Abstract
The performance of an amperometric biosensor, consisting of a subcutaneously implanted miniature (0.29 mm diameter, 5 × 10−4 cm2 mass transporting area), 90 s 10–90% rise/decay time glucose electrode, and an on-the-skin electrocardiogram Ag/AgCl electrode was tested in an unconstrained, naturally diabetic, brittle, type I, insulin-dependent chimpanzee. The chimpanzee was trained to wear on her wrist a small electronic package and to present her heel for capillary blood samples. In five sets of measurements, averaging 5 h each, 82 capillary blood samples were assayed, their concentrations ranging from 35 to 400 mg/dl. The current readings were translated to blood glucose concentration by assaying, at t = 1 h, one blood sample for each implanted sensor. The rms error in the correlation between the sensor-measured glucose concentration and that in capillary blood was 17.2%, 4.9% above the intrinsic 12.3% rms error of the Accu-Chek II reference, through which the illness of the chimpanzee was routinely managed. Linear regression analysis of the data points taken at t>1 h yielded the relationship (Accu-Chek) = 0.98 × (implanted sensor) + 4.2 mg/dl, r2 = 0.94. The capillary blood and the subcutaneous glucose concentrations were statistically indistinguishable when the rate of change was less than 1 mg/(dl⋅min). However, when the rate of decline exceeded 1.8 mg/(dl⋅min) after insulin injection, the subcutaneous glucose concentration was transiently higher.
Research and development programs toward continuous and intermittent glucose monitoring devices for management of diabetes are in progress. These programs include noninvasive monitoring through infrared spectroscopy (1–4), light scattering, (5, 6), transdermal monitoring through iontophoretic transport of a small but proportional fraction of glucose in the subcutaneous interstitial fluid (7,8), enhanced transdermal transport through suction (9), microdialysis (10–14) or ultrafiltration (15–17) of the subcutaneous interstitial fluid through subcutaneously implanted tubings and measurement of the glucose concentration in a downstream sensor external to the body, and subcutaneously implanted amperometric sensors (18–34).
Three types of amperometric subcutaneous glucose sensors, all involving glucose oxidase catalyzed processes (Eqs. 1-3),
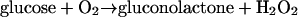 |
1 |
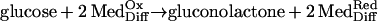 |
2 |
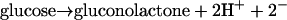 |
3 |
but with different detection schemes have been described:
(i) Sensors based on transducing the glucose concentration-dependent flux to hydrogen peroxide through reaction 1, followed by measurement of the current of electrooxidation of H2O2 to O2 (18–21) or by measurement of the current of O2 electroreduction (22–25);
(ii) Sensors transducing the glucose flux into a flux of a reduced diffusional electron carrying mediator (MedDiff), such as a ferrocene derivative (reaction 2), with measurement of the electrooxidation current of the diffusional mediator (26, 27);
(iii) Sensors based on the electrical connection (“wiring”) of reaction centers of immobilized glucose oxidase through an electron conducting hydrogel, enabling the direct electrooxidation of glucose (reaction 3) (29, 32-34).
In the latter, electrons are transferred from the glucose-reduced FAD/FADH2 cofactor comprising reaction centers of glucose oxidase to redox centers of the crosslinked polymer scaffold of the redox hydrogel, then through the redox centers of the polymer to the electrode. The electrons diffuse in the hydrogel through motion of segments of the hydrated polymer, whereby the scaffold-bound redox centers periodically approach each other sufficiently for electron transfer (35–37). The high current density, providing the basis for miniaturization of the mass transporting area, derives of the simultaneous achievement of adequate electron conductivity, and permeability of the water-soluble reactant (glucose) and product (gluconolactone) in the hydrogel. The concentration of oxygen can be two orders of magnitude lower in the body fluid of a diabetic patient than the concentration of glucose. Consequently, the minimal area through which oxygen is transported in a device producing a given current is also about two orders of magnitude larger than that of a device that does not consume oxygen but directly electrooxidizes glucose. In devices based on reaction 2, the mediator often is leached. This leaching shortens the life of the sensor and causes release of an unwanted chemical. Here we report the results of tests of “wired” glucose oxidase electrodes in a type I diabetic chimpanzee.
EXPERIMENTAL PROCEDURES
A 7-year-old, female, 40-kg, insulin-dependent, brittle diabetic chimpanzee (Pan troglodytes), whose illness was routinely managed by heel-prick blood testing by using an Accu-Chek II glucometer, was trained, through positive reinforcement, to wear a wrist-mounted, potentiostatic glucose monitor. Training sessions consisted of introducing and acclimating the chimpanzee to the wearing of the monitor. Only six training sessions, lasting a total of 20 h, were required.
