Newcastle disease (ND) is an OIE (World Organization for Animal Health) notifiable disease affecting many species of birds and causing severe economic losses in the poultry sector. The disease is caused by virulent avian paramyxoviruses of serotype 1 (APMV-1). As for many other RNA viruses, the genetic variability of APMV-1 is significant. Genetic analysis has revealed the existence of two main clades, namely classes I and II, with distinct lineages and sublineages (2). Class I typically includes low-virulence strains recovered from live-bird markets in the United States or wild waterfowl worldwide (3, 4). Class II comprises the vast majority of viruses isolated from poultry, including the virulent strains. The official method for ND diagnosis is virus isolation in embryonated eggs (5). Recently, molecular tests have become more common, including the USDA-validated real-time reverse transcription-PCR (rRT-PCR) which is widely used in North America and Europe. This assay targets a conserved region of the matrix gene and correlates well with virus isolation in the study in which it was originally used (6). However, it is not expected to detect both avirulent and virulent ND viruses of all the genetic lineages included in classes I and II (3, 6). In this study we report discrepancies between the molecular and virus isolation results with samples collected from three continents. We also report the development of an improved probe resulting in enhanced diagnostic performance.
Virus isolation (5) and rRT-PCR (6) were done to confirm the presence of ND viruses in suspect samples submitted to the OIE/FAO Reference Laboratory for avian influenza and ND viruses. Discrepant results between the two laboratory tests were revealed. In particular, 34 samples collected from domestic birds, doves, and pigeons in Italy, Nepal, Mauritania, and Niger yielded positive results by virus isolation but tested negative by rRT-PCR on the clinical sample and on the virus isolate. The identification, pathotyping, and genotyping of the isolates were confirmed by genetic sequencing of the hypervariable part of the fusion (F) gene (1). All the isolates were identified as virulent viruses belonging to distinct genetic lineages within class II. Specifically, they clustered in lineage 4 (10 isolates from Italy), in lineage 5 (9 isolates from Nepal), and in a separate novel lineage, tentatively named lineage 7 (15 isolates from Mauritania and Niger).
To investigate the reasons for these discrepancies, the fragment of the matrix (M) gene targeted by the rRT-PCR protocol was sequenced and analyzed. Multiple sequence alignments revealed several nucleotide mismatches in the primer and probe regions. To verify whether the primers or the probe was responsible for the false-negative results of the rRT-PCR, the samples were loaded on an acrylamide gel after rRT-PCR analysis. Distinct and specific amplification products of the expected size (121 bp) were visualized on the gel (Fig. 1), indicating the adequate performance of the primers and demonstrating that the probe mismatches were likely the cause for the false rRT-PCR results (i.e., absence of a fluorescent signal in the rRT-PCR assay).
FIG. 1.
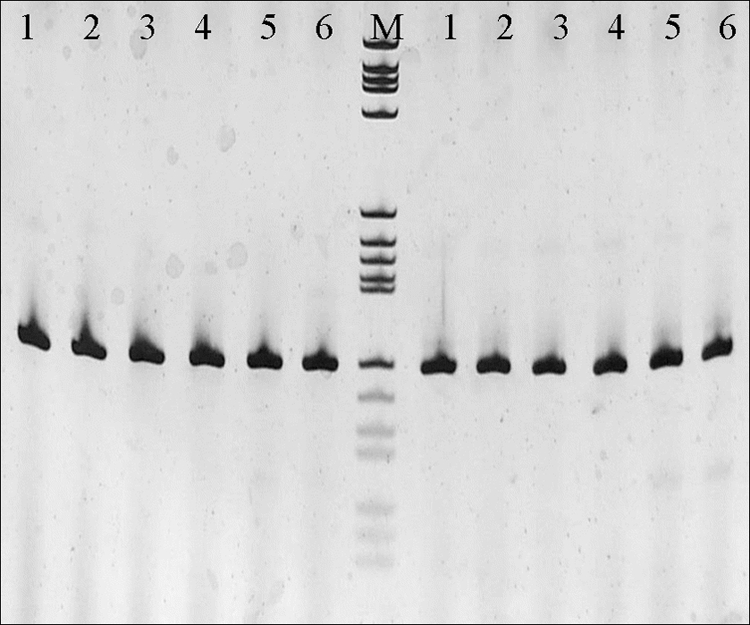
Sodium dodecyl sulfate-acrylamide gel electrophoresis of the rRT-PCR products. Samples loaded in this gel tested negative by rRT-PCR with the original primers/probe set (6). Lanes: 1, 1377-1/chicken/Niger/2006; 2, 1532-14/chicken/Mauritania/2006; 3, 2407-135/avian/Nepal/2006; 4, 1210/dove/Italy/2007; 5, 50/pigeon/Italy/2008; 6, positive control (ND virus Ulster 2C). M, molecular marker V (8 to 587 bp; Roche). Lanes to the left of lane M, PCR products resulting from the application of the original primer-and-probe set; lanes to the right of lane M, PCR products resulting from the application of the modified probe. Primers are identical to those used in the original protocol (6).
