Abstract
An outbreak of infectious keratoconjunctivitis (IKC) occurred in semidomesticated reindeer (Rangifer tarandus tarandus) in Troms County, Norway, in February 2009. Twenty-eight animals with clinical symptoms and 12 apparently healthy animals were investigated. They ranged in age from calves of the year to 4-year-old animals (mean, 1.9 years; standard deviation, ±0.9). The seroprevalence of antibodies against cervid herpesvirus 2 (CvHV2) was 86% in animals with IKC and 42% in unaffected animals. For the 28 clinically affected animals, CvHV2 was detected by PCR in swabs obtained from the eye (82%), nose (64%), and vagina (24%), and CvHV2 was isolated from eye swabs from 8 animals. Virus was not isolated from clinically unaffected animals but was detected by PCR in eye swab samples from five of them. The viral activity, assessed by the ability to cause a cytopathic effect in cell culture, increased with the severity of clinical symptoms, but in severe clinical cases, virus was absent and secondary bacterial infections were dominant. Moraxella sp. isolates were obtained from seven animals, and those from two animals were identified as Moraxella bovoculi. Staphylococcus aureus, Streptococcus sp., and Arcanobacterium pyogenes were also isolated. It is concluded that CvHV2, which is endemic in reindeer, can cause IKC, probably most commonly as a primary infection of calves. This can be a very painful and devastating disease of economic importance for reindeer herders. This is the first report of CvHV2 as the primary agent of IKC in reindeer. This is also the first isolation of this virus in reindeer under natural herding conditions.
Infectious keratoconjunctivitis (IKC) is a highly contagious ocular infection in domestic animals, regarded as a multifactorial disease with low mortality and high morbidity. IKC may be subclinical or restricted to mild clinical symptoms. More-severe cases are characterized by increased lacrimation, edema of the conjunctiva and the periorbital region, and opacity and ulceration of the cornea. Infectious bovine keratoconjunctivitis (IBK, or “pinkeye”) is the most important ocular disease among cattle worldwide, causing significant reductions in cattle industry production (22). IBK is most common during the summer. The gram-negative coccoid bacterium Moraxella bovis is reported as the primary cause, whereas during winter outbreaks, Neisseria sp. infections also seem to be involved (26). Other infectious agents can also be involved, as well as other factors, such as sunlight (UV radiation) and the presence of dust and flies (22, 26). IKC can also be a common disease in sheep and goats (19). In Norway, Moraxella ovis/Moraxella spp. and Mycoplasma conjunctivae have been isolated from clinically affected animals and from animals with no disease symptoms (1). Keratoconjunctivitis has also been described in many wildlife species, such as Alpine chamois (Rupicapra rupicapra rupicapra) and Alpine ibex (Capra ibex ibex) (14), moose (Alces alces) (10), and red deer (Cervus elaphus) (15); in the latter species, the disease is also associated with infections with a herpesvirus, cervid herpesvirus 1 (CvHV1), which seems to be a common pathogen in farmed red deer in the United Kingdom (20).
In semidomesticated reindeer (Rangifer tarandus tarandus), IKC was first reported more than a century ago (5), and the disease has a specific name in the local indigenous Saami language, “Ĉalbmevikke” (30). IKC occurs sporadically in reindeer and causes unilateral or bilateral ocular disease and, in severe cases, blindness. In some years, transmissible IKC outbreaks can be especially severe, affecting calves in particular (25). Affected animals are sensitive to light, have increased lacrimation, develop an opaque bluish or whitish cornea, and have edema in the eyelids and the periorbital region. In later stages, the ocular secretions become purulent, in wintertime resulting in a frozen lump of tears and pus in the fur below the affected eye. If the disease proceeds from this stage, panophthalmitis is seen, with perforation of the cornea and the infection damaging the whole eye. Usually, however, IKC outbreaks in reindeer are more benign, with high morbidity but with a high percentage of recovery (30).
No specific etiological agent of IKC in reindeer has yet been determined. M. ovis has been isolated from reindeer with IKC in Finland (21), and during a recent outbreak in reindeer in Norway, a mixed bacterial flora including Arcanobacterium (Actinomyces) pyogenes, Staphylococcus spp., and Escherichia coli was isolated from the affected eyes of two animals (4). During an evaluation of 660 reindeer calves in western Alaska, where outbreaks of IKC in calves are reported to be common, clinical signs were found in 26 individuals. Since no primary viral or bacterial agent could be identified, a multifactorial cause was suggested, including the involvement of stress, ocular foreign bodies, and arthropods (13).
Alphaherpesviruses serologically related to bovine herpesvirus 1 (BoHV1) have been detected in a wide range of wildlife species and are also widely distributed in populations of semidomesticated reindeer in Norway (7, 34), Sweden (24, 27), and Finland (11), as well as in reindeer and caribou populations in North America (12). Recent viral characterization of isolates from Norway has shown that cervid herpesvirus 2 (CvHV2) is the alphaherpesvirus circulating in the reindeer populations (7). The clinical impact of CvHV2 infection in reindeer is not fully understood, and it has been assumed that it is usually a subclinical genital infection and that the pathology and disease potential may be limited to a primary infection in the upper respiratory tract and mouth, facilitating a secondary infection with bacteria such as Fusobacterium necrophorum (alimentary necrobacillosis) (27). However, recent investigations conducted on slaughtered reindeer from Finnmark County, Norway, as well as experimental studies, have revealed that CvHV2 is endemic in reindeer populations (47%), where it is latent in the trigeminal nerve ganglion (7, 8). CvHV2 has been isolated both from the respiratory tract and from the genital tract, and it has been shown that the virus is transferred from the mother to the fetus and has abortogenic potential (8, 9). From experimental studies, it has been shown that latent CvHV2 infections can be reactivated by immunosuppressive corticosteroid treatment and that the virus may cause conjunctivitis (27), as well as multiple lesions on the junction of the dermis and the mucosa of the lip (9).
