the results obtained with the CTX-BA disks, the CTX-CA-BA disks failed to detect four isolates carrying SHV-type ESBLs, although they detected all CTX-M-type producers. None of the 37 non-ESBL-producing isolates showed a ≥5-mm increase in the zone diameter of either the CTX-CA-BA or the CAZ-CA-BA disks (specificity, 100%; Table 3).
FIG. 2.
Increase in the inhibition zone diameters of CTX-CA-BA disks versus those of CTX-BA disks for 118 isolates producing both KPCs and ESBLs and 37 isolates producing KPCs but not ESBLs.
FIG. 3.
Increase in the inhibition zone diameters of CAZ-CA-BA disks versus those of CAZ-BA disks for 118 isolates producing both KPCs and ESBLs and 37 isolates producing KPCs but not ESBLs.
(iii) DDSTs.
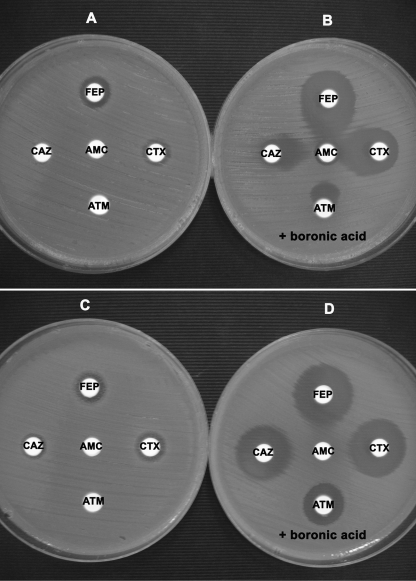
Table 4 shows the results of a comparative evaluation of various forms of DDSTs applied to the screening of ESBLs in KPC-producing isolates. The DDST performed by placing disks of CTX, CAZ, aztreonam, or cefepime at distance of 20 mm (center to center) from a disk containing AMC was able to detect only 8 of the 118 ESBL producers (sensitivity, 6.8%). The application of this DDST with the 37 non-ESBL producers gave negative results for all isolates (specificity, 100%; Fig. 4).
TABLE 4.
Comparative evaluation of various forms of DDSTs applied to screening of ESBLs in 118 ESBL- and KPC-producing isolatesa
| DDSTb | No. (%) of tests positive for ESBL- and KPC-producing isolates (n = 118) |
|---|---|
| AMC 20 mm from: | |
| CAZ | 0 |
| CTX | 3 (2.5) |
| ATM | 0 |
| FEP | 7 (5.9) |
| At least one combination | 8 (6.8) |
| AMC-BA 30 mm from: | |
| CAZ-BA | 6 (5.1) |
| CTX-BA | 13 (11.0) |
| ATM-BA | 2 (1.7) |
| FEP-BA | 26 (22.0) |
| At least one combination | 28 (23.7) |
| AMC-BA 25 mm from: | |
| CAZ-BA | 44 (37.3) |
| CTX-BA | 86 (72.9) |
| ATM-BA | 45 (38.1) |
| FEP-BA | 101 (85.6) |
| At least one combination | 101 (85.6) |
| AMC-BA 20 mm from: | |
| CAZ-BA | 103 (87.3) |
| CTX-BA | 111 (94.1) |
| ATM-BA | 93 (78.8) |
| FEP-BA | 114 (96.6) |
| At least one combination | 116 (98.3) |
All forms of DDSTs gave negative results for the 37 ESBL PCR-negative KPC-producing isolates.
Abbreviations: ATM, aztreonam; FEP, cefepime. All other abbreviations are defined in the text.
FIG. 4.
Representative results of DDSTs performed by placing antibiotic disks at a distance of 20 mm (A and C) and the DDST modification performed by using antibiotic disks containing BA (B and D) for ESBL PCR-positive (A and B) and ESBL PCR-negative (C and D) KPC-possessing isolates. ATM, aztreonam; FEP, cefepime.