The monitor was a single-channel, in-house-built potentiostat with digital display output in nA or, when calibrated in situ by analysis of one sample of withdrawn blood, the predicted glucose concentration in units of mg/dl of glucose. The monitor measured 80 × 55 × 28 mm, weighed approximately 120 g, and was powered by a 12-V, size A23 battery. It was set to maintain the sensing (working) electrode +300 mV with respect to the Ag/AgCl electrocardiogram reference electrode placed on the shaven skin of the wrist.
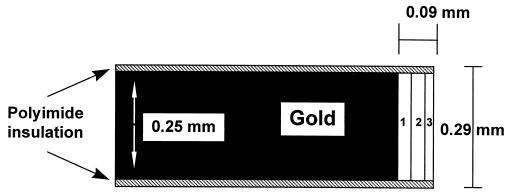
The implanted electrodes were those described earlier, shown schematically in Fig. 1 (29). The 5 × 10−4 cm2 mass transporting recessed area at the tip of a 0.29 mm diameter insulated gold wire was coated with three layers, weighing 2.5 × 10−6 g when dry. The “wired” enzyme sensing layer (Fig. 1, layer 1) was formed by crosslinking glucose oxidase to poly[(1-vinylimidazole)osmium(4,4′-dimethylbipyridine)2Cl] via poly(ethylene glycol) diclycidyl ether 400 (Polysciences). The glucose flux restricting layer (Fig. 2, layer 2) was formed by sequentially filling the 90 μm deep, 250 μm diameter recess at the wire’s tip and curing (at room temperature for 20 min) twice with 1% solution of cellulose acetate in cyclohexanone; once with a 0.5% solution of Nafion (Aldrich) in n-propanol; and once with a freshly prepared solution of poly(vinylpyridine) acetate (25 mg/ml in water) and polyfunctional aziridine (XAMA-7, E.I.T., Lakewillie, SC) (30 mg/ml in water) in a 1:2 volume ratio, this layer being cured for at least 8 h.
Figure 1.
Schematic drawing of the sensor. 1: “Wired” enzyme sensing layer. 2: Mass transport controlling membrane. 3: Biocompatible polyethylene oxide film. [Reproduced with permission from ref. 29 (Copyright 1998, American Chemical Society)].
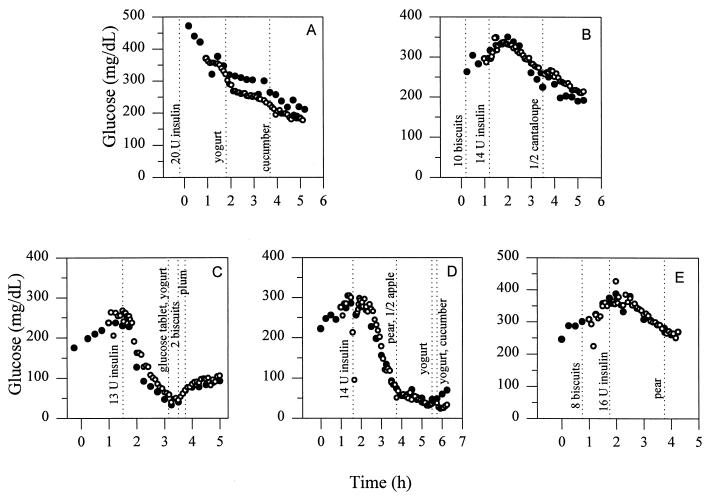
Figure 2.
Comparison of the Accu-Chek (•) and sensor estimated (○) glucose readings for the five individual experiments. The implanted subcutaneous glucose sensors were calibrated at t = 1 h by a one-point in vivo calibration.
The timing of the runs was defined by the availability of the chimpanzee and her trainer, not by the glycemia of the animal. By using a needle, the tough mid-dorsal aspect of the left wrist of the chimpanzee was pierced, and the sensing electrode was inserted through a 22-gauge catheter introducer (Bard Access Systems, Salt Lake City, UT) to a depth of less than 2 mm below the skin, the angle of insertion being about 15°. A new electrode was used for each run. Before insertion the sensors were disinfected by wiping with alcohol-soaked gauze. The electrode was held in place with a 2-inch × 3-inch adhesive pad. The Ag/AgCl electrocardiogram skin electrode (LecTec, Minnetonka, MN), served as both reference and counter electrode. Readings were recorded at 5-min intervals after implantation and every 2 min immediately after meals or insulin injections. Blood samples were taken from the heel of the chimpanzee every 15 min. These samples were assayed with the Accu-Chek II glucose monitor (Boehringer-Mannheim), for which an rms error of 12.3% was reported (38). The average duration time of the experiments, which were spaced approximately 2 weeks apart, was 5 h. The duration was limited to the periods in which the chimpanzee remained cooperative, as demanded by the protocol approved by The University of Texas at Austin Institutional Animal Care and Use Committee.