In February 2009, an outbreak of IKC occurred in a flock of semidomesticated reindeer that was corralled for supplementary feeding in Troms County, Norway. The aim of this study was to look for the primary infectious agent causing transmissible IKC in reindeer, with a special focus on the role of CvHV2.
MATERIALS AND METHODS
Sampling of animals.
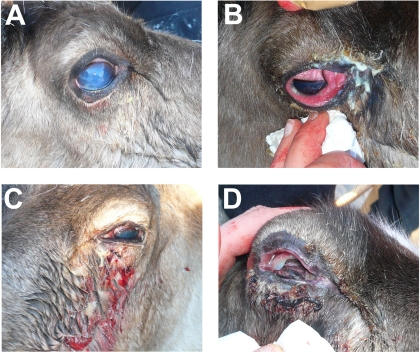
A total of 40 animals, 28 of which had clinical symptoms of IKC, were investigated. The clinical symptoms of the eyes were classified as mild, with increased lacrimation (grade 1); mild to moderate, with an opaque and bluish or whitish cornea (grade 2); moderate, with purulent exudation and periorbital edema (grade 3); moderate to severe, with severe edema and purulent exudation (grade 4); and severe, with a penetrating ulcus of the cornea and severe panophthalmitis with complete loss of structure and function (grade 5), usually on one side only (see Fig. 1A to D). Twelve of the animals sampled had no clinical symptoms (classified as grade zero). The animals ranged in age from calves of the year (9 months old) to approximately 4-year-old animals (i.e., 45 months) (mean, 1.9 years; standard deviation [SD], ±0.9 year). Clinically affected animals had a mean age of 2.0 years (SD, ±0.9 year), whereas unaffected animals had a mean age of 1.9 years (SD, ±0.6 year).
FIG. 1.
Clinical stages of IKC in semidomesticated reindeer during an outbreak in Norway in 2009. Pictures show different animals with different levels of disease severity. (A) Increased lacrimation and a bluish, opaque cornea (grade 2). (B) Purulent exudation from the eye and periorbital edema (grade 3). (C) Purulent exudation, bleeding, and severe bacterial infection (grade 4). (D) Late stage of IKC, with complete loss of structure and function (grade 5).
Blood was sampled from the jugular vein, and serum was stored at −20°C. For two reindeer, a swab sample from the conjunctival sac was obtained before treatment began and was investigated by PCR for DNA specific to Mycoplasma spp. (Vet Med Lab ApS, Taastrup, Denmark).
For virological investigations, sterile swab samples were obtained from the conjunctival sac, the nose, and, for females, the vagina. The swabs were placed in cryotubes with 1 ml of Eagle's minimum essential medium, with antibiotics in final concentrations of 100 IU/ml of penicillin, 100 μg/ml of streptomycin, 50 μg/ml of gentamicin, and 2.5 μg/ml of amphotericin B. The cryotubes were stored at −80°C.
For bacteriological investigations, a swab sample [Transwab ENT for Aerobes and Anaerobes; Medical Wire and Equipment Co. (Bath) Ltd., Wiltshire, United Kingdom] was obtained from the conjunctival sac and placed in its transport medium container.
Three animals were slaughtered 1 week later because their affected eyes were completely destroyed. Blood and trigeminal ganglia were sampled, and a nasal swab sample was obtained. One of these animals (identification number [ID] 39) had also been sampled the week before.
Serology.
Serum samples were investigated for the presence of antibodies against alphaherpesvirus by a commercial bovine enzyme-linked immunosorbent assay (ELISA) kit (gB Blocking LSI; Laboratoire Service International, France) based on BoHV glycoprotein B (gB) as the antigen. This kit has been validated previously for the testing of reindeer serum samples for antibodies against CvHV2, and all serum samples were tested in duplicate and evaluated against bovine and reindeer positive-control sera (6).
Serum samples were also investigated for antibodies against gammaherpesvirus by an ELISA (17) previously used for testing reindeer serum samples (35).
PCR for detection of alphaherpesvirus.
DNA was extracted from all swab samples using a DNA MiniPrep kit (Qiagen, Hilden, Germany). A nested panalphaherpesvirus PCR was conducted as described elsewhere (8, 28), with primers amplifying a 294-bp region of the UL27 gene, a highly conserved gene among ruminant alphaherpesviruses, encoding gB (29). BoHV1 (strain Iowa) (18) and CvHV2 (strain Salla 82) (11) were used as positive controls. PCR products were separated by agarose gel electrophoresis and visualized with SYBR green (Roche Applied Biosciences, Basel, Switzerland). Amplicons of the expected sizes were purified and sequenced as described previously (8).
