Abstract
Sexually transmitted infections are a major public health problem in France and other European countries. Particularly, surveillance data about Neisseria gonorrhoeae infections have clearly indicated an increase in the incidence of gonorrhoea in France in 2006. The French laboratories participated on voluntary basis in the RENAGO (Réseau National du Gonocoque) network and sent all of their collected strains to the National Reference Center for Neisseria gonorrhoeae. In this first French molecular epidemiological study, 93 isolates collected in 2006 and representative of the French gonorrhoea epidemiology were selected. Antibiotic susceptibility to six antibiotics was determined, and serotyping and N. gonorrhoeae multiantigen sequence typing (NG-MAST) were performed. NG-MAST identified 53 sequence types (STs), of which 13 STs contained 2 to 16 isolates. The major STs identified in France were previously described elsewhere. However, two newly described STs, ST1479 and ST1987, had only been found in France until now. ST1479 was characterized by a multiple-resistance phenotype, whereas ST1987 presented a susceptibility phenotype. Moreover, among the predominant French STs, ST225, which had already been described in many countries, comprised isolates (14/16) resistant to ciprofloxacin and with reduced susceptibility to ceftriaxone. Thus, the surveillance of resistance to antibiotics is a priority in order to adapt treatment and decrease the transmission of resistant strains. Of note, no predominant ST was identified among rectal isolates from men who have sex with men.
Gonorrhoea, caused by the gram-negative bacterium Neisseria gonorrhoeae, remains one of the most common sexually transmitted infections (STIs), causing cervicitis, urethritis, ano-rectitis, and conjunctivitis of the newborn. Complications are epididymitis, prostatitis, salpingitis, and endometritis, which can lead to infertility, ectopic pregnancy, chronic pelvic pain, and cecity. Even though gonorrhoea is treatable and curable, this disease contributes to a substantial burden of morbidity, mortality, and infertility worldwide (10).
Over the last few years, the number of gonorrhoea cases has increased regularly in many European countries (1-3, 6), the United States (15), and China (7). The French National Reference Centre (NRC) for Neisseria gonorrhoeae receives isolates of gonorrhoea diagnosed in France, through a voluntary national network, RENAGO (Réseau National du Gonocoque), including 235 laboratories located across the entire national territory. Through this network, and particularly based on 211 active laboratories that send all of their collected isolates, an increase from 100 collected isolates in 1995 to more than 600 in 2006 was observed. With a constant number of participating laboratories, the average number of N. gonorrhoeae isolates per active laboratory and per year has significantly increased: e.g., there were 3.75 isolates/lab in 2006 versus 2.5 isolates/lab in 2005 (4). In the same way, the number of other STIs (including syphilis and Chlamydia trachomatis infections, including lymphogranuloma venereum) has increased, reflecting the resurgence of risky sexual behavior in France and more generally in Europe (3, 4).
One cause of concern is the development of resistance to antimicrobial therapy (7, 11, 14, 17). Since the first report of tetracycline resistance in 1985, gonococci that are resistant to tetracycline have spread globally, with the coexistence of chromosomally and plasmid-mediated resistance being observed in the majority of resistant isolates (16). During a sentinel surveillance study in Western Europe in 2004, a total of 1,055 gonococcal isolates were analyzed, showing 21.3% resistance to penicillin (8). In addition, high levels of ciprofloxacin-resistant gonococcal isolates have been reported worldwide, initially (in the early 1990s) in the Southeast Asia and Pacific regions and more recently in different parts of Europe. For example, in Scotland, episodes of gonorrhoea caused by ciprofloxacin-resistant strains reached 11% in 2002 and increased to 15.3% in 2003, prompting a change of first-line therapy from ciprofloxacin to broad-spectrum cephalosporins (11, 13). The levels of resistance to penicillins, tetracyclines, fluoroquinolones, and oral cephalosporins have also recently begun to increase in Japan. In this context, Tanaka et al. recently described an N. gonorrhoeae isolate with reduced susceptibility to ceftriaxone and a chromosomally mediated multidrug resistance phenotype (18). Isolates with high-level azithromycin resistance were recently reported in Scotland (14).
