Abstract
The aim of this prospective randomized controlled clinical trial was to assess the impact of immediate incubation of blood cultures delivered to the laboratory outside its hours of operation on turnaround times, antibiotic prescription practices, and patient outcomes. A continuously monitoring blood culture incubator was placed outside the laboratory, which was switched on (intervention arm) and off (control arm) in a randomized manner. Included were new bacteremia episodes of patients older than 18 years. During the 30-week study period, the first positive blood culture specimen of an episode had to be brought to the laboratory outside its hours of operation. The median time from specimen collection until growth detection was reduced by 10.1 h in the intervention arm (P < 0.001). For 46 of 66 (70%) episodes in the intervention arm and for 51 of 85 (60%) episodes in the control arm, the antibiotic regimen was changed (not significant). The median time until the first change in the antibiotic regimen was 42.8 h in the intervention arm and 64.0 h in the control arm (P, 0.024). There was no difference in length of stay or hospital mortality. Immediate incubation of blood cultures outside laboratory hours reduces turnaround times and accelerates antibiotic switching.
Appropriate antibiotic therapy for bacteremia is associated with a decrease in mortality (10). Shortening the period during which no therapy or only empirical therapy is given may result in improved patient outcomes. A major step in reducing mortality rates (6), costs (1), and antibiotic use was the introduction of microbiological diagnostic systems that reduce the turnaround time of tests for the identification and susceptibility testing of bacterial pathogens.
Probably the most important moment for changing antibiotic therapy is when the results of blood culture specimens from patients with signs and symptoms of severe sepsis become available. Blood culture specimen analysis was considerably improved by the introduction of automated blood culture systems.
Our hypothesis was that immediate incubation of blood culture bottles would shorten the time until antibiotic therapy is changed and therefore might improve patient outcomes. With this aim, we placed a continuously monitoring blood culture incubator (Bactec 9120; Becton Dickinson, Sparks, MD) outside the laboratory. We assessed the impact of immediate incubation of blood cultures delivered to the laboratory after the hours of operation on turnaround times, the time to antibiotic regimen change, in-hospital mortality, and hospital stay in a randomized, controlled clinical trial.
MATERIALS AND METHODS
Setting.
The Erasmus University Medical Centre is a 1,200-bed tertiary-care university medical center. The Department of Medical Microbiology and Infectious Diseases has its laboratory integrated with an active infectious-disease (ID) consultation service run by a team of M.D. microbiologists and infectious-disease specialists. This ID consultation service operates 24 h per day, 7 days per week. The laboratory is open on weekdays from 7:30 am to 5:00 pm and on Saturdays and Sundays from 8:30 am to 1:00 pm. During its hours of operation, all blood culture specimens are processed immediately. During evening and night shifts, a technician and an ID consultant are on call for emergency purposes. Blood culture bottles are inoculated directly at the bedside using a closed Vacutainer needle system. A standard blood culture set consists of an aerobic and an anaerobic bottle (Bactec Plus Aerobic/F and Anaerobic/F bottles).
Intervention.
A continuously monitoring blood culture incubator (Bactec 9120; Becton Dickinson, Sparks, MD) was placed in the hall directly outside the laboratory facility. This enabled the loading of blood culture bottles into the incubator outside the laboratory hours of operation. This incubator was randomly switched on and off. During the periods when the incubator was switched on (Bactec-ON periods, or intervention periods), negative bottles were transferred to the main Bactec system inside the laboratory, and positive bottles were processed at the start of the working day. During the Bactec-OFF periods (control periods), the outside Bactec incubator functioned as a blood culture storage cabinet at ambient temperature, and all bottles were transferred to the main Bactec system in the morning.
Randomization.
An independent epidemiologist provided a computer-generated list defining the status (ON/OFF) of the outside Bactec incubator; permuted block randomization was used (three randomization blocks of 8 weeks and one of 6 weeks). The setting of the instrument was changed weekly, by two senior technicians, in accordance with the computer-generated list. These senior technicians did not have any direct involvement with patient diagnostics.