Virus isolation and characterization.
For virus isolation, eye swab samples were inoculated on MDBK cell monolayers as described previously (9). Each swab sample was also plated on blood agar and incubated aerobically and anaerobically at 37°C to check for bacterial contamination that could interfere with the herpesvirus-induced cytopathic effect (CPE) in cells. CPEs were classified as absent or were graded from mild (grade 1) to severe (grade 4), depending on how soon the CPE was visible and how destructive it was for the cell monolayer, as an indirect indication of the amount of virus in the original sample. If no CPE was observed after 5 days of incubation, a second passage in MDBK cells was performed. Samples that did not exhibit a CPE after 2 passages were considered negative. DNA was extracted from CPE-positive samples to confirm the presence of CvHV2 by PCR.
One virus isolate (ID 39) was chosen for characterization. After propagation, the virus was subjected to ultracentrifugation through a 30% sucrose cushion (32) before DNA extraction. Restriction endonuclease analysis was performed using BamHI and BstEII (New England Biolabs, England) as described previously (9), and the results were compared to those for Norwegian CvHV2 isolates obtained from reindeer in an experimental reactivation study (9), CvHV2 strain Salla 82, and BoHV1 strain Iowa.
A set of PCR primers amplifying a 624-bp region of the US8 gene, encoding gE, was used in order to better characterize the new isolate (32). Amplicons were purified and sequenced (8), and sequences were compared to corresponding sequences from GenBank. Sequences were assembled and aligned with MEGA, version 4 (31). The phylogenetic distances between sequences were estimated, and a tree was drawn using the neighbor-joining method. The reliability of the tree was assessed by bootstrapping (1,000 random data set repeats).
The following gene sequences (US8) from GenBank were used for comparison with the virus gene sequences generated in this study: BoHV5 N569 (accession number EF624468), bubaline herpesvirus 1 b6 (EF624469), elk herpesvirus 1 (EF624473), CvHV1 Anlier (EF624474), CvHV1 Banffshire 82 (EF624471), CvHV2 Salla 82 (EF624472), caprine herpesvirus 1 Ba-1 (EF624470), CvHV2 Norway isolates 1, 2, and 3 (FJ917366 to FJ917368), BoHV1 Norway (FJ917360), and caprine herpesvirus 1 Norway (FJ917362).
Bacteriology.
On the day of sampling, each bacteriological eye swab sample was plated on one 5% bovine blood agar plate and one MacConkey agar plate, both of which were incubated aerobically, and on one blood agar plate that was incubated anaerobically. The incubation temperature was 37°C. The plates were inspected after 24 and 48 h. Selected colonies were subcultured for purity and identified to the genus or species level. Two strains, identified as Moraxella sp. strains using standard bacteriological criteria, were further identified to the species level by 16S rRNA sequence determination. The proximal portion of the 16S rRNA gene was sequenced for one strain, whereas the sequence of the entire 16S rRNA gene was determined for the other strain. The proximal part of the 16S rRNA gene was amplified and sequenced on both strands using MicroSeq 500 16S rDNA bacterial identification kits (Applied Biosystems, Foster City, CA). The entire 16S rRNA gene was amplified using the primers described by Weisburg et al. (36). Nucleotide sequences were determined using the BigDye Terminator cycle sequencing kit (version 3.1; Applied Biosystems) with oligonucleotide primers by primer walking. The sequencing reactions were run on a capillary sequencer (3130xl Genetic Analyzer; Applied Biosystems). The sequences were edited using the CLC Main Workbench, version 5.0 (CLC bio, Aarhus, Denmark), and a homology search was performed using NCBI-BLAST2.
Pathology.
Necropsy was performed on the heads of three slaughtered animals. Eyes were dissected, fixed in neutral buffered 10% formalin, sectioned, and stained with hematoxylin and eosin.
Nucleotide sequence accession numbers.
The virus gene sequences generated in this study have been deposited in GenBank under accession numbers GQ118967 (gB) and GQ118968 (gE).
RESULTS
Case report.
The herd of semidomesticated reindeer (Troms County, Norway) that had the outbreak of IKC is seasonally moved between winter and summer pastures within its reindeer herding district. A restricted outbreak of keratoconjunctivitis, affecting only a few animals, had appeared in September to October 2008 and again in December 2008, both times among reindeer gathered from summer pastures. The main outbreak reported here started in February 2009, 4 weeks after a flock of reindeer had been taken from their winter pasture and corralled for supplementary feeding with pelleted reindeer food (Reinsdyrfôr; Norske Felleskjøp, Oslo, Norway). An early sign of infection was a brownish discoloration of the fur around the medial eye angle, caused by enhanced lacrimal production. This was especially visible on light-colored animals. Inspection of affected animals revealed a bluish, and later a whitish, cornea (Fig. 1A), purulent exudation around the eyes and in the conjunctival sac, and edema (Fig. 1B). Many animals seemed to recover completely from this stage. In other animals, however, the amount of pus increased and a more invasive bacterial infection was established (Fig. 1C and D). In some cases, the lens had protruded through a penetrating ulcus of the cornea, and the eyeball was collapsed. Clinically affected animals were restrained for inspection, sampling, and treatment. They were treated once or twice per day as long as the clinical symptoms persisted. Treatment consisted of cleaning the eye and surrounding area with paper cloths, flushing the eye with sterile physiological salt water, and applying an antibiotic ointment (Fucithalmic Vet 1%; VetXX, Uldum, Denmark). Severely affected animals were euthanized.