In France, the NRC for Neisseria gonorrhoeae has observed that the number of isolates with high-level resistance to penicillin due to penicillinase production increased from 7.2% in 2004 to 11.2% in 2006. The number of isolates with high resistance to tetracycline (MIC, ≥16 mg/liter) increased from 6.8% to 18%. More than 75% of isolates with high levels of penicillin resistance were also resistant to high levels of tetracycline. Resistance to ciprofloxacin reached more than 40%, and ciprofloxacin-resistant isolates were significantly more resistant to penicillin and tetracycline than were susceptible isolates. Resistance to ceftriaxone was not observed, but 20% of strains had increased MICs (0.016, 0.023, or 0.032 mg/liter) to this agent (4).
At present, there is no effective vaccine against N. gonorrhoeae. The control of gonococcal infections depends on the surveillance of populations at risk, on public health measures to limit the spread of infection, and on early intervention to treat infected individuals. For the prevention and control of infection and for the efficient surveillance of antibiotic resistance, it is crucial to have precise knowledge about the isolates that circulate in different communities, including the emergence of particular clones.
A number of typing methods have been used for molecular epidemiology studies of N. gonorrhoea. Some of these methods are based on growth requirements for specific nutrients, antibiotic susceptibility, differences in multilocus enzyme electrophoresis, and serological reactivity against surface antigens. Because the above approaches present several limitations, molecular typing methods have been developed with improved discrimination among gonococcal isolates. Of the currently available sequence-based methods, N. gonorrhoeae multiantigen sequence typing (NG-MAST) is one of the most advantageous because it generates a simple numerical sequence type (ST) based on the combined sequences of two genes (por and tbpB). In addition, an internationally accessible web database allows strain comparison worldwide (9).
The aim of our study was to analyze the N. gonorrhoeae populations circulating in France in 2006. In order to identify clusters of isolates from high-risk groups and clusters with particular antibiotic resistance phenotypes, we used the NG-MAST method. This study represents the first molecular epidemiological study of N. gonorrhoeae isolates from France.
MATERIALS AND METHODS
N. gonorrhoeae isolates.
All laboratories participating voluntarily in the RENAGO network sent all strains isolated to the NRC for Neisseria gonorrhoeae (Institut Alfred Fournier, Paris, France). In parallel, epidemiological data (patient gender, age, geographical location, and sampling type) were sent to the French Institute for Public Health Surveillance (InVS). For each isolate, phenotypic analysis (serotype and antibiotic phenotype) was performed. Among 295 isolates submitted to RENAGO between January and June 2006, 93 isolates were selected for this study in order to be representative of the entire population based on antibiotic resistance and populations of patients. A first group of 67 isolates (group I) was selected from different antibiotic resistance phenotypes (Table 1) and then from different geographic origins, from male and female patients, and finally from different sampling locations (cervix, urethra, urine, and knee fluid), in order to identify possible clusters. A second group of 26 isolates (group II) was selected from rectal samples from men who have sex with men (MSM), without of antibiotic phenotype restriction criteria, in order to identify specific clusters in this high-risk population.
TABLE 1.
Characteristics of antibiotic susceptibility phenotypes of the French N. gonorrhoeae isolates studied
| Phenotype (no. of strains) | Antibiotic susceptibility (MIC [mg/liter]) toa: |
||||||
|---|---|---|---|---|---|---|---|
| Penicillin | β-Lactamase | Tetracycline | Ciprofloxacin | Ceftriaxone | Spectinomycin | Erythromycinb | |
| 1 (10) | R (1-4) | Positive | hR (16-32) | I or R (0.5-4) | S (≤0.004) | S (4-8) | S |
| 2 (6) | I (0.064-0.19) | Negative | hR (16-32) | I or R (0.5-2) | S (≤0.004) | S (4-6) | S |
| 3 (39) | S or I (≤1) | Negative | S or I (0.125-1) | S (<0.002-0.012) | S (≤0.004) | S (4-12) | S |
| 4 (31) | I (1-1.5) | Negative | I or R (1.5-3) | hR (≥32) | rS (0.016-0.032) | S (4-8) | S |
| 5 (2) | I (0.5-0.75) | Negative | R (2) | S (0.008-0.012) | rS (0.012-0.016) | S (6-8) | S |
| 6 (5) | I (0.125-0.75) | Negative | I or R (1-2) | R or hR (4-32) | S (0.003-0.016) | S (4-8) | S |
The antimicrobial MIC interpretative standards and breakpoints for susceptibility and resistance (CLSI) for the antibiotics shown are as follows: penicillin MIC, ≥0.06 and ≥2; tetracycline MIC, ≥0.25 and ≥2; ciprofloxacin MIC, ≥0.06 and ≥1; ceftriaxone MIC, ≥0.5 and ≥1; and spectinomycin MIC, ≥32 and ≥64. S, susceptible; I, intermediate; R, resistant; rS, reduced susceptibility (ceftriaxone MIC, ≥0.012 and ≤0.032 mg/liter); hR, high level of resistance (tetracycline MIC, ≥16 mg/liter; ciprofloxacin MIC, ≥32 mg/liter).