RESULTS
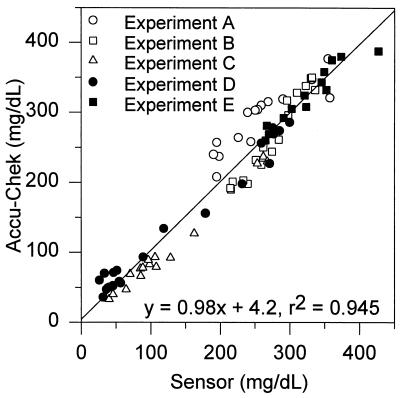
All postcalibration data of all runs are presented (Fig. 2 A-E). The currents recorded were converted to glucose concentrations through one-point in vivo calibration exactly 1 h after implantation, irrespective of whether the current (or glucose concentration) was steady, increasing, or decreasing at the time. At the t = 1 h point the Accu-Chek-read capillary blood glucose concentration of the chimpanzee was in Fig. 2: (A) 371 mg/dl, (B) 296 mg/dl, (C) 237 mg/dl, (D) 276 mg/dl, and (E) 308 mg/dl. At the time of calibration it was rising in two experiments, steady (at a maximum) in two, and declining in one experiment. The in vivo sensitivities of the sensors were in Fig. 2: (A) 0.013 nA/(mg/dl), (B) 0.08 nA/(mg/dl), (C) 0.18 nA/(mg/dl), (D) 0.12 nA/(mg/dl), and (E) 0.18 nA/(mg/dl). All sensors were functional but could not be reliably calibrated during the first 20 min after implantation. However, at t = 1 h all sensors could be calibrated, and further delay of calibration did not improve the accuracy. The errors were conservatively calculated by assuming that the Accu-Chek monitor readings were true or that any difference between the sensor and the Accu-Chek readings resulted from sensor inaccuracy. In fact, the rms error of this reference is 12.3% (38). The slope, intercept, and (squared) correlation coefficient (r2) were determined by two-parameter regression analysis. The 82 points of the combined linear regression plot of all postcalibration data points of all runs are presented in Fig. 3. The analysis (± 95% confidence interval) yielded the relationship, (Accu-Chek/capillary blood) = 0.98 (± 0.05) (sensor/subcutaneous fluid) + 4.2 (± 12.6) mg/dl, with a correlation coefficient r2 = 0.94, and an rms error of 17.2%.
Figure 3.
Overall correlation of the Accu-Chek-measured capillary blood and the sensor-measured subcutaneous glucose concentrations.
DISCUSSION
The results show that the “wired” glucose oxidase-based, (reaction 3) subcutaneously implanted, 0.29-mm electrodes track the capillary blood glucose concentration after one-point calibration at any arbitrarily chosen point in time 1 h past implantation (Fig. 3). Although their diameter was similar to that of sensors based on reaction 1, their much smaller mass transporting area of 5 × 10−4 cm2 vs. the 10−2 cm2 mass transporting area of reaction 1-based sensors (19) suggests that their further miniaturization is possible. The blood and subcutaneous glucose concentrations were correlated (r2 = 0.94) through the entire 35–400 mg/dl range of relevance in the management of diabetes. The implanted sensor’s readings would have been nearly as accurate (17.2% rms error) as those of the Accu-Chek monitor, for which an rms error of 12.3% has been reported (38), were it not for the transient true physiological discrepancy between the blood and the subcutaneous fluid glucose concentrations when the concentrations in blood declined particularly rapidly after insulin injection (39). The transient difference was consistent with that observed in the nondiabetic rat after intravenous insulin injection (39). On omission of 1 h of data during the period of steepest decline in the two runs, respectively 1.8 and 2.4 mg/(dl⋅min) (segments of the curves of Fig. 2 C and D), the overall rms error decreased to 15.5%. Under all other conditions, including rise or decline of the blood glucose concentration at a moderate rate of 1 mg/(dl⋅min), the two concentrations were not statistically distinguishable, consistent with reported results in humans and other mammals (40–43). At a higher rate of change a correction algorithm (39), based on knowledge of the instantaneous first derivative, improves the correlation. Thus, fast sensing of the subcutaneous glucose concentration is important for providing the instantaneous rate of change required for the correction terms.