The results of serology, PCR of eye, nasal, and vaginal swabs, and isolation of CvHV2 from eye swabs are summarized in Table 1 .
TABLE 1.
Results from serology (ELISA), PCR,a and isolation of CvHV2 in cell culture for unaffected animals and for each group of animals with clinical symptoms of IKCb
| Group (no. of animals) | No. of ELISA-positive animals/total no. tested (%) | No. of PCR-positive swab samples/total no. tested |
Virus isolation |
|||
|---|---|---|---|---|---|---|
| Eye | Nasal | Vaginalc | No. of eye swabs for which CvHV2 was isolated | Sum of CPE scoresd | ||
| Unaffected animals (grade 0) (12) | 5/12 (42) | 5/12 | 2/12 | 3/11 | 0 | 0 |
| Animals with clinical symptoms | ||||||
| Grade 1 (6) | 5/6 | 5/6 | 3/6 | 1/4 | 1 | 3 |
| Grade 2 (5) | 4/5 | 4/5 | 2/5 | 1/5 | 3 | 9 |
| Grade 3 (7) | 5/7 | 6/7 | 5/7 | 1/6 | 3 | 11 |
| Grade 4 (3) | 3/3 | 3/3 | 2/3 | 0/3 | 1 | 3 |
| Grade 5 (5) | 5/5 | 3/5 | 5/5 | 3/5 | 0 | 0 |
| NAe (2) | 2/2 | 2/2 | 1/2 | 0/2 | 0 | 0 |
| Total (28) | 24/28 (86) | 23/28 | 18/28 | 6/25 | 8/28 | 26 |
| All animals (40) | 29/40 (73) | 28/40 | 20/40 | 9/36 | 8/40 | 26 |
Of the UL27 gene, encoding gB.
Animals with clinical symptoms are grouped according to the severity of the symptoms, from mild (grade 1) to severe (grade 5).
For vaginal swabs, the total number of animals tested is the number of the females in each group.
Calculated by adding the individual CPE scores for all animals from whose eye swabs CvHV2 was isolated. Individual CPE scores range from 1 (slightly cytopathic) to 4 (highly cytopathic).
NA, not assessed.
Serology.
Seroprevalence was 73% for all 40 animals investigated, 86% for clinically affected animals (n = 28), and 42% for animals that were not clinically affected (n = 12). One of the animals that were slaughtered (ID 39) had seroconverted since the first time of sampling, increasing the competition percentage in the ELISA from −1.3% (negative) to 78% (positive). One of the 40 animals had antibodies against gammaherpesvirus (2.5%).
PCR for detection of alphaherpesvirus.
PCR produced amplicons of the expected size (data not shown). CvHV2 DNA was detected in the trigeminal ganglia from two of the three euthanized animals. The trigeminal ganglion of the animal that seroconverted (ID 39) was PCR positive at the time of euthanasia. Direct sequencing of PCR amplicons from the UL27 gene revealed 100% homology with previous CvHV2 isolates from reindeer in Norway (9) and 99.6% homology (one single-nucleotide difference) with the Finnish CvHV2 isolate (Salla 82) (data not shown).
Virus isolation and characterization.
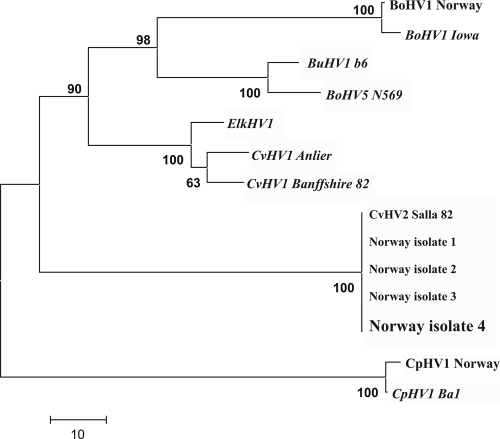
CvHV2 was isolated from eye swab samples from eight animals and was further confirmed by PCR and partial UL27 gene sequencing. The isolate chosen for characterization (designated Norway isolate 4; animal ID 39) showed a restriction endonuclease analysis profile identical to those of previous Norwegian reindeer isolates and CvHV2 Salla 82, whereas BoHV1 Iowa generated fragments similar to those of the BoHV1.1 type but clearly different from those of CvHV2 (data not shown). A 577-bp sequence of the US8 gene of the isolate obtained in this study had 100% homology with those of other Norwegian CvHV2 reindeer isolates (Norway isolates 1 to 3) and CvHV2 Salla 82, as shown also by phylogenetic analysis (Fig. 2).
FIG. 2.