Only the disk diffusion method was used for erythromycin.
Culture and identification.
The isolates were cultured on PVX chocolate agar (bioMérieux, Marcy-l'Étoile, France) supplemented with 1% IsoVitaleX and incubated overnight at 37°C with 5% carbon dioxide. Identification was performed with the API NH system (bioMérieux, Marcy-l'Étoile, France).
Phenotypic analysis.
Isolates were tested by routine analysis for antibiotic susceptibility and serotype. Susceptibility to six antibiotics (penicillin, ceftriaxone, tetracycline, ciprofloxacin, spectinomycin, and erythromycin) was measured using the disk diffusion method (bioMérieux, Marcy-l'Étoile, France). β-Lactamase production was detected by the cefinase test (bioMérieux, Marcy-l'Étoile, France). Etests (AB Biodisk, Sweden) were used to determine MICs of penicillin, ceftriaxone, tetracycline, ciprofloxacin, and spectinomycin. Results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) (Table 1). Isolates were serotyped with the Phadebact GC serovar test using monoclonal antibodies (Orgentec SAS, Trappes, France).
DNA extraction.
Isolates were retrieved from storage at −80°C in glycerol broth, cultured on PVX chocolate agar supplemented with 1% IsoVitaleX, and incubated overnight at 37°C with 5% carbon dioxide. A turbid suspension of the gonococcal subculture was made in phosphate-buffered saline (optical density of ∼2.0). The bacteria were pelleted by centrifugation at 2,000 × g for 5 min, washed once, resuspended in phosphate-buffered saline, and boiled for 5 min to lyse the cells. The lysate was centrifuged for 5 min at 2,000 × g, and the supernatant was stored at −20°C until required.
Molecular typing by NG-MAST.
Briefly, internal regions of the por and tbpB genes were amplified by PCR from the DNA extracts, and both strands were sequenced using with Big Dye v.1.1 chemistry on an ABI3730XL capillary sequencer (Applied BioSystems). Sequences were aligned, edited, and trimmed to a fixed length from conserved positions as described previously (9) using BioNumerics software (Applied Maths, Belgium). Alleles were assigned to each sequence variant of por and tbpB, and the corresponding ST was assigned through the NG-MAST website (http://www.ng-mast.net) based on the combination of the alleles of the two loci. All French isolate data were deposited in this database.
RESULTS
Our purpose was to provide a first overview of the epidemiology of N. gonorrhoeae in France in 2006, based on analysis of 93 isolates. The 67 group I isolates (from a heterosexual population and various geographic and clinical sources) came from Paris and its surroundings (25 isolates [37%]) and from other regions (42 isolates [63%]). Sixty-one isolates were from males and 6 from women. This low number of isolates from women could be explained by the asymptomatic or paucisymptomatic gonococcal infections observed in women who did not attend an STI clinic. The diagnosis of N. gonorrhoeae infection is more often casual, generally on the occasion of a gynecological checkup. Moreover, carriage in women is characterized by a low number of bacteria, which are particularly difficult to isolate, among the commensal vaginal bacteria. The ages were known for 56 men (mean, 32 years; range, 18 to 57 years) and 5 women (mean, 31 years; range, 18 to 62 years) and unknown for 6 other patients (5 men and 1 woman). Fifty-six isolates were obtained from male urethra, 6 from cervix, 4 from male urine, and 1 from the fluid of a male's knee. The last isolate was included because it was collected from an exceptional location and characterized by the rare antibiotic resistance phenotype 2. The majority of the group II isolates (MSM) came from Paris and its surrounding area (88%), with an average patient age of 33 years (range, 20 to 54 years).