The DDSTs performed by placing disks of CTX, CAZ, aztreonam, or cefepime in combination with BA at distances of 20 mm (center to center) from a disk containing AMC-BA were able to detect 111, 103, 93, and 114 of the 118 ESBL producers, respectively (Table 4; Fig. 4). In total, the test was able to detect 116 ESBL producers with at least one combination (sensitivity, 98.3%; Table 3). Application of the test to the 37 non-ESBL producers gave consistently negative results for all isolates (specificity, 100%; Table 3). DDSTs performed by placing disks of CTX, CAZ, aztreonam, or cefepime in combination with BA at distances of 25 and 30 mm (center to center) from a disk containing AMC-BA were able to detect 101 (85.6%) and 28 (23.7%) of the 118 ESBL producers, respectively; they showed no false-positive results for non-ESBL producers (Tables 3 and 4). It should be noted that when the distance of 30 mm (center to center) was used, none of the CTX-M producers was detected.
DISCUSSION
The complex epidemiology of β-lactamases among multidrug-resistant isolates of the Enterobacteriaceae family complicates the accurate phenotypic detection of different types of β-lactamases. KPC enzymes have recently disseminated among K. pneumoniae isolates in different regions worldwide (12, 13, 15, 21). These β-lactamases have also been sporadically detected in other enterobacterial species (3, 15). KPC enzymes efficiently hydrolyze all β-lactam molecules but lack any significant catalytic activity for CAZ (15, 18, 30). Thus, the coproduction of ESBLs, such as the SHV and CTX-M derivatives, seems to contribute to the hydrolytic activity of KPCs and the levels of resistance to broad-spectrum cephalosporins (15, 18). Moreover, KPC genes may be cotransferred with ESBL genes (18, 26), and both KPC and ESBL genes are often associated with plasmid-mediated fluoroquinolone and aminoglycoside resistance determinants (15, 20). This may possibly contribute to the dissemination of additional resistance mechanisms among KPC producers. Therefore, the phenotypic detection of ESBLs in KPC-possessing isolates is important for epidemiological purposes, as well as from an infection control perspective, adding to the studies that may be performed for the preliminary characterization of antimicrobial resistance mechanisms.
In the present study the CLSI ESBL confirmatory test was initially evaluated for its ability to differentiate the ESBLs from a large collection of KPC-possessing clinical isolates of the Enterobacteriaceae. This combined test was able to identify almost 70% of the ESBL producers, in contrast to the DDSTa with disks of CTX, CAZ, aztreonam, and cefepime at a distance of 20 mm from a disk of AMC, which failed to detect ESBLs in the vast majority of ESBL PCR-positive KPC-producing isolates. In a previous study, the CLSI ESBL confirmatory test was able to detect a similar percentage of ESBL-positive isolates among a collection of AmpC-producing isolates of the Enterobacteriaceae (11). However, in that study, both CTX-CA and CAZ-CA disks identified equal numbers of ESBL-positive isolates, whereas in our study, CAZ-CA detected a considerably greater number of ESBL-positive isolates, possibly because the hydrolytic activity of the KPCs is greater for CTX than it is for CAZ.
Herein different phenotypic tests based on BA were evaluated for their ability to detect ESBLs in KPC producers. Disk tests based on the inhibitory activity of BA have originally been described for the identification of class C AmpC-type β-lactamase-producing enterobacterial pathogens (5). These tests were found to enhance considerably the growth-inhibitory zone around disks of cefamycins, allowing the differentiation of AmpC-producing isolates. It has also recently been described that BA disk tests with carbapenems or cefepime can be used for the accurate identification of KPC carbapenemases (6, 16, 27). Additionally, BA disk tests have been shown to exhibit high sensitivities and specificities for the detection of ESBLs even when they are potentially masked by the presence of a derepressed chromosomal or plasmid-mediated AmpC β-lactamase (11, 25). Corresponding to the findings presented in those studies, in the present study we proposed a modification of the CLSI confirmatory test based on BA in order to detect ESBLs in KPC-producing isolates. The inhibitory activity of BA on KPC enzymes seems to influence the sensitivities of the CTX-CA and CAZ-CA disks compared with those of the CTX and CAZ disks for the detection of ESBLs in KPC producers. The method was able to identify all ESBL PCR-positive isolates and did not give false-positive results for any of the ESBL PCR-negative isolates. It is also remarkable that the assay successfully detected ESBLs in E. aerogenes isolates with mutants with derepressed AmpC mutations that coproduced ESBL and KPC enzymes. This might indicate that the proposed modification of the CLSI ESBL confirmatory method could detect ESBLs even in isolates that harbor both KPCs and that overproduce AmpC enzymes.