Phylogenetic analysis comparing the new CvHV2 isolate (Norway isolate 4), obtained from a reindeer with a clinical case of IKC, with previous Norwegian (Norway isolates 1 to 3) and Finnish (CvHV2 Salla 82) reindeer isolates, as well as with corresponding sequences from other alphaherpesvirus isolates and host species (GenBank). The analysis is based on a 577-bp fragment of the US8 gene, encoding gE. Phylogenetic relationships were inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to each branch. The unrooted tree is drawn to scale, with branch lengths in the same units as the evolutionary distances used to infer the phylogenetic tree (nucleotide differences). All positions containing gaps and missing data were eliminated from the data set (complete-deletion option). BuHV1, bubaline herpesvirus 1; CpHV1, caprine herpesvirus 1.
Bacteriology.
A hemolytic Moraxella sp. was isolated from 7 of 20 animals with clinical symptoms, and this bacterium dominated in mixed cultures. Two of the Moraxella sp. isolates were identified as Moraxella bovoculi on the basis of 16S rRNA sequencing. The sequences obtained (463 bp and 1,417 bp) were 100% identical to M. bovoculi sequences deposited in GenBank. Mixed, unspecific cultures grew in samples from eight animals, while two samples displayed dominant growth of a Streptococcus sp. and a Staphylococcus sp., respectively. Arcanobacterium pyogenes was also isolated from several animals.
In samples from five animals without symptoms of eye infection, no bacteria grew. No mycoplasma-specific DNA was amplified by PCR from the samples obtained from the conjunctival sacs of two individuals presenting mild clinical signs of IKC at the early stage of the outbreak (prior to antibiotic treatment).
Pathology.
The three animals that were euthanized each had a severe infection of a single eye. Two animals had a chronic infection, with a collapsed and totally destroyed eye consisting mostly of fibrinous tissue. One animal had a more active infection, with edema and mild hyperemia in the conjunctiva, displaying a hemorrhagic and purulent exudate, which was also present in the fur located ventrally to the infected eye. The eyeball was partly collapsed; the cornea was ulcerated; and the ulcus was filled with pus and fibrin. A fibrinous and purulent exudation on the surface of the sclera could be seen when the eye was removed from the orbit. When the eye was sectioned, the vitreous was filled with blood and fibrin, and normal structures, such as the lens and retina, could not be identified. Histological sections showed edema and infiltration of mononuclear leukocytes in the conjunctiva. In lacrimal glands in the third eyelid, there were neutrophil leukocytes in the interstitium and in the lumen, as well as degeneration and necrosis of the epithelial cells. The cornea showed a loss of epithelium, edema, and numerous neutrophil lymphocytes, as well as bleeding in the stroma. The iris and ciliary process were adherent to the cornea, and a large clot consisting of fibrin, neutrophil lymphocytes, and bacteria filled the vitreous. Infiltration of fibrinous tissue and mononuclear leukocytes were present in the uvea.
DISCUSSION
The clinical signs and the development of the outbreak of IKC in semidomesticated reindeer reported here are concordant with previous descriptions (4, 5, 25, 30). This is the first report of CvHV2 as the primary infectious agent of IKC in reindeer and the first report of isolation of CvHV2 from reindeer under ordinary herding conditions. To our knowledge, this is also the first report of isolation of Moraxella bovoculi in reindeer.
The US8 gene, encoding gE, which was used for the phylogenetic comparison in this study, shows a lower degree of homology between alphaherpesviruses than, for instance, UL27 (gB) and has been used to distinguish between strains of the same virus species (32). In our study, the sequences obtained from the outbreak of IKC confirmed that the virus that was present was CvHV2, and not other alphaherpesviruses known to be associated with cattle and goats (Fig. 2). Further, the sequence from the isolate obtained was identical with those of previous CvHV2 isolates from Norway and Sweden, indicating a close relationship between these isolates.
To summarize the results of this investigation (Table 1), it is clear that seroprevalence was higher in clinically affected animals (86%) than in unaffected animals (42%) and that the percentage of seropositive animals rose with increasing severity of clinical symptoms of IKC, reaching 100% for animals with a clinical score of 4 or 5. PCR amplification of CvHV2 DNA from swab samples also showed that prevalence was higher in the group of animals with clinical symptoms of IKC than in unaffected animals, except for vaginal swabs, where the percentages of PCR-positive swab samples in the two groups were comparable. Interestingly, the activity of the virus, as assessed by its ability to cause CPE in MDBK cells and assumed to reflect the amount of virus in the swab sample, was higher for animals with clinical scores of 1 to 3 (particularly scores of 2 and 3) than for the most severely affected animals (with scores of 4 and 5), in which the viral activity seemed to fade. This is in line with results from an experimental inoculation of reindeer with CvHV2, where the excretion of the virus in the vagina and the nose and the potential to cause CPE were centered between days 4 and 10 postinoculation, with a subsequent quick decline in the frequency of successful detection of CvHV2 (unpublished data). Quantification of viral activity by comparison of PCR and CPE detection has also been used in similar studies with BoHV1 in cattle, revealing similar patterns, with early detection of the virus by PCR, followed by isolation and CPE, and at later stages again only detection by PCR (28, 38). In experimental studies of cattle, it was also shown that BoHV1 had its highest activity between days 2 and 12 postinoculation (16). These reports of BoHV1 activity in cattle, as well as our knowledge from experimental infection of reindeer with CvHV2, together with the clinical and laboratory findings of this study, strongly indicate that CvHV2 was the primary infectious agent in this outbreak of IKC and that the activity of the virus ceased as the severity of the secondary bacterial infections, which caused the later stages of IKC, increased. This conclusion is further supported by additional investigations of one of the three animals that were euthanized (ID 39). This animal displayed mild keratoconjunctivitis in one of the eyes at the time of sampling. A swab sample from the nose was PCR positive, and CvHV2 was isolated from the eye swab sample. At necropsy 1 week later, the trigeminal ganglion was PCR positive; the infected eye now showed a penetrating ulcus of the cornea and severe panophthalmitis; and the animal had seroconverted during this week. The monitoring of this animal over a week during the development of clinical IKC revealed that CvHV2 was indeed the primary agent, followed by bacterial infections.
