Abstract
Salmonella enterica serovar Typhimurium is an important pathogen in swine and is also a frequently reported zoonotic agent. The objective of this study was to characterize isolates of S. enterica serovar Typhimurium associated with septicemia in swine and to compare them to isolates recovered from clinically healthy pigs. We were particularly interested in comparing the two groups of isolates for their ability to adhere to and invade host cells, to be phagocytized and survive in monocyte cells, to induce apoptosis, and to adhere to intestinal mucus. Their surface properties were also evaluated by interactions with solvents. The isolates recovered from diseased animals were shown to invade intestinal epithelial cell lines at a higher rate (P = 0.003) than isolates from healthy pigs. Septicemic isolates were phagocytized by human monocytes at a higher rate than isolates from healthy pigs (P = 0.009). The mean percentages of phagocytosis were significantly lower for human monocytes than for porcine monocytes (P = 0.02 and P = 0.008, respectively) for isolates from both diseased and healthy animals. Healthy animal isolates were phagocytized more by porcine monocytes at 15 min (P = 0.02) than septicemic isolates. No difference between isolates from septicemic pigs and isolates from healthy pigs was detected for other tested parameters. These results suggest that septicemic isolates have a particular pattern of invasion.
In pigs, Salmonella strains are related to significant animal infections associated with clinical signs and economic losses but are mainly associated with a carrier state, becoming a reservoir for human infections (3). Infection and/or silent carriage of Salmonella in pigs is an important public health concern. Multiresistance to antibiotics is often associated with Salmonella enterica serovar Typhimurium (14). In order to develop control measures, it is important to characterize these isolates and better understand the pathogenesis of infection.
The pattern of infection by Salmonella is oral ingestion of the bacteria followed by passage through the mucus which covers the epithelial cells to invade mucous membranes and cause disease (35). The intestinal mucus can then serve as the initial binding site for bacteria. Initial adhesion is mainly a physicochemical process founded on nonspecific interactions (van der Waals and coulombic interactions) (17, 33). This type of adhesion can be reversible or not, and surface properties of some bacteria have been show to influence nonspecific interactions with host cells. The first steps of infection are adhesion on the surface, firm attachment, and penetration into intestinal epithelial cells. The invasion of intestinal epithelial cells is believed to be a very important step related to the virulence of Salmonella strains associated with infections (5).
After invasion of the epithelial cells, the bacteria reach the subepithelial lymph tissue and the lamina propria, where Salmonella cells meet host immune cells (28). The overall phagocytic process can be divided into at least two main parts. First, bacteria must adhere to the phagocyte surface in a process called adherence. The second step of phagocytosis involves internalization or ingestion of the adherent particle. Following initial adhesion to phagocytes, special bacterial cell surface structures recognize receptors on the target cell surface (17, 33). The virulence genes of S. enterica serovar Typhimurium located on SPI-1, which encode a type III protein export machinery, are necessary for invasion of either nonphagocytic (5) or phagocytic (28) cells. Salmonella is able to induce cell death in macrophages in two different ways (32). Rapid activation of programmed macrophage cell death depends on SipB and SPI-1, whereas delayed induction of apoptosis in infected macrophages is SPI-1 independent (32). The results reported by van der Velden et al. indicate that ompR and a functional SPI-2-encoded type III protein secretion apparatus are required for delayed induction of apoptosis (32). The survival in phagocytes is an important step to induce septicemia in pigs, causing clinical signs similar to those in humans (16, 31); therefore, this animal model may be used to study human salmonellosis. Salmonella is able to survive and replicate in phagocytic cells, and this is an essential component of the virulence of these bacteria (1). S. enterica serovar Typhimurium cells that have invaded the macrophage by phagocytosis are able to replicate intracellularly (28) and induce apoptosis (32). In the past 10 to 15 years, an increased number of cases of clinical salmonellosis associated with Salmonella serovar Typhimurium were observed in pigs (11).
The aim of this study was to characterize isolates of S. enterica serovar Typhimurium associated with septicemia in swine and to compare them to isolates recovered from clinically healthy pigs. We were particularly interested in comparing the two groups of isolates in regard to their abilities to adhere to host cells, invade host cells, be phagocytized, survive in cells, generate apoptosis, adhere to intestinal mucus, and adhere to solvents.
MATERIALS AND METHODS
Bacterial isolates.
Salmonella isolates (n = 33) were recovered from extraintestinal organs or feces of dead pigs with an anamnesis of diarrhea and sudden death and typical lesions associated with salmonellosis and septicemia at necropsy. A diagnosis of salmonellosis was established following necropsy. These isolates from pigs with clinical signs (CS isolates) were obtained from S. Messier (Veterinary Medicine Faculty, Université de Montréal, Saint-Hyacinthe, Québec, Canada). Isolates (n = 33) from healthy pigs without clinical sign (WCS isolates) were collected at slaughterhouses from apparently healthy animals from farms without a history of clinical signs and without macroscopic lesions observed at this time (24). Sampled animals originated from a total of 55 farms; 7 farms were sampled more than once (4). S. enterica serovar Typhimurium strain SL1344, which was previously described as highly invasive in in vitro invasion assays and virulent for mice, was used as a positive control (18, 20), and the noninvasive Escherichia coli strain 862B, kindly provided by J. M. Fairbrother (Veterinary Medicine Faculty, Université de Montréal, Saint-Hyacinthe, Québec, Canada), was used as a negative control. This strain was isolated from the intestinal content of a pig and is considered nonvirulent (9). All strains and isolates used in this study were sensitive to colistin (polymyxin E; Sigma-Aldrich Canada Ltd., Ontario, Canada). Unless otherwise mentioned, bacterial cultures were grown at 37°C in Luria-Bertani (LB) Muller broth (Difco Laboratories, Detroit, MI) or on LB containing 1.5% (wt/vol) agar. Isolates were stored at −70°C in LB supplemented with 35% (vol/vol) glycerol.