Acknowledgments
We thank the National Institutes of Health for their financial support through Grant DK42015, Dr. Jerry Fineg for suggesting the collaboration of the Austin and Bastrop groups, and Dr. Chaim Yarnitzky for the wrist-worn electronic package.
References
- 1.Arnold M A. Curr Opin Biotechnol. 1996;7:46–49. doi: 10.1016/s0958-1669(96)80093-0. [DOI] [PubMed] [Google Scholar]
- 2.Spanner G, Niessner R. Fresenius’ J Anal Chem. 1996;355:327–328. doi: 10.1007/s0021663550327. [DOI] [PubMed] [Google Scholar]
- 3.Berger A J, Itzkan I, Feld M S. Spectrochim Acta, Part A. 1997;53:287–292. doi: 10.1016/s1386-1425(96)01779-9. [DOI] [PubMed] [Google Scholar]
- 4.Heise H M. Horm Metab Res. 1996;28:527–534. doi: 10.1055/s-2007-979846. [DOI] [PubMed] [Google Scholar]
- 5.Kohl M, Essenpreis M, Cope M. Phys Med Biol. 1995;40:1267–1287. doi: 10.1088/0031-9155/40/7/009. [DOI] [PubMed] [Google Scholar]
- 6.Bruulsema J T, Essenpreis M, Schmelzeisen-Redeker G, Heinemann L, Hayward J E, Bocker D, Berger M, Koschinsky T, Sandahl-Christiansen J, Orskov H, et al. Opt Lett. 1997;22:190–192. doi: 10.1364/ol.22.000190. [DOI] [PubMed] [Google Scholar]
- 7.Tamada J A, Bohannon N J V, Potts R O. Nat Med. 1995;1:1198–1201. doi: 10.1038/nm1195-1198. [DOI] [PubMed] [Google Scholar]
- 8.Rao G, Guy R H, Glikfeld P, LaCourse W R, Leung L, Tamada J, Potts R O, Azimi N. Pharm Res. 1995;12:1869–1873. doi: 10.1023/a:1016271301814. [DOI] [PubMed] [Google Scholar]
- 9.Kayashima S, Arai T, Noritake M, Nagata N, Kikuchi M, Ito N, Matsumoto Y, Kaneyoshi A, Kimura J, Kuriyama T. Clin Chim Acta. 1995;240:11–19. doi: 10.1016/0009-8981(95)06122-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Kissinger P T, Ohara T. Curr Sep. 1995;14:31–35. [Google Scholar]
- 11.Sternberg F, Meyerhoff C, Mennel F J, Bischof F, Pfeiffer E F. Diabetes Care. 1995;18:1266–1269. doi: 10.2337/diacare.18.9.1266. [DOI] [PubMed] [Google Scholar]
- 12.De Boer J, Korf J, Plijter-Groendijk H. Int J Artif Organs. 1994;17:163–170. [PubMed] [Google Scholar]
- 13.Hashiguchi Y, Sakakida M, Nishida K, Uemura T, Kajiwara K, Shichiri M. Diabetes Care. 1994;17:387–396. doi: 10.2337/diacare.17.5.387. [DOI] [PubMed] [Google Scholar]
- 14.Bolinder J, Ungerstedt J, Arner P. Lancet. 1993;30:1080–1085. doi: 10.1016/0140-6736(93)92063-y. [DOI] [PubMed] [Google Scholar]
- 15.Ash S R, Rainier J B, Zopp W E, Truitt R B, Janle E M, Kissinger P T, Poulos J T. ASAIO J. 1993;39:M699–M705. [PubMed] [Google Scholar]
- 16.Barnikol W K R, Weiler N. Biomed Technol. 1995;40:114–120. doi: 10.1515/bmte.1995.40.5.114. [DOI] [PubMed] [Google Scholar]
- 17.Moscone D, Venema K, Korf J. Med Biol Eng Comp. 1996;34:290–294. doi: 10.1007/BF02511240. [DOI] [PubMed] [Google Scholar]
- 18.Fischer, U. (1995) Acta Anesthesiol. Scand. 104, Suppl., 21–29. [DOI] [PubMed]
- 19.Thome-Duret V, Gangnerau M N, Zhang Y, Wilson G S, Reach G. Diabetes Metab. 1996;22:174–178. [PubMed] [Google Scholar]
- 20.Johnson K W, Allen D J, Mastrototaro J J, Morff R J, Nevin R S. ACS Symp Ser. 1994;556:84–95. [Google Scholar]