Clinically unaffected animals in whose eyes CvHV2 was present (Table 1) may have experienced clinical symptoms of IKC later on.
In a previous investigation of free-ranging cervids in Norway, pathological findings corresponding to malignant catharral fever, including corneal opacity and conjunctivitis, were evident (35). It was concluded that malignant catharral fever virus from sheep and goats may cause serious disease in wild moose, roe deer, and red deer, and a serological screening of 250 wild reindeer revealed a seroprevalence of 4% (35). Since only one of the animals investigated in this study had antibodies against malignant catharral fever virus, we have no reason to believe that gammaherpesviruses contributed to this outbreak of ocular disease in reindeer.
Although IKC in reindeer is reported to be caused by M. ovis (21), this bacterium may not be essential for the development of IKC in reindeer, as also indicated by a study from Alaska in which no such bacteria were found (13). We found that a Moraxella sp. was the dominant bacterium in eye swab samples from seven animals, but it is likely that other bacteria, such as Streptococcus spp., Staphylococcus spp., and an Arcanobacterium sp., which were detected in severe clinical cases in this study, may also contribute to the later stages of the disease. Such bacteria may act as opportunistic agents, invading the eye tissues once the cell lining of the conjunctiva and cornea has been damaged by CvHV2. The virus thus seems to account for the transmissible nature of IKC, whereas the invasion of bacteria of different kinds may be more dependent on the bacterial flora present.
Moraxella bovoculi is a recently described species (2), previously isolated from dairy and beef cattle suffering from IKC in the United States (2, 3). Virulent Moraxella sp. strains have capsular pili that facilitate corneal colonization, and most of the virulent strains are hemolytic. Moraxella spp. also produce a potent cytotoxin, which accelerates neutrophil cell destruction (37). The finding of M. bovoculi in reindeer may indicate that this pathogen may also occur among other wild ruminant species.
From a description of a similar but larger outbreak in Sweden (25), it was concluded that feeding with insufficiently pelleted feed, introducing plant particles into the eyes, caused the initial conjunctivitis, followed by infection with bacteria normally found in the oral and nasal mucosae, in that case Pasteurella multocida, Arcanobacterium (Actinomyces) pyogenes, Moraxella sp., and Klebsiella sp. (23, 25). It was also pointed out that stress, caused by lack of food, high competition for feed, severe cold, and intensive capture and handling procedures, might have played an important role. For that outbreak, virological investigations (serology and virus isolation) for bovine viral diarrhea virus and alphaherpesvirus gave negative results, but only very few animals were investigated. These facts, together with the considerations that the virus may have been present and subsequently disappeared, and that the animals investigated had not yet managed to seroconvert, may not exclude a virus as an etiological factor in that outbreak.
Animal density has been proven to be a risk factor for infection with CvHV2 (7). However, different herding situations, such as corralling and handling, antiparasite treatment, and selection of animals for slaughter, may be even more important factors affecting virus transmission. Latent alphaherpesvirus infections are reactivated upon stress, and such herding situations may create heavy stress for the animals and an opportunity for the endemic CvHV2 agent to reactivate and infect new susceptible individuals in the herd, i.e., calves and young animals. These animals undergo a primary infection, more likely to cause a disease such as IKC than reactivation of latent CvHV2 infection in older animals, since the latter are able to mount a quick and effective immune response. Depending on nutritional status and the individual immune response, as well as other possible factors, such as UV light (sunlight), dust, and flies, the viral infection may be followed by secondary bacterial infections, with clinical expressions such as those described for this outbreak.
Corralling of semidomesticated reindeer for supplementary feeding may be necessary to save animals from starvation when the pasture is insufficient for the number of animals present or when winter pastures are locked by heavy ice and snow cover. However, gathering animals in dense settings over an extended period also facilitates the spread of infectious agents, both by inducing stress and by immunosuppression, but also by facilitating the horizontal transmission of infectious agents (4, 25, 33). Given the endemic status of CvHV2 in reindeer and its ability to establish latency and lifelong infections, it will be impossible to eradicate the virus from the reindeer population. Since IKC is multifactorial, preventive measures should focus on avoiding stress in terms of food deprivation, strenuous relocations over long distances by motor vehicles, and unnecessary gathering and handling of animals.
From the data obtained in this study, we conclude that in this outbreak of IKC in reindeer, CvHV2 was the initial infectious agent, followed by M. bovoculi and other bacteria. These findings thus shed light on the etiology of a disease of semidomesticated reindeer that has been reported for more than a century and for which the causative agent hitherto has been unknown. It is thus reasonable to believe that CvHV2 may play a greater role in reindeer health and the reindeer herding economy than previously thought. The annual loss of calves reported by the reindeer herding authority is not fully understood, and the role of CvHV2, and specifically the disease IKC, should be further investigated in order to find preventive measures, both for animal welfare and for the economy of the reindeer herding industry.