DDSTs based on BA were also evaluated for their ability to identify ESBLs. We found that the sensitivity of the test was significantly improved by reducing the distance between the tested disks to 20 mm and using cefepime disks. Similar to those findings, we have previously proposed that the sensitivity of the DDST for the detection of ESBLs in Enterobacter spp. with stably derepressed AmpCs can be improved when the distance between the cefepime and the AMC disks is reduced to 20 mm (28). However, in the present study, none of the DDSTs based on BA were able to identify all ESBL producers, whereas the modified CLSI ESBL confirmatory test accurately differentiated all isolates. It should also be noted that the interpretation of DDST results may be difficult and subjective in some cases, whereas interpretation of the results of the combined tests is always objective (7).
In our study and despite previous implications that the inhibition of KPCs by β-lactam inhibitors might give false-positive phenotypic results for ESBL detection (19), the combination disk methods as well as the different DDSTs did not give false-positive results for the 37 ESBL PCR-negative KPC-producing isolates. This could be attributed to the weak inhibitory activity of CA referred to previously (15), which possibly does not interfere with the interpretation of our test results. The present findings are also in accordance with recent observations showing that the commercially used β-lactamase inhibitors (CA and tazobactam) are unable to lower the MICs of β-lactams for clinical KPC-producing isolates (8). In conclusion, our results demonstrate that BA compounds are useful tools in the clinical laboratory not only for the detection of KPCs but also for the identification of possibly coproduced ESBLs by means of a modified CLSI ESBL confirmatory test.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Bradford, P. A., S. Bratu, C. Urban, M. Visalli, N. Mariano, D. Landman, J. J. Rahal, S. Brooks, S. Cebular, and J. Quale. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55-60. [DOI] [PubMed] [Google Scholar]
- 2.Bratu, S., D. Landman, R. Haag, R. Recco, A. Eramo, M. Alam, and J. Quale. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430-1435. [DOI] [PubMed] [Google Scholar]
- 3.Cai, J. C., H. W. Zhou, R. Zhang, and G. X. Chen. 2008. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob. Agents Chemother. 52:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing, 19th informational supplement. M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Coudron, P. E. 2005. Inhibitor-based methods for detection of plasmid-mediated AmpC β-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J. Clin. Microbiol. 43:4163-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi, Y., B. A. Potoski, J. M. Adams-Haduch, H. E. Sidjabat, A. W. Pasculle, and D. L. Paterson. 2008. Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type β-lactamase by use of a boronic acid compound. J. Clin. Microbiol. 46:4083-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drieux, L., F. Brossier, W. Sougakoff, and V. Jarlier. 2008. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clin. Microbiol. Infect. 14(Suppl. 1):90-103. [DOI] [PubMed] [Google Scholar]
- 8.Endimiani, A., A. M. Hujer, F. Perez, C. R. Bethel, K. M. Hujer, J. Kroeger, M. Oethinger, D. L. Paterson, M. D. Adams, M. R. Jacobs, D. J. Diekema, G. S. Hall, S. G. Jenkins, L. B. Rice, F. C. Tenover, and R. A. Bonomo. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J. Antimicrob. Chemother. 63:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin, C., L. Liolios, and A. Y. Peleg. 2006. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative bacilli in the clinical laboratory. J. Clin. Microbiol. 44:3139-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikonomidis, A., D. Tokatlidou, I. Kristo, D. Sofianou, A. Tsakris, P. Mantzana, S. Pournaras, and A. N. Maniatis. 2005. Outbreaks in distinct regions due to a single Klebsiella pneumoniae clone carrying a blaVIM-1 metallo-β-lactamase gene. J. Clin. Microbiol. 43:5344-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong, S. H., W. Song, M. J. Park, J. S. Kim, H. S. Kim, I. K. Bae, and K. M. Lee. 2008. Boronic acid disk tests for identification of extended-spectrum β-lactamase production in clinical isolates of Enterobacteriaceae producing chromosomal AmpC β-lactamases. Int. J. Antimicrob. Agents 31:467-471. [DOI] [PubMed] [Google Scholar]