Serotyping was performed on all isolates, which were divided into 29 serovars. The four largest groups were represented by 14 isolates (serovar Boprstvy), 11 isolates (serovar Boprsty), 10 isolates (serovar Ast), and 9 isolates (serovar Arst). Fourteen other serovars comprised 2 to 5 isolates, while 11 serovars were represented by a single isolate.
Based on the MICs obtained for penicillin (with or without β-lactamase production), tetracycline, ciprofloxacin, ceftriaxone, and spectinomycin, the 93 isolates could be classified into six phenotypes (Table 1), representing an overview of the diversity of antibiotic resistance phenotypes observed in France. Thirty-nine (42%) isolates had antibiotic susceptibility phenotype 3, characterized by a susceptibility to all tested antibiotics, whereas 31 (33%) isolates had phenotype 4 (with reduced susceptibility to ceftriaxone and high-level resistance to ciprofloxacin). The remaining isolates were divided into the four other susceptibility phenotypes, which were distinguished by their combination of resistance to tetracycline, ciprofloxacin, and ceftriaxone.
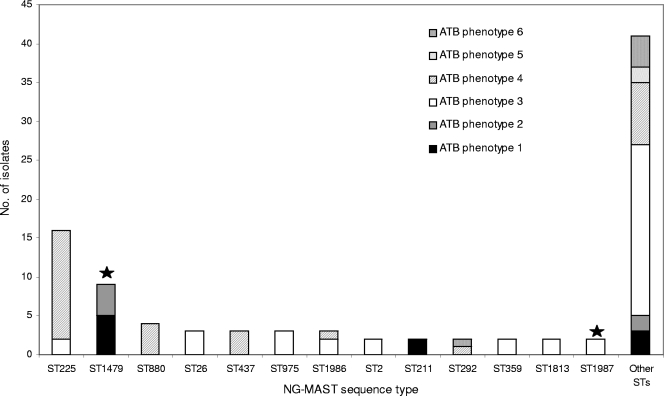
Based on NG-MAST, the 93 isolates were subdivided into 53 different STs, including previously described STs as well as new STs. The majority of STs (75%) were only represented by single isolate, whereas the remaining STs were represented by 2 to 16 isolates. The 13 most common STs were ST225 (16 isolates [17.2%]); ST1479 (9 isolates [9.7%]); ST880 (4 isolates [4.3%]); ST26, ST437, ST975, and ST1986 (3 isolates [3.2%]); and ST2, ST211, ST292, ST359, ST1813, and ST1987 (2 isolates [2.1%]). These STs had been previously described in other parts of the world, except for ST1479 and ST1987, which were not found in the NG-MAST database and thus have only been described in France so far (Table 2). There was no apparent geographic clustering of STs according to regions of France. Among the 67 group I isolates, we could identify 36 different STs, 10 of which included more than one isolate (ST225, ST1479, ST26, ST975, ST437, ST1986, ST2, ST1987, ST211, and ST880), together clustering 61% of the isolates (Table 2). Regarding the 26 isolates from group II, six STs were found with more than one isolate (ST225, ST880, ST292, ST359 and ST1813), grouping 38% of the isolates (Table 2). The diversity indices were similar in the two groups (Simpson's index, 92.3% versus 94.7%, respectively). Fourteen isolates from group I and two isolates from group II belonged to ST225, among which 14 isolates were characterized by antibiotic resistance phenotype 4. ST1479 grouped eight isolates from different geographic regions from group I and one isolate from group II. The eight isolates presented the two similar antibiotic resistance phenotypes 1 and 2 (differing by the presence or absence of a β-lactamase). Of note, the isolate from knee fluid was ST1479. ST880 was represented by two isolates from group I and two isolates from group II, all of which exhibited antibiotic phenotype 4. Antibiotic resistance phenotype 5 (with a reduced susceptibility to ceftriaxone) was observed only in two isolates from group I (ST21 and ST1985). These STs were closely related, since they share the same tbpB allele (allele 33) and their porB alleles differed by two single nucleotide polymorphisms (allele 14 for ST21 and allele 467 for ST1985). Antibiotic phenotype 6 (resistance to tetracycline and ciprofloxacin) was observed only in group II, in five isolates of distinct STs.