After the 30-week study period was completed, the primary investigator assessed each entry for eligibility according to predefined inclusion criteria (see below).
A reduction in turnaround time cannot be blinded; thus, no formal blinding was attempted. However, the attending physicians and the consulting ID specialists were not informed that a particular patient was included in the trial. The monitor displaying the outside Bactec status was switched off throughout the entire study period.
Inclusion criteria.
Included were new bacteremia episodes of patients older than 18 years who were hospitalized or seen at the emergency department. The first positive blood culture specimen of an episode had to be brought to the laboratory outside its hours of operation. Only new bacteremia episodes were included. To qualify as a new bacteremia episode, there had to be at least 7 days between positive cultures with the same organism, and at least 24 h if different species of microorganisms were recovered.
Outcomes and data collection.
The primary outcome measure was the time from specimen collection to the first change in the antibiotic regimen, defined as the starting, switching, addition, or stopping of an antibiotic, that occurred after the detection of growth. Secondary outcome parameters were turnaround times (defined as the time from collection to growth detection, identification, and susceptibility testing results), total duration of hospital stay, and hospital mortality.
Episodes were followed until 48 h after the susceptibility testing results were available for the primary outcome, or 48 h after identification if no susceptibility testing was performed. Changes after this period were considered not to be related to culture results. For the secondary outcomes, mortality and hospital stay, the first episode of each patient was followed until hospital discharge.
For all patients included, data on microbiological cultures, age, gender, duration and department of stay, mortality, and antibiotic use were collected from the hospital information system and the intensive care units' information systems.
The time of detection of blood culture growth was defined as the time at which the positive results of the main Bactec system were actively transferred electronically to our laboratory information system by a technician. Times of identification and susceptibility testing were extracted from the laboratory information system.
Therapeutic systemic antibiotic prescriptions were collected from the time of specimen collection until 48 h after susceptibility testing results were available, or 48 h after identification if no susceptibility testing was performed. No data were collected on antibiotics given topically or antibiotics given for prophylactic indications.
For patients with positive blood cultures, ID diagnoses were classified by the consulting physician using a preprogrammed list in our electronic consultation system. This list is based on the ICD-10 criteria (http://www.who.int/classifications/icd/en/). For each episode included in the study, the ID diagnosis was retrieved from this system.
The study was approved by the Medical Ethics Committee of the Erasmus University Medical Centre, and no informed consent was required.
Statistical methods.
All randomized episodes that met the eligibility criteria were analyzed on their assigned trial arm (intention to treat). Patient characteristics, culture isolates, and infections at baseline were analyzed by Pearson chi-square tests with Fisher's exact test in case of small numbers (noncontinuous variables) and Student t tests (continuous variables). Differences in the turnaround time, the time from specimen collection to the first change in the antibiotic regimen, and the length of hospital stay were analyzed using nonparametric tests (Mann-Whitney U test). Both the time from specimen collection to the first change in the antibiotic regimen and mortality were analyzed univariately using Kaplan-Meier survival analysis. A P value of <0.05 was considered statistically significant.
RESULTS
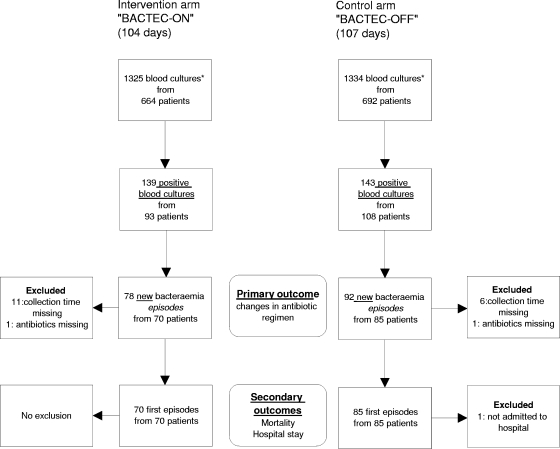
The inclusion period was from March to October 2005. The Bactec incubator was switched on for 104 days (intervention period) and off for 107 days (control period). Figure 1 details the Bactec ON/OFF switch regimen and the flow of blood cultures delivered to the laboratory during the hours when the laboratory was closed. A total of 170 new bacteremia episodes were identified: 78 episodes (70 patients) in the Bactec-ON arm and 92 episodes (85 patients) in the Bactec-OFF arm.