Cell cultures.
The Int-407 human embryonic intestinal epithelial cell line (CCL-6; ATCC) (also known as Henle 407) was used in this study. Cells were routinely grown and maintained in minimal essential medium (MEM) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Canada Inc., Ontario, Canada). The culture medium was changed every 3 to 4 days for up to 15 passages. For adhesion and invasion assays, 105 cells/1.5 ml/dish (medium without antibiotic) were seeded and allowed to grow to confluence (approximately 5.5 × 105 cells/dish) in 24-well plates.
Growth and preparation of bacteria for adhesion and invasion assays.
All isolates from a previous study (4) were analyzed for adhesion and invasion assays. LB agar plates were first inoculated with bacteria from frozen glycerol stocks and incubated at 37°C overnight. Late-logarithmic-phase cultures of bacteria (optical density [OD] at 600 nm of ∼0.650) were prepared by inoculating LB with a single colony and incubating the bacteria with shaking for 20 h at 37°C. The bacteria were centrifuged at low speed and resuspended in MEM at an OD at 600 nm of ∼0.290 (108 CFU/ml). Dilutions were prepared to result in a multiplicity of infection (MOI) of 10 bacteria per cell in 1 ml of inoculum.
Adhesion and invasion assays.
For adhesion assays, confluent monolayers of Int-407 cells were washed once with prewarmed Dulbecco's phosphate-buffered saline (D-PBS) (pH 7.4) (Invitrogen). The bacterial inoculum was added, and the infected cells were incubated at 37°C for 30 min in a 5% CO2 atmosphere. Cells were rinsed three times with D-PBS to remove the nonadherent bacteria. Cells were then overlaid with 0.1% (wt/vol) sodium deoxycholate in D-PBS and incubated for 5 min at room temperature to release bacteria. The cell lysate was then diluted and plated on LB agar to determine viable bacterial counts.
For invasion assays, confluent monolayers of Int-407 cells were washed and the bacterial inoculum was added to the cells as described above for the adhesion assay. The infected cells were incubated at 37°C for 1 h in a 5% CO2 atmosphere. Cells were rinsed three times with D-PBS and then incubated for an another 2 h with fresh MEM containing 150 μg/ml colistin. Colistin was chosen instead of the commonly used gentamicin since some isolates belonging to Salmonella serovar Typhimurium are resistant to this antibiotic. Kusters et al. (20) validated the use of colistin for killing extracellular bacteria. Cells were washed three times with D-PBS and subsequently overlaid with 0.1% (wt/vol) sodium deoxycholate in D-PBS and incubated for 5 min at room temperature. The cell lysate was then diluted and plated on LB agar to determine viable bacterial counts. The adhesion and invasion rates were calculated according to the following method: percent adhesion or invasion = (number of bacteria recovered/total number of bacteria added) × 100.
Evaluation of phagocytosis level.
For logistical reasons, selected isolates were chosen for the remaining experiments according to the results of the invasion assay. From the group of septicemic pigs, the six most invasive isolates and three isolates with an invasion rate similar to that of the isolates from the group of healthy pigs were chosen. From the group of healthy pigs, six isolates among the eight least invasive isolates were chosen.
LB agar plates were first inoculated with bacteria from frozen glycerol stocks and incubated at 37°C overnight. LB was then inoculated with a single colony and incubated with shaking for 16 h at 37°C. Late-logarithmic-phase cultures of bacteria (OD at 600 nm of ∼0.650; 109 CFU/ml) were prepared by inoculating 200 ml of LB with 10 ml of overnight culture and incubated with shaking for 4 to 5 h at 37°C. The bacteria were concentrated using low-speed centrifugation and marked with the fluorochrome fluorescein isothiocyanate (FITC) (Sigma-Aldrich). The bacteria, resuspended in 1 ml carbonate-bicarbonate buffer (Sigma-Aldrich) and FITC at a concentration of 20 mg/ml (100 μl), were incubated with shaking in darkness at room temperature for 30 min. Bacteria were washed three times with D-PBS to remove excess FITC (23). The OD of the bacterial suspension was then determined at 600 nm and adjusted to an absorbance reading of 0.5 (108 CFU/ml). An aliquot of the suspension was used to determine the number of viable cells by diluting and plating samples on LB agar. Confirmation of the bacterial fluorescence was provided by flow cytometry and fluorescence microscopy.
The protocol was based on reports by Busque et al. and Letellier et al. (6, 23). Porcine blood was collected at the slaughterhouse, and human blood was collected from volunteer. The institutional review board approved the protocol, and all patients provided written informed consent. Whole blood (porcine or human) was incubated with FITC-labeled Salmonella at a phagocyte/bacterium ratio of 1:10 for 15, 30, and 60 min at 4°C and 37°C. The reaction was stopped with ice-cold D-PBS, the erythrocytes were lysed with Coulter whole-blood lysing reagents (Beckman Coulter Canada Inc., Ontario, Canada), and the cells were washed and fixed with paraformaldehyde (2%, wt/vol). The phagocytosis level was calculated as the percentage of phagocytosis by monocytes at 37°C minus the percentage of phagocytosis at 4°C (adherent control). The analyses were done by flow cytometry (BD FACSCalibur System; BD Biosciences Pharmagen, Ontario, Canada). Three assays were done with cells from various blood donors.