TABLE 2.
Characteristics of French N. gonorrhoeae isolates associated with main NG-MAST sequence types
| ST | Total no. of isolates | Isolate groupa | No. of isolates | Serotype | Geographic originb | Antibiotic susceptibility phenotypec | Antibiotic susceptibility tod: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin | β-Lactamase | Tetracycline | Ciprofloxacin | Ceftriaxone | Spectinomycin | Erythromycin | |||||||
| 225 | 16 | I | 12 | B | Paris + OR | 4 | I | Negative | I or R | hR | rS | S | S |
| I | 2 | B | Paris + OR | 3 | S or I | Negative | I | S | S | S | S | ||
| II | 2 | B | Paris | 4 | I | Negative | R | hR | rS | S | S | ||
| 1479 | 9 | I | 4 | A | Paris + OR | 1 | R | Positive | hR | I or R | S | S | S |
| I | 2 | A | Paris + OR | 2 | I | Negative | hR | I | S | S | S | ||
| I | 2 | B | Paris + OR | 2 | I | Negative | hR | R | S | S | S | ||
| II | 1 | A | Paris | 1 | R | Positive | hR | I | S | S | S | ||
| 880 | 4 | I | 2 | B | Paris + OR | 4 | I or R | Negative | R | hR | rS | S | S |
| II | 2 | B | Paris + OR | 4 | I | Negative | R | hR | rS | S | S | ||
| 26 | 3 | I | 2 | B | Paris | 3 | I or S | Negative | I | S | S | S | S |
| II | 1 | B | Paris | 3 | I | Negative | I | S | S | S | S | ||
| 437 | 3 | I | 3 | B | Paris + OR | 4 | I or R | Negative | R | R | rS | S | S |
| 975 | 3 | I | 3 | A | Paris + OR | 3 | S or I | Negative | S | S | S | S | S |
| 1986 | 3 | I | 2 | B | Paris + OR | 3 | S | Negative | S | S | S | S | S |
| I | 1 | B | 4 | I | Negative | R | hR | rS | S | S | |||
| 2 | 2 | I | 1 | A | Paris | 3 | S | Negative | S | S | S | S | S |
| I | 1 | B | OR | 3 | I | Negative | S | S | S | S | S | ||
| 211 | 2 | I | 2 | A | Paris + OR | 1 | R | Positive | hR | R | S | S | S |
| 292 | 2 | II | 1 | B | Paris | 6 | I | Negative | I | R | S | S | S |
| II | 1 | B | Paris | 4 | I | Negative | R | hR | rS | S | S | ||
| 359 | 2 | II | 2 | B | Paris | 3 | S | Negative | I | S | S | S | S |
| 1813 | 2 | II | 2 | B | Paris | 3 | I | Negative | I | S | S | S | S |
| 1987 | 2 | I | 1 | A | Paris | 3 | S | Negative | S | S | S | S | S |
| I | 1 | B | Paris | 3 | I | Negative | I | S | S | S | S | ||
As defined in Materials and Methods.
Paris, Paris and its surroundings; OR, other regions outside Paris and its surroundings.
As described in Table 1.
S, susceptible; I, intermediate; R, resistant; hR, high level of resistance (tetracycline MIC, ≥16 mg/liter; ciprofloxacin MIC, ≥32 mg/liter); rS, reduced susceptibility (ceftriaxone MIC, ≥0.012 and ≤0.032 mg/liter.
Notably, the genetic diversity differed according to the susceptibility phenotype of the isolates. Isolates with antibiotic phenotype 3 (susceptible to all tested antibiotics) were associated with many single STs, whereas a majority (22/31 [71%]) of isolates with antibiotic phenotype 4 (with reduced susceptibility to ceftriaxone and high resistance to ciprofloxacin) were clustered in the STs ST225, ST880, and ST437 (Fig. 1). In addition, these three STs were closely related, as the allele sequences of tbpB gene were identical (allele 4). Moreover, the por allele of ST225 (allele 4) differed from that of ST437 (allele 14) and ST880 (allele 489) by a single nucleotide polymorphism. These observations indicate a common ancestral origin of these isolates, from which they acquired their phenotype.