Acknowledgments
We thank the reindeer herders for patience while working with the animals. We also acknowledge the cooperation and assistance of veterinarians Lars Haugene and Ingebjørg Helena Nymo and Master's student Hanne Ihlebæk in the field and the cooperation and assistance of Karin-Elisabeth Holmgren, Ellinor Hareide, Eva Marie Breines, and Jannice Schau Slettemeås in the laboratory.
This investigation was funded by the Norwegian Reindeer Development Fund (RUF).
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Åkerstedt, J., and M. Hofshagen. 2004. Bacteriological investigation of infectious keratoconjunctivitis in Norwegian sheep. Acta Vet. Scand. 45:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelos, J. A., P. Q. Spinks, L. M. Ball, and L. W. George. 2007. Moraxella bovoculi sp. nov., isolated from calves with infectious bovine keratoconjunctivitis. Int. J. Syst. Evol. Microbiol. 57:789-795. [DOI] [PubMed] [Google Scholar]
- 3.Angelos, J. A., and L. M. Ball. 2007. Differentiation of Moraxella bovoculi sp. nov. from other coccoid moraxellae by the use of polymerase chain reaction and restriction endonuclease analysis of amplified DNA. J. Vet. Diagn. Investig. 19:532-534. [DOI] [PubMed] [Google Scholar]
- 4.Aschfalk, A., T. D. Josefsen, H. Steingass, W. Müller, and R. Goethe. 2003. Crowding and winter emergency feeding as predisposing factors for kerato-conjunctivitis in semi-domesticated reindeer in Norway. Dtsch. Tierarztl. Wochenschr. 110:295-298. [PubMed] [Google Scholar]
- 5.Bergman, A. 1912. Contagious keratitis in reindeer. Scand. Vet. J. 2:145-177. [Google Scholar]
- 6.Das Neves, C. G., M. Roger, N. G. Yoccoz, E. Rimstad, and M. Tryland. 2009. Evaluation of three commercial bovine ELISA kits for detection of antibodies against alphaherpesviruses in reindeer (Rangifer tarandus tarandus). Acta Vet. Scand. 51:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das Neves, C. G., J. Thiry, E. Skjerve, N. Yoccoz, E. Rimstad, E. Thiry, and M. Tryland. 21 June 2009. Alphaherpesvirus infections in semidomesticated reindeer: a cross-sectional serological study. Vet. Microbiol. doi: 10.1016/j.vetmic.2009/06.013. [DOI] [PubMed]
- 8.Das Neves, C. G., E. Rimstad, and M. Tryland. 2009. Cervid herpesvirus 2 causes respiratory and fetal infections in semidomesticated reindeer. J. Clin. Microbiol. 47:1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das Neves, C. G., T. Mørk, J. Thiry, J. Godfroid, E. Rimstad, E. Thiry, and M. Tryland. 21 August 2009. Cervid herpesvirus 2 experimentally reactivated in reindeer can produce generalized viremia and abortion. Virus Res. doi. 10.1016/j.virusres.2009.08.002. [DOI] [PubMed]
- 10.Dubay, S. A., E. S. Williams, K. Mills, and A. M. Boerger-Fields. 2000. Association of Moraxella ovis with keratoconjunctivitis in mule deer and moose in Wyoming. J. Wildl. Dis. 36:241-247. [DOI] [PubMed] [Google Scholar]
- 11.Ek-Kommonen, C., S. Pelkonen, and P. F. Nettleton. 1986. Isolation of a herpesvirus serologically related to bovine herpesvirus 1 from a reindeer (Rangifer tarandus). Acta Vet. Scand. 27:299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elazhary, M. A., J. L. Frechette, A. Silim, and R. S. Roy. 1981. Serological evidence of some bovine viruses in the caribou (Rangifer tarandus caribou) in Quebec. J. Wildl. Dis. 17:609-612. [DOI] [PubMed] [Google Scholar]
- 13.Evans, A., R. F. Bey, J. V. Schoster, J. E. Gaarder, and G. L. Finstad. 2008. Preliminary studies on the etiology of keratoconjunctivitis in reindeer (Rangifer tarandus tarandus) calves in Alaska. J. Wildl. Dis. 44:1051-1055. [DOI] [PubMed] [Google Scholar]
- 14.Giacometti, M., M. Janovsky, L. Belloy, and J. Frey. 2002. Infectious keratoconjunctivitis of ibex, chamois and other caprinae. Rev. Sci. Tech. 21:335-345. [DOI] [PubMed] [Google Scholar]
- 15.Gortázar, C., D. Fernández-de-Luco, and K. Frölich. 1998. Keratoconjunctivitis in a free-ranging red deer (Cervus elaphus) population in Spain. Z. Jagdwiss. 44:257-261. [Google Scholar]
- 16.Lemaire, M., F. Schynts, G. Meyer, and E. Thiry. 1999. Antibody response to glycoprotein E after bovine herpesvirus type 1 infection in passively immunised, glycoprotein E-negative calves. Vet. Rec. 144:172-176. [DOI] [PubMed] [Google Scholar]
