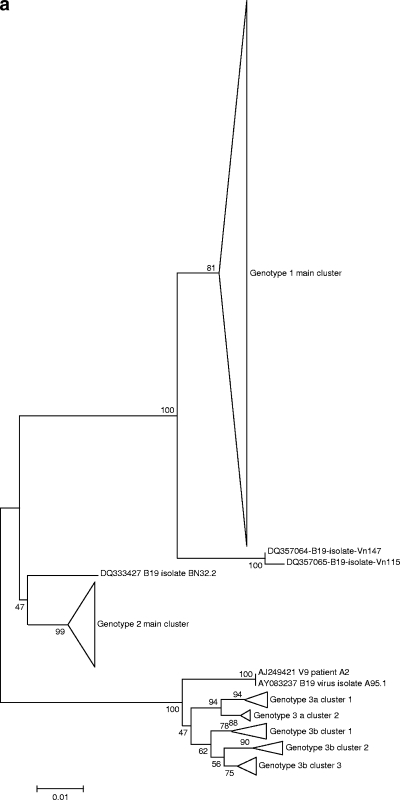
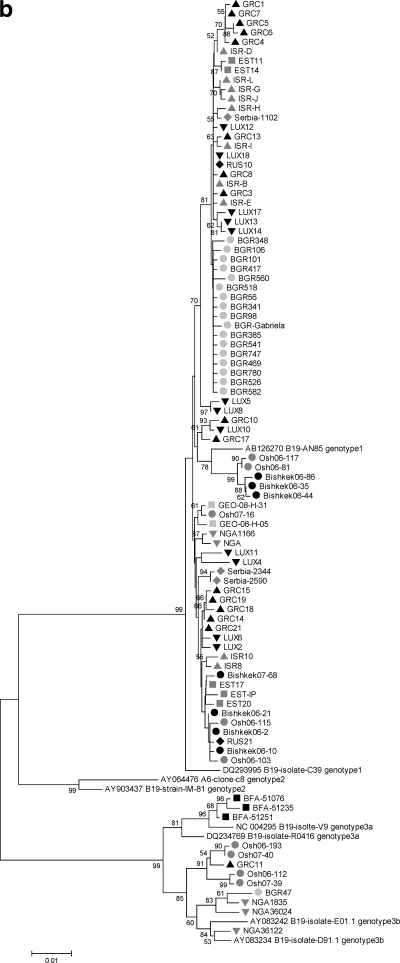
Judith M Hübschen
Judith M Hübschen
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
1,
Zefira Mihneva
Zefira Mihneva
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
2,
Andreas F Mentis
Andreas F Mentis
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
3,
François Schneider
François Schneider
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
4,
Yair Aboudy
Yair Aboudy
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
5,
Zehava Grossman
Zehava Grossman
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
5,
Hagit Rudich
Hagit Rudich
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
5,
Kalia Kasymbekova
Kalia Kasymbekova
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
6,
Inna Sarv
Inna Sarv
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
7,
Jasminka Nedeljkovic
Jasminka Nedeljkovic
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
8,
Marc C Tahita
Marc C Tahita
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
9,
Zekiba Tarnagda
Zekiba Tarnagda
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
9,
Jean-Bosco Ouedraogo
Jean-Bosco Ouedraogo
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
9,
A G Gerasimova
A G Gerasimova
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
10,
T N Moskaleva
T N Moskaleva
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
10,
Nina T Tikhonova
Nina T Tikhonova
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
10,
Nazibrola Chitadze
Nazibrola Chitadze
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
11,
J C Forbi
J C Forbi
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
1,12,
Adedayo O Faneye
Adedayo O Faneye
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
13,
Jesse A Otegbayo
Jesse A Otegbayo
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
14,
Emilie Charpentier
Emilie Charpentier
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
1,
Claude P Muller
Claude P Muller
Institute of Immunology, Laboratoire National de Santé/Centre de Recherche Public-Santé, Luxembourg, Luxembourg,1 National Center of Infectious & Parasitic Diseases, Department of Virology, Measles/Rubella Laboratory, Sofia, Bulgaria,2 Laboratory of Medical Microbiology, Institut Pasteur Hellenique, Athens, Greece,3 Division of Microbiology, Laboratoire National de Santé, Luxembourg, Luxembourg,4 Central Virology Laboratory, Sheba Medical Center, Ramat Gan, Israel,5 National Virological Laboratory, Department of the State Sanitary and Epidemiological Surveillance, Ministry of Health of Kyrgyz Republic, Bishkek, Kyrgyzstan,6 Central Laboratory of Communicable Diseases, Health Protection Inspectorate, Tallinn, Estonia,7 Respiratory Department, TORLAK Institute of Immunology and Virology, Belgrade, Serbia,8 Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso,9 Gabrichevsky G.N. Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation,10 Measles/Rubella Group, National Center for Disease Control and Public Health, Tbilisi, Georgia,11 Virology Laboratory, Innovative Biotech-Keffi No. 1, Keffi, Nasarawa State, Nigeria,12 Department of Virology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria,13 Department of Medicine, University College Hospital & University of Ibadan, Ibadan, Nigeria14
1,*