FIG. 1.
Distribution of NG-MAST STs of the French N. gonorrhoeae isolates obtained in 2006 (n = 93) and the antibiotic (ATB) phenotypes of the isolates. Others STs were represented only by one isolate. STs indicated by black stars are newly described STs in the NG-MAST database.
DISCUSSION
The increase in gonorrhoea in many developed countries and the occurrence of resistance to antimicrobial treatment constitute an important public health problem. Sequence-based genotyping methods have proven helpful to understand the transmission patterns of infection and to follow the emergence and spread of antibiotic resistance.
The 93 French isolates analyzed in this study revealed the same antibiotic phenotype trend observed in others parts of the world. All French isolates were susceptible to spectinomycin. In France, this antibiotic is only used in case of β-lactam allergy. Even though azithromycin was not tested, the probability that isolates were all susceptible to this agent is strong, since no resistance to erythromycin was observed for any isolate. In France, azithromycin is not used for N. gonorrhoeae treatment. In contrast, N. gonorrhoeae azithromycin resistance increased in Scotland (14). It may have occurred as a response to exposure to sublethal levels of azithromycin resulting from the treatment of C. trachomatis infections. Possibly another reason was azithromycin being prescribed alone in N. gonorrhoeae infection, as suggested by Habib and Fernando (5).
Reduction of susceptibility to ceftriaxone is worrying, especially in combination with ciprofloxacin resistance. As a consequence, since 2005, French guidelines have not recommended fluoroquinolones for N. gonorrhoeae treatment, which could hopefully result in a steady state or a decrease of resistance over the next few years. Ceftriaxone became the recommended treatment, always associated with azithromycin for C. trachomatis infections (Groupe de Travail sur les Antibiotiques, GTA no. 188, 6 June 2005; http://www.afsapps.sante.fr).
This study presents the first molecular epidemiology analysis of N. gonorrhoeae strains that are currently circulating in France. As in previous studies, the NG-MAST method revealed numerous single STs, probably due to the lack of effective procedures for an exhaustive diagnosis in partners or in the community. In addition, high recombination rates in N. gonorrhoeae result in a rapid evolution of genotypes. Only six isolates from women were analyzed, and all had different STs, excluding any interpretation about genotype variability related to gender.
No geographic clustering was observed, and even though 88% of isolates (23/26) from group II were isolated from MSM from the same area (Paris), no predominant ST was identified in this group. A particular antibiotic-resistant phenotype (phenotype 6) was only found in this group, with chromosomal resistance to tetracycline, high-level resistance to ciprofloxacin, and high susceptibility to ceftriaxone.
ST225, one of the main STs identified in France (16 isolates), is characterized by 87.5% of isolates with resistance to tetracycline and ciprofloxacin and by slightly reduced susceptibility to ceftriaxone (0.016 to 0.032 mg/liter). This ST was previously found to be prevalent in Denmark (S. H. Hoffman, L. Lambertsen, L. Berthelsen, and S. Cowan, Presented at the International Society for Sexually Transmitted Disease Research 16th Meeting, Amsterdam, The Netherlands, 10 to 13 July 2005), Scotland (12), and Australia (19, 20) and has been reported in Greece and Hong Kong (http://www.ng-mast.net). In Scotland, ST225 was the most prevalent ST (with 85 out of 1,526 isolates), where it had the same predominant antibiotic phenotype as in France (except that ceftriaxone was not reported) (12). In Australia, Todd et al. (19) described 10 out of 14 isolates of ST225 that showed the same antibiotic resistance phenotype as that in the present study, including the increased MIC of ceftriaxone (0.03 mg/liter). The two prevalent STs ST880 and ST437, which are genetically closely related to ST225, presented the same antibiotic resistance phenotype in our study as well as in other countries (http://www.ng-mast.net). These results are clearly indicative of the international spread of the antimicrobial-resistant clone that comprises ST225, ST880, and ST437. In contrast, French isolates from ST292, resistant to ciprofloxacin (phenotypes 4 and 6), did not share the same antibiotic resistance phenotype as ST292 isolates from Scotland (12), suggesting a recent acquisition of resistance in France.