FIG. 1.
Blood cultures brought to the laboratory outside its hours of operation from March to October 2005, and selection of positive episodes to be included in the study. *, a blood culture consists of two bottles: an aerobic and an anaerobic bottle.
Table 1 summarizes the baseline demographic and clinical characteristics of each group. There were no significant differences between the two groups at baseline.
TABLE 1.
Characteristics of patients and bacteremia episodes at baseline
| Characteristic | Value for group |
P | |
|---|---|---|---|
| Bactec-ON | Bactec-OFF | ||
| Patient characteristics (first episode) | |||
| No. of patients | 70 | 85 | |
| Age (yr) (±SD) | 57.4 (19) | 56.6 (14.3) | 0.73 |
| No. (%) male | 48 (69) | 55 (65) | 0.73 |
| No. (%) in the following ward: | 0.15 | ||
| Medical | 25 (36) | 32 (38) | |
| General surgery | 7 (10) | 16 (19) | |
| Neurology and neurosurgery | 5 (7) | 2 (2) | |
| Cardiology and thorax surgery | 6 (9) | 10 (12) | |
| Emergency department | 2 (3) | 6 (7) | |
| Intensive care unit | 25 (36) | 19 (22) | |
| Episode characteristics | |||
| No. of episodes | 78 | 92 | |
| No. (%) with the following microorganism: | 0.10 | ||
| Coagulase-negative staphylococci | 24 (31) | 34 (37) | |
| S. aureus | 14 (18) | 7 (8) | |
| Streptococci and enterococci | 2 (3) | 11 (12) | |
| Enterobacteriaceae and Pseudomonas spp. | 25 (30) | 22 (24) | |
| Other | 4 (5) | 8 (9) | |
| Yeast | 1 (1) | 2 (2) | |
| Mixed culture | 8 (10) | 8 (9) | |
| No. (%) with the following type of underlying infection: | 0.28 | ||
| Urinary tract | 6 (8) | 7 (8) | |
| Intravenous catheter related | 27 (35) | 28 (30) | |
| Respiratory tract | 1 (1) | 5 (5) | |
| Intra-abdominal | 19 (24) | 21 (23) | |
| Bloodstream, of unknown origin | 9 (11) | 5 (5) | |
| Other | 7 (9) | 4 (4) | |
| Contamination | 9 (12) | 22 (24) | |
For 67 (86%) episodes in the intervention arm and 86 (92%) episodes in the control arm, the time of specimen collection was available. The median time from blood culture collection to growth detection was reduced by 10.1 h in the Bactec-ON arm (P < 0.001). Subsequently, median times from specimen collection to identification and susceptibility testing results were also significantly reduced (Table 2).
TABLE 2.
Time intervals between blood culture collection and growth detection, identification, and susceptibility testing results for new bacteremia episodes
| Result | Median (IQR) time (h) from specimen collection |
P | |
|---|---|---|---|
| Bactec-ON (n = 67) | Bactec-OFF (n = 86) | ||
| Growth detection | 29 (18-39) | 39 (29-51) | <0.001 |
| Identification | 44 (36-61) | 63 (44-86) | <0.001 |
| Susceptibility testing | 62 (45-81) | 70a (60-92) | 0.009 |
n = 82 new bacteremic episodes.