Survival.
In order to evaluate survival rates, the phagocytosis was stopped with ice-cold D-PBS and cells were centrifuged. The pellet was suspended and incubated with D-PBS-colistin (600 μg/ml) for elimination of extracellular bacteria. The cells were washed with ice-cold D-PBS. The pellet was resuspended in D-PBS and incubated at 37°C for 6 and 18 h. Water was added to the cells and left for 10 min, and the cell lysate was then diluted and plated on LB agar for 20 to 24 h at 37°C to determine viable bacterial counts.
Apoptosis.
At 2, 4, and 6 h after the beginning of phagocytosis, the reaction was stopped with ice-cold D-PBS. The erythrocytes were lysed with Coulter whole-blood lysing reagents. The cells were washed with D-PBS, colored with annexin V-allophycocyanin (APC) and 7-amino-actinomycin D (7-AAD) (BD Biosciences) in accordance with the manufacturer's instructions, and analyzed by flow cytometry within 1 hour.
Mucosal preparations.
The mucus was prepared by using a modified protocol based on previous reports (21, 22). Briefly, the porcine intestinal mucus was isolated from the small intestines (segments of 10 cm) of five pigs from a slaughterhouse. The small intestines were placed in sterile petri dishes containing 10 mmol/liter HEPES-Hanks buffer (HH) (pH 7.4) and cut into 2- to 3-cm lengths. Any feces and partially digested food were removed from each intestinal section manually. The intestines were shifted to a second set of petri dishes containing HH and split open longitudinally with scissors. The sections were shaken to remove any further debris and transferred to a third set of petri dishes. The layer of mucus gel covering the intestinal lumen was collected by gently scraping the mucosa with a rubber spatula.
After the intestinal sections were discarded, the collected mucus gel was centrifuged twice at 28,000 × g for 15 min at 4°C to remove the cells debris and bacteria. Mucus was stored at −70°C after measurement of the protein concentrations (protein assay, dye reagent concentrate; Bio-Rad Laboratories Inc., Ontario, Canada) and used for binding studies. A culture was made to verify the presence of Salmonella in the mucus gel.
Adhesion assay with mucus.
A modification of the protocol of Laux et al. (21, 22) was used for the adhesion assay with mucus. Bacteria were labeled using the same method as for phagocytosis and apoptosis. Assays, in triplicate, were performed in small petri dishes (tissue culture dish; Sarstedt Inc., Québec, Canada). Mucosal preparations (10 mg/ml) were immobilized in petri dishes by incubation for 24 h at 4°C. Excess mucus was removed by two washes with ice-cold HH. The fixed mucus was used for binding assays with S. enterica serovar Typhimurium. The labeled bacteria were added to each petri dish. After incubation for 1 h at 37°C, the petri dishes were rinsed three times with ice-cold HH to remove unattached bacteria. Adherent bacteria were released by adding 5% sodium dodecyl sulfate to each petri dish and incubating for 1 h at 37°C. Samples were removed from each petri dish and fixed with paraformaldehyde (2%, wt/vol) before examination by flow cytometry (25). The level of adherence to mucus was calculated as the percentage of relative fluorescence by bacteria minus the autofluorescence by the mucus.
Affinity of strains for solvent.
In order to study microbial surface properties that can affect interaction with host cells, microbial adhesion to solvents (MATS) was used as described previously (2). This method compares the microbial cell affinities to a monopolar solvent and an apolar solvent. The monopolar solvent can be an electron acceptor or an electron donor, but both solvents should have similar van der Waals' surface tension components (2). Two pairs of solvents were used, as described by Bellon-Fontaine et al. (2), to determine acidic and basic microbial surface properties: (i) chloroform, an electron acceptor solvent, and hexadecane, a nonpolar solvent, and (ii) ethyl acetate, a strong electron donor solvent, and decane, a nonpolar solvent. All solvents were obtained from Sigma-Aldrich.
The protocol is based on reports by Bellon-Fontaine et al. and Planchon et al. (2, 30). Three successive subcultures were completed for all strains in LB broth, and the third one was collected in stationary phase. The bacteria were washed and resuspended in saline water (8.5 g/liter NaCl) to obtain an OD at 400 nm of between 0.6 and 0.7 (A0). In all assays, 2.4 ml of this suspension was vortexed for 90 s with 0.4 ml of solvent. The mixture was placed at room temperature for 15 min to ensure the separation of two phases. The OD at 400 nm (A) was measured using 1 ml of aqueous phase. The percentage of bound cells was calculated by using the following equation: percent affinity = [1 − (A/A0)] × 100.
Flow cytometry.
For flow cytometry, data for all samples were obtained on a BD FACSCalibur and analyzed using a BD FACStation and BD Cell Quest Pro software (BD Biosciences Pharmagen). For each experimental condition, 10,000 cells were acquired.
We used a dot plot display of forward scatter (FSC) versus side scatter (SSC) for analysis of blood and bacteria. Computerized gating in FSC and SSC was set to exclude cell debris and residual free bacteria. The data analysis was performed on fluorescence histograms. After gating, for the monocyte population, the number of monocytes participating in the phagocytosis could be determined. The upper limit of the autofluorescence of the monocytes was measured in a control sample containing blood only. A marker was positioned after the upper FL1 value of the autofluorescence, and the portion of monocytes in the histogram to the right of the marker represented the signal for cells with associated FITC-labeled bacteria (12). Phagocytosis was expressed as the percentage of monocyte-associated fluorescent cells. For the apoptosis analysis, events were collected in list mode format for each sample with FSC versus SSC and 4-decade logarithmic amplification of fluorescence (29).