ST1479 comprised isolates resistant to ciprofloxacin, with plasmidic resistance to penicillin and tetracycline. This ST, exclusively identified in nine French isolates (including knee fluid sample), was characterized by the multiresistance phenotype 1 or 2. ST211, which was also associated with phenotype 1 in France (2 isolates), was previously found in Scotland (13), London, Australia, Greece, and New Zealand (http://www.ng-mast.net). However, the sequences of both genes (tbpB and por) were distant between ST1479 and ST211. Therefore, it cannot be determined whether antimicrobial resistance phenotype 1 was acquired independently in these two genomic backgrounds or whether the genes tbpB and por evolved from a common antimicrobial-resistant ancestor. Expectedly, the susceptible isolates (antibiotic phenotype 3) were diverse. Many STs of susceptible isolates (ST2, ST26, ST359, ST975, ST1813, and ST1986) were observed in other countries (14; http://www.ng-mast.net). In contrast, some STs that were described as being frequent in other countries did not appear in this study (ST661, ST547, ST835, and ST338) or were represented only once (ST147) (11-13, 17, 21). Thus, ST225 stands out as the only widespread international clone, probably selected by the widespread use of ciprofloxacin and oral cephalosporins.
In conclusion, this study provides a first overview of the characteristics of N. gonorrhoeae infections in France. Even though a large diversity of genotypes was found, few clones associated with particular antimicrobial resistance phenotypes were observed. Comparison with European and international data identifies ST225 as one international multiresistant clone, whereas ST1479 was prevalent in France but has not been described elsewhere so far. Our results show that the worrying increase in resistance is due to a combination of clonal spread and emergence of resistance in distinct genomic backgrounds.
Epidemiological studies based on antibiotic susceptibility testing remain a good phenotypic approach for the surveillance of N. gonorrhoeae infections. NG-MAST provides improved capacity for the detection of chains of transmission and helps in predicting antibiotic susceptibility trends, as the antibiotic resistance phenotype is generally conserved for a given ST.
Acknowledgments
This study received financial support from Institut Fournier (Paris, France), Institut Pasteur (Paris, France), and Institut de Veille Sanitaire (St. Maurice, France).
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Bilek, N., I. M. Martin, G. Bell, G. R. Kinghorn, C. A. Ison, and B. G. Spratt. 2007. Concordance between Neisseria gonorrhoeae genotypes recovered from known sexual contacts. J. Clin. Microbiol. 45:3564-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhury, B., C. L. Risley, A. C. Ghani, C. J. Bishop, H. Ward, K. A. Fenton, C. A. Ison, and B. G. Spratt. 2006. Identification of individuals with gonorrhoea within sexual networks: a population-based study. Lancet 368:139-146. [DOI] [PubMed] [Google Scholar]
- 3.Fenton, K. A., and C. M. Lowndes. 2004. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex. Transm. Infect. 80:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallay, A., A. Bouyssou-Michel, F. Lassau, B. Basselier, P. Sednaoui, and Laboratoires du Réseau Renago. 2008. Neisseria gonorrhoeae infections in France in 2006: significant progression in women and persistent increase of ciprofloxacin resistance. Bull. Epidémiol. Hebdomadaire 5-6:33-36. (In French.) [Google Scholar]
- 5.Habib, A. R., and R. Fernando. 2004. Efficacy of azithromycin 1g single dose in the management of uncomplicated gonorrhoea. Int. J. STD AIDS 15:240-242. [DOI] [PubMed] [Google Scholar]