- 12.Kitchel, B., J. K. Rasheed, J. B. Patel, A. Srinivasan, S. Navon-Venezia, Y. Carmeli, A. Brolund, and C. G. Giske. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53:3365-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leavitt, A., S. Navon-Venezia, I. Chmelnitsky, M. J. Schwaber, and Y. Carmeli. 2007. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob. Agents Chemother. 51:3026-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moland, E. S., N. D. Hanson, V. L. Herrera, J. A. Black, T. J. Lockhart, A. Hossain, J. A. Johnson, R. V. Goering, and K. S. Thomson. 2003. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711-714. [DOI] [PubMed] [Google Scholar]
- 15.Nordmann, P., G. Cuzon, and T. Naas. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228-236. [DOI] [PubMed] [Google Scholar]
- 16.Pasteran, F., T. Mendez, L. Guerriero, M. Rapoport, and A. Corso. 2009. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J. Clin. Microbiol. 47:1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Pérez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrella, S., N. Ziental-Gelus, C. Mayer, M. Renard, V. Jarlier, and W. Sougakoff. 2008. Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A β-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob. Agents Chemother. 52:3725-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai, D. R., R. Melano, P. Rawte, S. Lo, N. Tijet, M. Fuksa, N. Roda, D. J. Farrell, and S. Krajden. 2009. Klebsiella pneumoniae carbapenemase, Canada. Emerg. Infect. Dis. 15:827-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel, L., C. Leviandier, and P. Nordmann. 2006. Prevalence and genetic analysis of plasmid-mediated quinolone resistance determinants QnrA and QnrS in Enterobacteriaceae isolates from a French university hospital. Antimicrob. Agents Chemother. 50:3992-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pournaras, S., E. Protonotariou, E. Voulgari, I. Kristo, E. Dimitroulia, D. Vitti, M. Tsalidou, A. N. Maniatis, A. Tsakris, and D. Sofianou. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 64:348-352. [DOI] [PubMed] [Google Scholar]
- 22.Preston, K. E., C. C. Radomski, and R. A. Venezia. 2000. Nucleotide sequence of the chromosomal ampC gene of Enterobacter aerogenes. Antimicrob. Agents Chemother. 44:3158-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuelsen, Ø., U. Naseer, S. Tofteland, D. H. Skutlaberg, A. Onken, R. Hjetland, A. Sundsfjord, and C. G. Giske. 2009. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J. Antimicrob. Chemother. 63:654-658. [DOI] [PubMed] [Google Scholar]
- 25.Song, W., I. K. Bae, Y. N. Lee, C. H. Lee, S. H. Lee, and S. H. Jeong. 2007. Detection of extended-spectrum β-lactamases by using boronic acid as an AmpC β-lactamase inhibitor in clinical isolates of Klebsiella spp. and Escherichia coli. J. Clin. Microbiol. 45:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsakris, A., I. Kristo, A. Poulou, F. Markou, A. Ikonomidis, and S. Pournaras. 2008. First occurrence of KPC-2-possessing Klebsiella pneumoniae in a Greek hospital and recommendation for detection with boronic acid disc tests. J. Antimicrob. Chemother. 62:1257-1260. [DOI] [PubMed] [Google Scholar]
- 27.Tsakris, A., I. Kristo, A. Poulou, K. Themeli-Digalaki, A. Ikonomidis, D. Petropoulou, S. Pournaras, and D. Sofianou. 2009. Evaluation of boronic acid disk tests for differentiating KPC-possessing Klebsiella pneumoniae isolates in the clinical laboratory. J. Clin. Microbiol. 47:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzelepi, E., P. Giakkoupi, D. Sofianou, V. Loukova, A. Kemeroglou, and A. Tsakris. 2000. Detection of extended-spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzelepi, E., C. Magana, E. Platsouka, D. Sofianou, O. Paniara, N. J. Legakis, A. C. Vatopoulos, and L. S. Tzouvelekis. 2003. Extended-spectrum β-lactamase types in Klebsiella pneumoniae and Escherichia coli in two Greek hospitals. Int. J. Antimicrob. Agents 21:285-288. [DOI] [PubMed] [Google Scholar]
- 30.Yigit, H., A. M. Queenan, G. J. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]