For adherence to mucus, we used a dot plot display of FSC versus SSC for analysis of mucus and bacteria, respectively. The device was set to exclude cellular debris and nonadherent bacteria. We also utilized an histogram to show the relative fluorescence of each cell sample and calculate the median fluorescence intensity for each peak. Median fluorescence intensity values were compared for two groups of isolates and controls. Bacteria bound to mucus are represented as a shift in fluorescence compared with the autofluorescence expressed by mucus (7, 25).
Statistical analysis.
All analyses were based on at least three independent replicates. We used SAS (version 9.1; SAS Institute, Cary, NC) for data analysis. For adhesion, invasion, phagocytosis, survival, apoptosis, mucus adhesion, and MATS assays, the mean and the standard deviation were used for descriptive statistics. Prior to analysis, survival data were log transformed and phagocytosis and apoptosis data were transformed with the arcsine square root to normalize distributions. For MATS, negative results were considered equal to zero. Linear models with isolates as fixed factors were used to examine differences among isolates. For adhesion, invasion, survival, and MATS assays, mixed linear models with the isolate type as a fixed factor and the isolate within a type as a random factor were used to compare types of isolates (CS and WCS). For decane, results were dichotomized (presence versus absence) due to the high prevalence of zero values. In this case, exact chi-square tests were used to compare the prevalence of positive results among isolates and to compare isolate types. For phagocytosis and apoptosis data, repeated-measures linear models, with time as a within-subject factor, were used to examine the effect of time, isolate, isolate type, and monocyte source (human or swine). Post hoc tests, with the sequential Bonferroni adjustment, were used to compare pairs of means. The statistical significance was set at a P value of <0.05.
RESULTS
Ability to adhere to Int-407 cells.
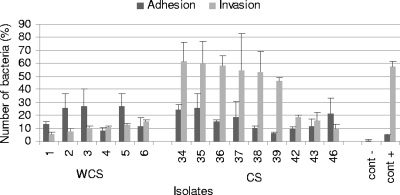
The adhesion of Int-407 cells was tested for two groups of isolates. It was not possible to detect any significant difference (P = 0.53) between the two groups of isolates in the ability to adhere to Int-407 cells. The means and standard errors were 16.00% ± 2.48% for septicemic isolates and 18.60% ± 3.13% for isolates from healthy pigs. The results are presented in Fig. 1.
FIG. 1.
Mean percentages of adhesion to and invasion into intestinal cell line Int-407 by Salmonella isolates from diseased and healthy animals. Data represent averages and standard deviations from at least three independent trials. cont−, negative control; cont+, positive control.
Ability to invade Int-407 cells.
We compared the invasion of Int-407 cells by S. enterica serovar Typhimurium isolates from septicemic animals with that by isolates from nonsepticemic pigs. A significant difference (P = 0.003) was observed between the two groups of isolates. Septicemic isolates (42.68% ± 5.54%) invaded epithelial cells at higher rates than isolates from healthy pigs (10.30% ± 6.84%). The results are presented in Fig. 1.
Phagocytosis.
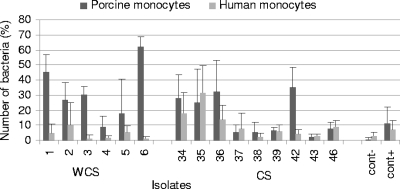
The percentage of monocytes that were able to phagocytize FITC-labeled Salmonella in porcine whole blood was measured. The mean values increased with time for CS isolates (16.40% ± 4.43%, 23.66% ± 3.21%, and 37.77% ± 3.11% for 15, 30, and 60 min, respectively) (P < 0.0001) as well as for WCS isolates (31.89% ± 7.81%, 28.65% ± 6.60%, and 36.34% ± 5.49% for 15, 30, and 60 min, respectively) (P = 0.02) but seemed to be more stable. The average was significantly lower for CS isolates than for WCS isolates at 15 min (P = 0.02) (Fig. 2) but not at 30 min (P = 0.70) or 60 min (P = 0.82), but globally, there was no difference. The percentage of phagocytosis was heterogeneous within CS isolates and within WCS isolates (P = 0.005 and P = 0.004, respectively).
FIG. 2.
Mean percentages of phagocytosis of Salmonella isolates from diseased and healthy animal by porcine and human monocytes at 15 min. Data represent averages and standard deviations from three independent trials. cont−, negative control; cont+, positive control.
The percentage of monocytes that were able to phagocytize FITC-labeled Salmonella in human whole blood was measured. Septicemic isolates were ingested at a higher level (10.63% ± 3.14%, 16.90% ± 2.19%, and 25.34% ± 3.64% for 15, 30, and 60 min, respectively) by human monocytes than isolates from healthy pigs (4.07% ± 1.39%, 5.84% ± 1.62%, and 11.82% ± 2.07% for 15, 30, and 60 min, respectively) at all times tested (P = 0.009). Figure 2 shows the results for 15 min. For WCS isolates, the mean values of intracellular bacteria increased with time for human monocytes (P = 0.005). The percentage of phagocytosis was heterogeneous within CS isolates (P = 0.02).
We compared the percentages of bacteria phagocytized by monocytes isolated from porcine blood and human blood. The mean percentages of phagocytosis were significantly lower for the human monocytes than for porcine monocytes for isolates from both diseased and healthy pigs at all times (P = 0.02 and P = 0.008, respectively).
Survival.