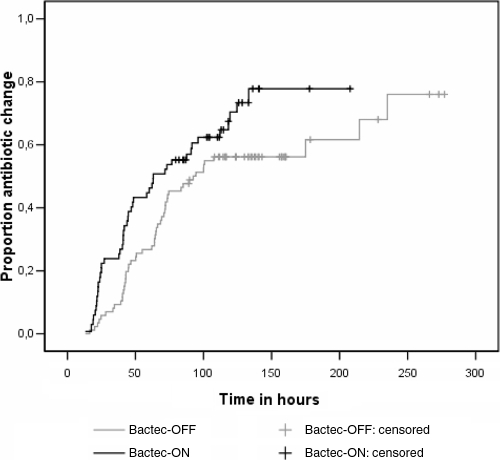
For two episodes (one in each of the two arms of the study), antibiotic use could not be assessed due to missing data. In 46 of 66 (70%) episodes in the Bactec-ON arm and in 51 of 85 (60%) episodes in the Bactec-OFF arm, the antibiotic regimen was changed (at least once) between growth detection and 48 h after susceptibility testing results became available. Of the first changes in the Bactec-ON arm, 14 (30%) were starts, 20 (43%) were switches, 8 (17%) were additions, and 4 (9%) were stops; in the Bactec-OFF arm, these numbers were 11 (22%), 21 (41%), 9 (18%), and 10 (20%), respectively. The median times from specimen collection until the first change in antibiotic therapy were 42.8 h (interquartile range [IQR], 23.5 to 72.1 h) in the Bactec-ON arm and 64.0 h (IQR, 41.8 to 74.5 h) in the Bactec-OFF arm (P = 0.024). Figure 2 presents the result of the Kaplan-Meier survival analysis, showing the significant reduction in the time to a change in the antibiotic regimen. In the Bactec-ON arm, 14 of 70 patients (20%) died during their hospital stays; in the Bactec-OFF arm, 11 of 84 patients (13%) died (P = 0.25). For the 56 surviving patients in the Bactec-ON arm, the median hospital stay was 28 days (IQR, 13.5 to 43.5 days); for the 73 surviving patients in the control arm, the median hospital stay was 23 days (IQR, 9.5 to 64.5 days) (P = 0.89).
FIG. 2.
Time from specimen collection to the first antibiotic regimen change after growth detection. Episodes were censored if patients left the hospital before the changes in therapy occurred. The differences between the arms are significant (P = 0.029).
DISCUSSION
Immediate incubation of blood cultures that arrive when the medical microbiology laboratory is closed significantly reduces turnaround times. This reduction, in turn, results in a significantly earlier change of antibiotic regimen. However, this earlier change in antibiotic regimen did not result in a reduction of mortality or length of hospital stay for these patients.
The limitations of our study are, first, that we used the time when the technician detected growth in the Bactec system as the start of the period in which changes in the antibiotic regimen were based on the culture result. We elected to use this point in time because this information was recorded without bias and was easily obtained. However, some delay exists between this time and the time when a result is brought to the attention of the attending physician; this time lag can range from 15 min (time to make a Gram stain and make a telephone call) to a couple of hours, depending on the workload and schedule of the ID consultant.
A second limitation of our study design is the follow-up period of 48 h after susceptibility testing results became available. This time frame may have been too short, such that we may have missed a number of later antibiotic changes based on the blood culture result. However, extending this time frame would have increased the chance of including changes in the antibiotic regimen that were unrelated to the reporting of the microbiological test results. However, both limitations will not have introduced bias or confounding into our comparison, due to the randomized nature of this trial.
A third limitation is that our sample size was too small to study the impacts of time to positivity (Gram stain), identification, and susceptibility testing separately on outcome. Munson et al. (7) have shown that notification of Gram stain results had a larger impact on antibiotic changes than notification of species identification and susceptibility testing data.
Although there were no significant differences between the two arms at baseline, the bacteremia episodes in the intervention arm (Bactec-ON) included more true infections and fewer episodes classified as contaminated blood cultures. Indeed, the percentages of isolates of Staphylococcus aureus and Enterobacteriaceae, which are always considered true pathogens, had a tendency to be higher in the intervention arm. Correction for these factors did not significantly influence the outcome (data not shown).
Riest et al. (8) demonstrated that the time benefit of continuously monitoring blood culture incubators for detection of positive blood cultures is lost if the loading and processing of the blood culture bottles is organized discontinuously. Also, the recovery of microorganisms is compromised if the loading of blood culture bottles is delayed, especially when they are stored at 36°C before loading into the blood culture system (9); therefore, preincubation without growth monitoring is not advised. Bengtsson et al. (2) found that with continuous (24 h per day, 7 days per week) loading of the BacT/Alert blood culture system, the median time from specimen collection until a Gram stain result was obtained was only 21.5 h in their setting. The 7.5-h difference from the 29 h we found is probably due to less-efficient transport in our setting.