- 6.Kolader, M.-E., N. H. T. M. Dukers, A. K. van der Bij, M. Dierdorp, J. S. A. Fennema, R. A. Coutinho, and S. M. Bruisten. 2006. Molecular epidemiology of Neisseria gonorrhoeae in Amsterdam, The Netherlands, shows distinct heterosexual and homosexual networks. J. Clin. Microbiol. 44:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao, M., K. Bell, W. M. Gu, Y. Yang, N. F. Eng, W. Fu, L. Wu, C. G. Zhang, Y. Chen, A. M. Jolly, and J. A. Dillon. 2008. Clusters of circulating Neisseria gonorrhoeae strains and association with antimicrobial resistance in Shanghai. J. Antimicrob. Chemother. 61:478-487. [DOI] [PubMed] [Google Scholar]
- 8.Martin, I. M., S. Hoffmann, and C. A. Ison. 2006. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J. Antimicrob. Chemother. 58:587-593. [DOI] [PubMed] [Google Scholar]
- 9.Martin, I. M., C. A. Ison, D. M. Aanensen, K. A. Fenton, and B. G. Spratt. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 189:1497-1505. [DOI] [PubMed] [Google Scholar]
- 10.Newman, L. M., J. S. Moran, and K. A. Workowski. 2007. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 44(Suppl. 3):S84-S101. [DOI] [PubMed] [Google Scholar]
- 11.Palmer, H. M., and H. Young. 2006. Dramatic increase in a single genotype of TRNG ciprofloxacin-resistant Neisseria gonorrhoeae isolates in men who have sex with men. Int. J. STD AIDS 17:254-256. [DOI] [PubMed] [Google Scholar]
- 12.Palmer, H. M., H. Young, C. Graham, and J. Dave. 2008. Prediction of antibiotic resistance using Neisseria gonorrhoeae multi-antigen sequence typing. Sex. Transm. Infect. 84:280-284. [DOI] [PubMed] [Google Scholar]
- 13.Palmer, H. M., H. Young, I. M. Martin, C. A. Ison, and B. G. Spratt. 2005. The epidemiology of ciprofloxacin resistant isolates of Neisseria gonorrhoeae in Scotland 2002: a comparison of phenotypic and genotypic analysis. Sex. Transm. Infect. 81:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer, H. M., H. Young, A. Winter, and J. Dave. 2008. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J. Antimicrob. Chemother. 62:490-494. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Losada, M., K. A. Crandall, J. Zenilman, and R. P. Viscidi. 2007. Temporal trends in gonococcal population genetics in a high prevalence urban community. Infect. Genet. Evol. 7:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starnino, S., A. Neri, and P. Stefanelli. 2008. Molecular analysis of tetracycline-resistant gonococci: rapid detection of resistant genotypes using a real-time PCR assay. FEMS Microbiol. Lett. 286:16-23. [DOI] [PubMed] [Google Scholar]
- 17.Starnino, S., B. Suligoi, V. Regine, N. Bilek, P. Stefanelli, I. Dal Conte, B. Flanchino, S. Delmonte, F. Robbiano, A. D'Antuono, E. Mirone, A. Matteelli, M. A. De Francesco, M. Cusini, L. Scioccati, A. Di Carlo, G. Prignano, and M. C. Salfa. 2008. Phenotypic and genotypic characterization of Neisseria gonorrhoeae in parts of Italy: detection of a multiresistant cluster circulating in a heterosexual network. Clin. Microbiol. Infect. 14:949-954. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka, M., H. Nakayama, K. Huruya, I. Konomi, S. Irie, A. Kanayama, T. Saika, and I. Kobayashi. 2006. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int. J. Antimicrob. Agents 27:20-26. [DOI] [PubMed] [Google Scholar]
- 19.Todd, K., D. Durrheim, R. Pickles, K. Eastwood, T. Merritt, J. Tapsall, S. Ray, and A. Limnios. 2007. Using epidemiological and molecular methods to investigate an outbreak of gonorrhoea associated with heterosexual contact in Newcastle, NSW, Australia. Sex. Health 4:233-236. [DOI] [PubMed] [Google Scholar]
- 20.Whiley, D. M., E. A. Limnios, S. Ray, T. P. Sloots, and J. W. Tapsall. 2007. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 51:3111-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong, W.-W., C.-T. Huang, L.-H. Li, C.-C. Chiang, B.-D. Chen, and S.-Y. Li. 2008. Molecular epidemiological identification of Neisseria gonorrhoeae clonal clusters with distinct susceptibility profiles associated with specific groups at high risk of contracting human immunodeficiency virus and syphilis. J. Clin. Microbiol. 46:3931-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]