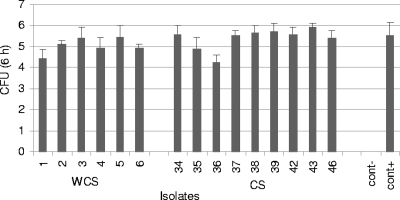
Since Salmonella is capable of surviving in monocyte cells, we then tested whether there was a difference in the survival rate between the two groups of isolates. Some isolates were able to survive after 6 h and 18 h. Negative control bacteria were not able to survive. It was not possible to detect any significant difference (P = 0.17) between the two groups of isolates in the ability to survive after 6 h (Fig. 3). The means (log transformed) and standard errors were 5.41 ± 0.15 for septicemic isolates and 5.05 ± 0.19 for isolates from healthy pigs. The results at 18 h were similar to those at 6 h.
FIG. 3.
Number of Salmonella organisms surviving in porcine blood at 6 h after the beginning of the phagocytosis. Data represent log-transformed mean values and standard deviations from three independent trials. cont−, negative control; cont+, positive control.
Apoptosis.
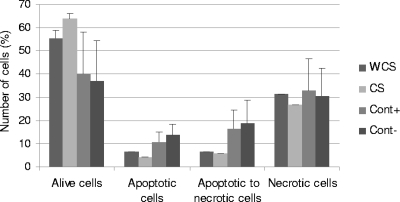
As S. enterica serovar Typhimurium was able to induce apoptosis of monocytes, we compared apoptosis levels in cells infected by CS and WCS isolates. The mean values for living cells infected with all isolates decreased with time for CS isolates (90.72% ± 3.28%, 77.91% ± 2.58%, and 63.69% ± 2.53 for 2, 4, and 6 h, respectively) (P < 0.0001) and WCS isolates (87.11% ± 2.71%, 74.66% ± 2.42%, and 55.55% ± 3.19% for 2, 4, and 6 h, respectively) (P < 0.0001). The results after 6 h of apoptosis are presented in Fig. 4 and 5. The percentage of living cells was heterogeneous within CS isolates (P < 0.0001). The apoptosis level was heterogeneous within CS isolates (P < 0.0001) and within WCS isolates (P = 0.005).
FIG. 4.
Mean percentages of bacteria at different times in the process of apoptosis for isolates from diseased and healthy animals at 6 h after beginning of phagocytosis. Data represent averages from three independent trials with standard errors (WCS and CS) or standard deviations (positive control [Cont+] and negative control [Cont−]).
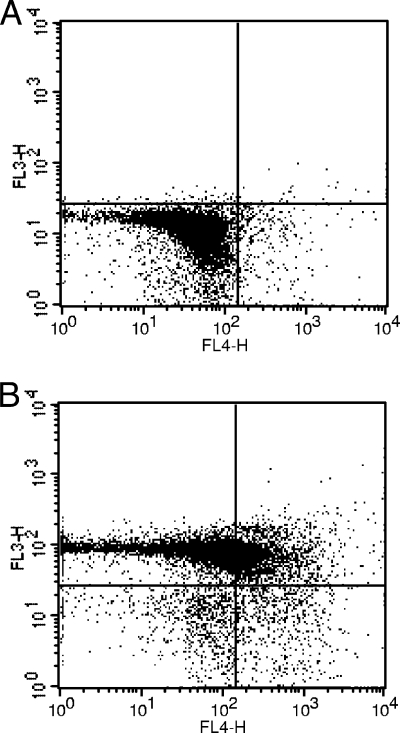
FIG. 5.
Invasion by isolate 4 induces apoptosis in porcine blood. Staining with annexin V-APC (FL4) and 7-AAD (FL3) determined phospholipid phosphatidylserine exposure and membrane integrity, respectively. (A) Two hours after the beginning of phagocytosis; (B) 6 h after the beginning of phagocytosis. When apoptosis is measured over time, cells can often be tracked from annexin V-APC and 7-AAD negative (viable, or no measurable apoptosis) (lower left) to annexin V-APC positive and 7-AAD negative (early apoptosis, membrane integrity present) (lower right) and finally to annexin V-APC and 7-AAD positive (end stage apoptosis and death) (upper right) (BD Biosciences).
The mean values of apoptotic cells toward necrosis infected with all isolates increased with time for CS isolates (2.07% ± 1.68%, 5.69% ± 1.50%, and 5.66% ± 2.19% for 2, 4, and 6 h, respectively) (P < 0.0001) and WCS isolates (1.62% ± 0.79%, 3.27% ± 1.07%, and 6.43% ± 2.52% for 2, 4, and 6 h, respectively) (P < 0.0001). The percentage of apoptotic cells toward necrosis was heterogeneous within CS and WCS isolates (P < 0.0001 and P < 0.0001, respectively). The mean values for necrotic cells infected with all isolates increased with time for CS isolates (4,52% ± 1.59%, 11.74% ± 1.78%, and 26.61% ± 2.82% for 2, 4, and 6 h, respectively) (P < 0.0001) and WCS isolates (6.69% ± 1.22%, 17.15% ± 1.63%, and 31.44% ± 4.29% for 2, 4, and 6 h, respectively) (P < 0.0001). The percentage of necrotic cells varied within CS and WCS isolates (P < 0.0001 and P < 0.0001, respectively).
The averages of the percentages of living cells, apoptotic cells, apoptotic cells toward necrosis, and necrotic cells did not differ between CS isolates and WCS isolates (P = 0.21, P = 0.51, P = 0.91, and P = 0.91, respectively).