- 17.Li, H., T. C. McGuire, U. Müller-Doblies, and T. B. Crawford. 2001. A simpler, more sensitive competitive inhibition enzyme-linked immunosorbent assay for detection of antibody to malignant catarrhal fever viruses. J. Vet. Diagn. Investig. 13:361-364. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. M., and M. J. van der Maaten. 1984. Reproductive tract lesions in heifers after intrauterine inoculation with infectious bovine rhinotracheitis virus. Am. J. Vet. Res. 45:790-794. [PubMed] [Google Scholar]
- 19.Motha, M. X., J. Frey, M. F. Hansen, R. Jamaludin, and K. M. Tham. 2003. Detection of Mycoplasma conjunctivae in sheep affected with conjunctivitis and infectious keratoconjunctivitis. N. Z. Vet. J. 51:186-190. [DOI] [PubMed] [Google Scholar]
- 20.Nettleton, P. F., J. A. Sinclair, J. A. Herring, D. M. Inglis, T. J. Fletcher, H. M. Ross, and M. A. Bonniwell. 1986. Prevalence of herpesvirus infection in British red deer and investigations of further disease outbreaks. Vet. Rec. 118:267-270. [DOI] [PubMed] [Google Scholar]
- 21.Oksanen, A., S. Laaksonen, and V. Hirvelä-Koski. 1996. Pink-eye in a winter-corralled reindeer herd. Suomen Eläinlääkärilehti 102:3. [Google Scholar]
- 22.Postma, G. C., J. C. Carfagnini, and L. Minatel. 2008. Moraxella bovis pathogenicity: an update. Comp. Immunol. Microbiol. Infect. Dis. 31:449-458. [DOI] [PubMed] [Google Scholar]
- 23.Rehbinder, C., and V. Glatthard. 1977. Keratitis in reindeer. Relation to bacterial infections. Acta Vet. Scand. 18:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehbinder, C., K. Belak, and M. Nordkvist. 1992. A serological, retrospective study in reindeer on five different viruses. Rangifer 12:191-195. [Google Scholar]
- 25.Rehbinder, C., and A. Nilsson. 1995. An outbreak of kerato-conjunctivitis among corralled, supplementary fed, semi-domesticated reindeer calves. Rangifer 15:9-14. [Google Scholar]
- 26.Riis, R. 2008. Ocular diseases, p. 561-589. In T. J. Divers and S. F. Peek (ed.), Rebhun's diseases of dairy cattle. Saunders Elsevier, Inc., St. Louis, MO.
- 27.Rockborn, G., C. Rehbinder, B. Klingeborn, M. Lefler, K. Klintevall, T. Nikkilä, A. Landèn, and M. Nordkvist. 1990. The demonstration of a herpesvirus, related to bovine herpesvirus 1, in reindeer with ulcerative and necrotizing lesions of the upper alimentary tract and nose. Rangifer Special Issue 3:373-384. [Google Scholar]
- 28.Ros, C., and S. Belak. 1999. Studies of genetic relationships between bovine, caprine, cervine, and rangiferine alphaherpesviruses and improved molecular methods for virus detection and identification. J. Clin. Microbiol. 37:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ros, C., and S. Belak. 2002. Characterization of the glycoprotein B gene from ruminant alphaherpesviruses. Virus Genes 24:99-105. [DOI] [PubMed] [Google Scholar]
- 30.Skjenneberg, S., and L. Slagsvold. 1968. Reindriften og dens naturgrunnlag. Universitetsforlaget, Oslo, Norway.
- 31.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 32.Thiry, J., F. Widen, F. Gregoire, A. Linden, S. Belak, and E. Thiry. 2007. Isolation and characterisation of a ruminant alphaherpesvirus closely related to bovine herpesvirus 1 in a free-ranging red deer. BMC Vet. Res. 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tryland, M., T. D. Josefsen, A. Oksanen, and A. Aschfalk. 2001. Parapoxvirus infection in Norwegian semi-domesticated reindeer (Rangifer tarandus tarandus). Vet. Rec. 149:394-395. [DOI] [PubMed] [Google Scholar]
- 34.Tryland, M., T. Mørk, K. A. Ryeng, and K. K. Sørensen. 2005. Evidence of parapox-, alphaherpes- and pestivirus infections in carcasses of semi-domesticated reindeer (Rangifer tarandus tarandus) from Finnmark, Norway. Rangifer 25:75-83. [Google Scholar]
- 35.Vikøren, T., H. Li, A. Lillehaug, C. M. Jonassen, I. Böckerman, and K. Handeland. 2006. Malignant catarrhal fever in free-ranging cervids associated with OvHV-2 and CpHV-2 DNA. J. Wildl. Dis. 42:797-807. [DOI] [PubMed] [Google Scholar]
- 36.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willock, B. P. 2007. Eye, eyelids, conjunctiva and orbit—diseases of the cornea, p. 1349-1413. In M. D. McGavin and J. F. Zachary (ed.), Pathologic basis of veterinary disease, 4th ed. Elsevier Mosby, St. Louis, MO.
- 38.Winkler, M. T. C., A. Doster, and C. Jones. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsils of latently infected calves. J. Virol. 74:5337-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]