To minimize variations in the protocol, an alternative probe was designed for the region within the two primers, which remained unchanged. To limit the number of nucleotide mismatches and the nucleotide degeneracy, the probe was shortened and degenerated in only two positions. To maintain probe stability and efficiency, the probe was locked in six nucleotide positions by the introduction of modified nucleotides (locked nucleic acid [LNA] probe). Primer and probe positions are illustrated in Fig. 2. The LNA probe was hexachloro-6-carboxyfluorescein (HEX)-GGGAcRgcHtGcTATcC-black hole quencher (BHQ) 3′ (lowercase letters indicate locked nucleotide positions).
FIG. 2.
Matrix (M) gene sequence alignment of representative viruses, illustrating the nucleotide sequences targeted by the primers and probe according to the original protocol (underlined). The nucleotide sequence targeted by the modified LNA probe is indicated in italic, bold type. AF077761, GenBank accession number of the sequence selected for the reference numbering (La Sota strain). The complete M gene sequences for isolates marked ND* have been deposited in GenBank. +, virus strains tested positive by the original protocol (6); −, virus strains tested negative by the original protocol (6).
The specificity of the modified rRT-PCR assay was tested on 9 APMV-1 strains (lineages 1, 2, 3, 4, and 5) and on unrelated microorganisms such as APMV serotypes 2 to 9 (7 strains), avian infectious bronchitis viruses (5 strains), avian influenza viruses (17 strains; subtypes H1 to H16), and avian pneumovirus types A and B. The modified test was capable of detecting all the distinct APMV-1 strains, and no cross-reactions with other viruses were found. The analytical sensitivity of the new protocol (62 × 102 gene copies/μl) was comparable to that of the original procedure (6). On clinical specimens, the threshold cycle (CT) values were similar to and in some cases lower than the CT values obtained with samples that tested positive by the original protocol. The modified rRT-PCR was capable of detecting all the variant strains, showing excellent performance and broadening the applicability of the original protocol to all known class II APMV-1 virus isolates.
The genetic diversity among APMV-1 isolates may produce false-negative results when molecular tests only are relied on. Our results suggest that virus isolation should not be abandoned and support the importance of monitoring circulating strains to rapidly identify possible antigenic and genetic variants.
Nucleotide sequence accession numbers.
The complete M gene sequences for isolates 913-1, 2415-580, 2602-348, 1377-8, and 1532-14 have been deposited in GenBank under accession numbers FJ772447, FJ772464, FJ772470, FJ772453, and FJ772456, respectively.
Footnotes
Published ahead of print on 16 September 2009.
REFERENCES
- 1.Aldous, E. W., J. K. Mynn, J. Banks, and D. J. Alexander. 2003. A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a partial nucleotide sequence of the fusion protein gene. Avian Pathol. 32:239-256. [DOI] [PubMed] [Google Scholar]
- 2.Czeglédi, A., D. Ujvári, E. Somogyi, E. Wehmann, O. Werner, and B. Lomniczi. 2006. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 120:36-48. [DOI] [PubMed] [Google Scholar]
- 3.Kim, L. M., D. J. King, P. E. Curry, D. L. Suarez, D. E. Swayne, D. E. Stallknecht, R. D. Slemons, J. C. Pedersen, D. A. Senne, K. Winker, and C. L. Afonso. 2007. Phylogenetic diversity among low-virulence Newcastle disease viruses from waterfowl and shorebirds and comparison of genotype distributions to those of poultry-origin isolates. J. Virol. 81:12641-12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim, L. M., D. J. King, D. L. Suarez, C. W. Wong, and C. L. Afonso. 2007. Characterization of class I Newcastle disease virus isolates from Hong Kong live bird markets and detection using real-time reverse transcription-PCR. J. Clin. Microbiol. 45:1310-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OIE. 14 August 2009, posting date. Manual of diagnostic tests and vaccines for terrestrial animals 2008. http://www.oie.int/eng/normes/mmanual/A_summry.htm.
- 6.Wise, M. G., D. L. Suarez, B. S. Seal, J. C. Pedersen, D. A. Senne, D. J. King, D. R. Kapczynski, and E. Spackman. 2004. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 42:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]