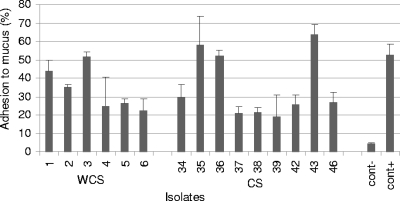
Adhesion with mucus.
Isolates were tested for their ability to adhere to the porcine intestinal mucus. It was not possible to detect any significant difference (P = 0.89) between the two groups of isolates in the ability to adhere to mucus. The means and standard errors were 35.36% ± 5.19% for septicemic isolates and 34.21% ± 6.36% for isolates from healthy pigs. The results are presented in Fig. 6.
FIG. 6.
Percentages of bacteria that adhere to swine intestinal mucus. Data represent averages and standard deviations from three independent trials. cont−, negative control; cont+, positive control.
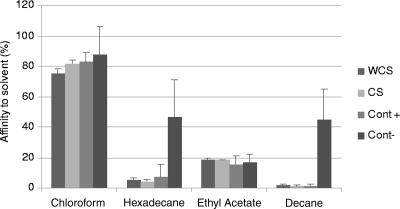
MATS.
Four different solvents were used to study the microbial surface properties of all isolates. Two monopolar solvents, chloroform and ethyl acetate, were chosen for the estimation of the Lewis acid/base character (i.e., electron donor/acceptor), while two apolar solvents, hexadecane and decane, were employed to estimate the hydrophobic/hydrophilic cell surface properties of isolates. All isolates of S. enterica serovar Typhimurium had maximal affinity with chloroform, the acidic solvent. The lowest affinity was for ethyl acetate, the basic solvent. These results showed that the cell surfaces of both groups of isolates were strong electron donors and weak electron acceptors. For all isolates, adhesion to the nonpolar solvent was near zero. Adhesion to chloroform was higher than that to hexadecane, the apolar solvent; the two solvents have comparable van der Waals properties. These differences showed electron donor and basic characters of S. enterica serovar Typhimurium. It was thus not possible to detect any difference between the groups of isolates in MATS (P = 0.10, P = 0.54, P = 0.97, and P = 0.75, respectively, for chloroform, hexadecane, ethyl acetate, and decane). The results are presented in Fig. 7.
FIG. 7.
Percentages of affinity to various solvents of selected WCS, CS, positive control (Cont+), and negative control (Cont−) Salmonella isolates. Data represent averages from three independent trials with standard errors (WCS and CS) or standard deviations (Cont+ and Cont−).
DISCUSSION
In this study, different procedures were used to discriminate between S. enterica serovar Typhimurium isolates recovered from diseased and clinically healthy pigs. Gianella et al. (15) have proposed an in vitro model for Salmonella invasion, and they found that only strains that penetrated HeLa cells were able to invade the mucosa of the rabbit ileum. Many parameters can influence the results and interpretation of this type of assay, such as the MOI. Kusters et al. (20) observed that the MOI has an impact on the fraction of the inoculum that adheres to or invades cells. Other authors enhanced the invasion by use of a low-speed centrifugation step (8, 19).
In our study, we used the same control strain (S. enterica serovar Typhimurium SL1344) and similar parameters (MOI, cell line, and growth conditions) as Galan and Curtiss (13) and found significant differences among septicemic and nonsepticemic isolates in invasion rates but not in adhesion rates. Septicemic isolates invaded epithelial cells at higher rate than isolates from healthy pigs. However, some strains isolated from clinically healthy animals also possessed a high invasiveness rates, suggesting that healthy animals may carry potentially pathogenic strains. Since pigs may carry Salmonella for prolonged period after onset of diarrhea and disease, it is possible that some septicemic isolates could have been recovered from clinically healthy animals. Another possibility is contamination during transport or lairage.
The greater morbidity and mortality caused by S. enterica serovar Typhimurium DT104 has led to the proposal that these strains may be more virulent (34). In our study, the prevalence of DT104 was 36.4% (12/33) for CS isolates and 51.5% (17/33) for WCS isolates (4). When we used invasion assays, we did not observe any increase in invasiveness for DT104. This is in accordance with the study by Allen et al. (1), who failed to show that S. enterica serovar Typhimurium DT104 was more virulent than S. enterica serovar Typhimurium ATCC 14028 using in vitro and in vivo experiments.
Overall, isolates were phagocytized more by porcine monocytes than by human monocytes. CS isolates were phagocytized less by porcine monocytes in 15 min than WCS isolates. One hypothesis would be that the phagocytosis is delayed with septicemic isolates. Although the mean values of phagocytosis were similar, it appeared that CS isolates are more heterogeneous than WCS isolates. On the other hand, when in contact with human monocytes, CS isolates are phagocytized more than WCS isolates. S. enterica serovar Typhimurium survival in macrophages is essential for virulence (10). Some authors, using cultured macrophages, demonstrated that invasive strains of S. enterica serovar Typhimurium invaded macrophages 10 times more than strains that have a noninvasive phenotype (28). Invasive S. enterica serovar Typhimurium strains can induce apoptosis, whereas noninvasive mutant strains did not (28). Interestingly, we did not observe any significant difference between the two groups of isolates in their ability to induce apoptosis. These results suggest that the apoptosis process is not associated with virulence in S. enterica serovar Typhimurium isolates that induce disease in pigs. We can speculate that these isolates take advantage of intracellular replication and use this mechanism to spread through the host.