In The Netherlands, the concept of de-escalation therapy (5) to a regimen with the narrowest spectrum providing adequate coverage is widely accepted, advised by the ID consultants, and implemented by the attending physicians. Earlier reporting of laboratory results will have less impact if these de-escalating policies are not in force. In countries with high rates of multiply resistant microorganisms causing bacteremic disease, streamlining will prove more difficult. However, rapid reporting of results could also reduce the time during which a patient is exposed to an ineffective regimen. Byl et al. (4) reported that empirical treatment was appropriate in only 63% of bacteremia episodes and that after blood culture results became available, the proportion of appropriate treatments increased to 94%.
The cost of implementing our intervention is low: just a small continuously monitoring blood culture incubator that occupies little space. The benefits could be substantial; Berild et al. (3) have shown that adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and decreased costs. Rapid availability of results by immediate incubation leads to an earlier adjustment and probably to even lower antibiotic costs.
In conclusion, we have shown that immediate incubation of blood culture bottles brought to the laboratory at odd hours results in significantly reduced turnaround times and that the reduced turnaround times result in significantly earlier changes in antibiotic therapy. This intervention is inexpensive and easy to integrate into the present procedures of medical microbiological laboratories.
Acknowledgments
Becton Dickinson provided the outside Bactec incubator used in this study but had no influence on the design, conduct, and analysis of the trial. Otherwise, no funding was received.
The authors declare no conflicts of interest.
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Barenfanger, J., C. Drake, and G. Kacich. 1999. Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing. J. Clin. Microbiol. 37:1415-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtsson, J., M. Wahl, and P. Larsson. 1998. Assessment of the BacT/Alert blood culture system: rapid bacteremia diagnosis with loading throughout the 24 h. Clin. Microbiol. Infect. 4:33-37. [DOI] [PubMed] [Google Scholar]
- 3.Berild, D., A. Mohseni, L. M. Diep, M. Jensenius, and S. H. Ringertz. 2006. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J. Antimicrob. Chemother. 57:326-330. [DOI] [PubMed] [Google Scholar]
- 4.Byl, B., P. Clevenbergh, F. Jacobs, M. J. Struelens, F. Zech, A. Kentos, and J. P. Thys. 1999. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin. Infect. Dis. 29:60-68. [DOI] [PubMed] [Google Scholar]
- 5.Deresinski, S. 2007. Principles of antibiotic therapy in severe infections: optimizing the therapeutic approach by use of laboratory and clinical data. Clin. Infect. Dis. 45(Suppl. 3):S177-S183. [DOI] [PubMed] [Google Scholar]
- 6.Doern, G. V., R. Vautour, M. Gaudet, and B. Levy. 1994. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J. Clin. Microbiol. 32:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munson, E. L., D. J. Diekema, S. E. Beekmann, K. C. Chapin, and G. V. Doern. 2003. Detection and treatment of bloodstream infection: laboratory reporting and antimicrobial management. J. Clin. Microbiol. 41:495-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riest, G., H. J. Linde, and P. M. Shah. 1997. Comparison of BacT/Alert and BACTEC NR 860 blood culture systems in a laboratory not continuously staffed. Clin. Microbiol. Infect. 3:345-351. [DOI] [PubMed] [Google Scholar]
- 9.Sautter, R. L., A. R. Bills, D. L. Lang, G. Ruschell, B. J. Heiter, and P. P. Bourbeau. 2006. Effects of delayed-entry conditions on the recovery and detection of microorganisms from BacT/ALERT and BACTEC blood culture bottles. J. Clin. Microbiol. 44:1245-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallés, J., J. Rello, A. Ochagavia, J. Garnacho, and M. A. Alcala. 2003. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest 123:1615-1624. [DOI] [PubMed] [Google Scholar]