- 21.Moussy F, Harrison D J, O’Brien D W, Rajotte R V. Anal Chem. 1993;65:2072–2077. doi: 10.1021/ac00063a023. [DOI] [PubMed] [Google Scholar]
- 22.Updike S J, Shults M C, Rhodes R K, Gilligan B J, Luebow J O, von Heimburg D. ASAIO J. 1994;40:157–163. [PubMed] [Google Scholar]
- 23.Gooding J J, Hall E A H. Electroanalysis. 1996;8:407–413. [Google Scholar]
- 24.Gamburzev S, Atanasov P, Wilkins E. Sens Actuators. 1996;B30:179–183. [Google Scholar]
- 25.Wilkins E, Atanasov P, Muggenburg B A. Biosens Bioelectron. 1995;10:485–494. doi: 10.1016/0956-5663(95)96894-5. [DOI] [PubMed] [Google Scholar]
- 26.Matthews D R, Bown E, Beck T W, Plotkin E, Lock L, Gosden E, Wickham M. Diabetic Med. 1988;5:248–252. doi: 10.1111/j.1464-5491.1988.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 27.Claremont D J, Sambrook I E, Penton C, Pickup J C. Diabetologia. 1986;29:817–821. doi: 10.1007/BF00873223. [DOI] [PubMed] [Google Scholar]
- 28.Heller A. Curr Opin Biotechnol. 1996;7:50–54. doi: 10.1016/s0958-1669(96)80094-2. [DOI] [PubMed] [Google Scholar]
- 29.Schmidtke D W, Heller A. Anal Chem. 1998;70:2149–2155. doi: 10.1021/ac970932u. [DOI] [PubMed] [Google Scholar]
- 30.Quinn C P, Pathak C P, Heller A, Hubbell J A. Biomaterials. 1995;16:389–396. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 31.Quinn C P, Pishko M V, Schmidtke D W, Ishikawa M, Wagner J G, Raskin P, Hubbell J A, Heller A. Am J Physiol. 1995;269:E155–E161. doi: 10.1152/ajpendo.1995.269.1.E155. [DOI] [PubMed] [Google Scholar]
- 32.Csöregi E, Schmidtke D W, Heller A. Anal Chem. 1995;67:1240–1244. doi: 10.1021/ac00103a015. [DOI] [PubMed] [Google Scholar]
- 33.Csöregi E, Quinn C P, Schmidtke D W, Lindquist S E, Pishko M V, Ye L, Katakis I, Hubbell J A, Heller A. Anal Chem. 1994;66:3131–3138. doi: 10.1021/ac00091a022. [DOI] [PubMed] [Google Scholar]
- 34.Ohara T J, Rajagopalan R, Heller A. Anal Chem. 1994;66:2451–2457. doi: 10.1021/ac00087a008. [DOI] [PubMed] [Google Scholar]
- 35.Aoki A, Heller A. J Phys Chem. 1993;97:11014–11019. [Google Scholar]
- 36.Aoki A, Rajagopalan R, Heller A J. Phys Chem. 1995;99:5102–5110. [Google Scholar]
- 37.Rajagopalan R, Aoki A, Heller A. J Phys Chem. 1996;100:3719–3729. [Google Scholar]
- 38.North D S, Steiner J F, Woodhouse K M, Maddy J A. Diabetes Care. 1987;10:360–366. doi: 10.2337/diacare.10.3.360. [DOI] [PubMed] [Google Scholar]
- 39.Schmidtke D W, Freeland A C, Heller A, Bonnecaze R T. Proc Natl Acad Sci USA. 1998;95:294–299. doi: 10.1073/pnas.95.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebrin, K., von Antwerp, W. P. & Mastrototaro, J. J. (1997) Diabetes 46, Suppl. 1, 158A (abstr.).
- 41.Bantle, J. & Thomas, W. (1997) Diabetes 46, Suppl. 1, 160A (abstr.).
- 42.Service, F. J., O’Brien, P. C., Wise, S. D., Ness, S. & Leblanc, S. (1997) Diabetes 46, Suppl. 1, 160A (abstr.).
- 43.Jensen B M, Bjerring P, Christiansen J S, Oerskov H. Scand J Clin Lab Invest. 1995;55:427–432. doi: 10.3109/00365519509104982. [DOI] [PubMed] [Google Scholar]