In this study, we also examined the binding of S. enterica serovar Typhimurium to pig intestinal mucus, since this was described as an important step leading to colonization. We observed that all isolates of S. enterica serovar Typhimurium can bind to crude mucus, with no significant difference between CS and WCS isolates. One study had indicated that both virulent and avirulent S. enterica serovar Typhimurium strains were able to bind to rat intestinal mucus; however, in this animal species, the binding of virulent strains was six times greater than that of avirulent S. enterica serovar Typhimurium (35). Those authors proposed that virulent Salmonella strains take advantage of a specific interaction with the mucus and possibly favor the colonization of the epithelial cells. Other studies reported that virulent strains of various bacterial species can bind to intestinal mucus from different animal species with a higher affinity than avirulent strains (21, 22, 26), in contrast to our findings and those of McCormick et al. (27). There are probably other virulence factors which were not examined in this study that could be implied. The examination of virulence-related genes by use of a microarray could be used to complete this study.
The MATS method was used for the characterization of the electron donor/electron acceptor properties of bacteria (2). In addition, this method confirmed that adhesion between the bacterial and cell surfaces is the result of an association between van der Waals, Lewis acid-base, and electrostatic interactions (2). According to our results, we can conclude that all isolates possess a higher affinity for chloroform. This high affinity for chloroform, an acidic solvent, it is due to the basic or electron donor character of the bacteria, while the weak affinity found in bacteria for a basic solvent is due to the weak acidic or electron acceptor property (2). This basic character can be attributed to the presence of carboxylic groups on the microbial surface (2) or to the negatively charged surface of microorganisms (30). Furthermore, most bacteria are negatively charged in the pH range frequently found in most food (pH of <7) (30). The fact that both types of isolates were found to possess similar surface properties suggests that virulence of S. enterica serovar Typhimurium in swine is linked not to a variation of their surface properties but probably to specific adhesins.
Whether the isolates (no. 42, 43, and 46) whose invasion is weaker are included or excluded, there is no difference in the results, indicating that the difference could be at the level of the invasion in epithelial cells.
In this study, we characterized isolates from septicemic animals in order to identify putative virulence factors. However, host factors may also affect this issue of infection by septicemic isolates, since a range of clinical signs can be observed during outbreaks in affected herds. One cannot rule out that host susceptibility may also influence the status of isolates. The influence of host factors in the outcome of infection by virulent isolates should be further studied.
In summary, in this study, using various methods to assess virulence of isolates from healthy or diseased animals, we were able to demonstrate that isolates from diseased animals possess an increased capacity to invade intestinal cells and were phagocytized at a lower level at early steps than isolates from healthy animals. However, most other classical virulence features of isolates related to survival within host cells, such as induction of apoptosis or survival within monocytes, were found to be identical, suggesting that early steps in the establishment of infection and spread within host phagocytes are most important for the outcome of the infection in animals. Further studies at the molecular level will be necessary to better elucidate the pathogeny of this infection in swine.
Acknowledgments
This work was supported by a grant from the NSERC to Sylvain Quessy.
We thank John M. Fairbrother, Serge Messier, and Nancy Rheault for contributing strains. We thank Nancy Blain, Annie DesRosiers, Guillaume Gauthier-Larivière, Peter Mueller, Frédéric Oligny, and Stéphanie St-Jean for technical assistance. We thank Guy Beauchamp of the Université de Montréal for the statistical analysis. We thank the blood donors for their contribution.
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Allen, C. A., P. J. Fedorka-Cray, A. Vazquez-Torres, M. Suyemoto, C. Altier, L. R. Ryder, F. C. Fang, and S. J. Libby. 2001. In vitro and in vivo assessment of Salmonella enterica serovar Typhimurium DT104 virulence. Infect. Immun. 69:4673-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellon-Fontaine, M. N., J. Rault, and C. J. van Oss. 1996. Microbial adhesion to solvents: a novel method to determine the electron-donor/electron acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B 7:47-53. [Google Scholar]
- 3.Berends, B. R., F. Van Knapen, J. M. A. Snijders, and D. A. A. Mossel. 1997. Identification and quantification of risk factors regarding Salmonella spp. on pork carcasses. Int. J. Food Microbiol. 36:199-206. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron, N., J. Corriveau, A. Letellier, F. Daigle, and S. Quessy. Can. J. Vet. Res., in press. [PMC free article] [PubMed]
- 5.Boyen, F., F. Pasmans, F. Van Immerseel, E. Morgan, C. Adriaensen, J. P. Hernalsteens, A. Decostere, R. Ducatelle, and F. Haesebrouck. 2006. Salmonella Typhimurium SPI-1 genes promote intestinal but not tonsillar colonization in pigs. Microbes Infect. 8:2899-2907. [DOI] [PubMed] [Google Scholar]
- 6.Busque, P., R. Higgins, S. Sénéchal, R. Marchand, and S. Quessy. 1998. Simultaneous flow cytometric measurement of Streptococcus suis phagocytosis by polymorphonuclear and mononuclear blood leukocytes. Vet. Microbiol. 63:229-238. [DOI] [PubMed] [Google Scholar]
- 7.Drudy, D., D. P. O'Donoghue, A. Baird, L. Fenelon, and C. O'Farrelly. 2001. Flow cytometric analysis of Clostridium difficile adherence to human intestinal epithelial cells. J. Med. Microbiol. 50:526-534. [DOI] [PubMed] [Google Scholar]
- 8.Ernst, R. K., D. M. Dombroski, and J. M. Merrick. 1990. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect. Immun. 58:2014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairbrother, J. M., A. Broes, M. Jacques, and S. Lariviere. 1989. Pathogenicity of Escherichia coli O115:K“V165” strains isolated from pigs with diarrhea. Am. J. Vet. Res. 50:1029-1036. [PubMed] [Google Scholar]
- 10.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley, S. L., A. M. Lynne, and R. Nayak. 2008. Salmonella challenges: prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 86(E Suppl.):E149-E162. [DOI] [PubMed] [Google Scholar]
- 12.Gaforio, J. J., M. J. Serrano, E. Ortega, I. Algarra, and G. Alvarez de Cienfuegos. 2002. Use of SYTOX green dye in the flow cytometric analysis of bacterial phagocytosis. Cytometry 48:93-96. [DOI] [PubMed] [Google Scholar]
- 13.Galan, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 86:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebreyes, W. A., C. Altier, and S. Thakur. 2006. Molecular epidemiology and diversity of Salmonella serovar Typhimurium in pigs using phenotypic and genotypic approaches. Epidemiol. Infect. 134:187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannella, R. A., O. Washington, P. Gemski, and S. B. Formal. 1973. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J. Infect. Dis. 128:69-75. [DOI] [PubMed] [Google Scholar]
- 16.Gordon, M. A. 2008. Salmonella infections in immunocompromised adults. J. Infect. 56:413-422. [DOI] [PubMed] [Google Scholar]
- 17.Heinzelmann, M., M. Scott, and T. Lam. 2002. Factors predisposing to bacterial invasion and infection. Am. J. Surg. 183:179-190. [DOI] [PubMed] [Google Scholar]
- 18.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 19.Iyoda, S., T. Kamidoi, K. Hirose, K. Kutsukake, and H. Watanabe. 2001. A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb. Pathog. 30:81-90. [DOI] [PubMed] [Google Scholar]
- 20.Kusters, J. G., G. A. W. M. Mulders-Kremers, C. E. M. van Doornik, and B. A. M. van der Zeijst. 1993. Effects of multiplicity of infection, bacterial protein synthesis, and growth phase on adhesion to and invasion of human cell lines by Salmonella typhimurium. Infect. Immun. 61:5013-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laux, D. C., E. F. McSweegan, and P. S. Cohen. 1984. Adhesion of enterotoxigenic Escherichia coli to immobilized intestinal mucosal preparations: a model for adhesion to mucosal surface components. J. Microbiol. Methods 2:27-39. [Google Scholar]
- 22.Laux, D. C., E. F. McSweegan, T. J. Williams, E. A. Wadolkowski, and P. S. Cohen. 1986. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12 (K88ab). Infect. Immun. 52:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letellier, A., S. Messier, L. Lessard, S. Chénier, and S. Quessy. 2001. Host response to various treatments to reduce Salmonella infections in swine. Can. J. Vet. Res. 65:168-172. [PMC free article] [PubMed] [Google Scholar]
- 24.Letellier, A., S. Messier, and S. Quessy. 1999. Prevalence of Salmonella spp. and Yersinia enterocolitica in finishing swine at Canadian abattoirs. J. Food Prot. 62:22-25. [DOI] [PubMed] [Google Scholar]
- 25.Logan, R. P. H., A. Robins, G. A. Turner, A. Cockayne, S. P. Borriello, and C. J. Hawkey. 1998. A novel flow cytometric assay for quantitating adherence of Helicobacter pylori to gastric epithelial cells. J. Immunol. Methods 213:19-30. [DOI] [PubMed] [Google Scholar]
- 26.Mantle, M., and S. D. Husar. 1993. Adhesion of Yersinia enterocolitica to purified rabbit and human intestinal mucin. Infect. Immun. 61:2340-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick, B. A., B. A. D. Stocker, D. C. Laux, and P. S. Cohen. 1988. Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestine of streptomycin-treated mice. Infect. Immun. 56:2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien, M. C., S. F. Healy, Jr., S. R. Raney, J. M. Hurst, B. Avner, A. Hanly, C. Mies, J. W. Freeman, C. Snow, S. K. Koester, and W. E. Bolton. 1997. Discrimination of late apoptotic/necrotic cells (type III) by flow cytometry in solid tumors. Cytometry 28:81-89. [PubMed] [Google Scholar]
- 30.Planchon, S., B. Gaillard-Martinie, S. Leroy, M. N. Bellon-Fontaine, S. Fadda, and R. Talon. 2007. Surface properties and behaviour on abiotic surfaces of Staphylococcus carnosus, a genetically homogeneous species. Food Microbiol. 24:44-51. [DOI] [PubMed] [Google Scholar]
- 31.Sirinavin, S., P. Jayanetra, and A. Thakkinstian. 1999. Clinical and prognostic categorization of extraintestinal nontyphoidal Salmonella infections in infants and children. Clin. Infect. Dis. 29:1151-1156. [DOI] [PubMed] [Google Scholar]
- 32.van der Velden, A. W. M., S. W. Lindgren, M. J. Worley, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, and A. J. B. Zehnder. 1990. Influence of interfaces on microbial activity. Microbiol. Rev. 54:75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villar, R. G., M. D. Macek, S. Simons, P. S. Hayes, M. J. Goldoft, J. H. Lewis, L. L. Rowan, D. Hursh, M. Patnode, and P. S. Mead. 1999. Investigation of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in Washington State. JAMA 281:1811-1816. [DOI] [PubMed] [Google Scholar]
- 35.Vimal, D. B., M. Khullar, S. Gupta, and N. K. Ganguly. 2000. Intestinal mucins: the binding sites for Salmonella typhimurium. Mol. Cell. Biochem. 204:107-117. [DOI] [PubMed] [Google Scholar]