Abstract
Blood culture contamination greatly affects clinical decisions. Hence, it is of interest to assess the influence of factors such as the volume of blood drawn and the site of blood draw on the rates of blood culture contamination. In a retrospective study, blood cultures from infants and children up to 18 years of age who had at least one positive blood culture during the year 2006 were analyzed for their volume of blood drawn, patient's weight, site of blood draw used, and blood culture results. Blood cultures were deemed adequate collections if they contained an appropriate weight-related volume of blood. Moreover, blood culture results were categorized as true pathogens, contaminants, and negative cultures; these were then compared and analyzed with respect to their volume and site of blood draw. A total of 5,023 blood cultures were collected during 2006, of which 843 were analyzed. There were 306 (36%) positive cultures among the 843 cultures analyzed. Of the 306 positive cultures, 98 (32%) were contaminants and 208 (68%) cultures grew significant pathogens. Thirty-five percent of the contaminant cultures had adequate volume compared to 60% in the true bacteremia group (P < 0.001). Also, of the 843 cultures, the rates of contamination among the different sites of blood draw were as follows: peripheral venipuncture, 36%; arterial, 10%; and central venous access, 7% (P = 0.155). The rate of contamination was higher with lower blood volumes, and there was no significant difference in the rates of contamination among the different sites of blood draw.
Blood cultures are vital for identifying pathogens causing serious infections and in directing appropriate antibiotic therapy. Moreover, they remain the standard method for detecting bacteremia in the evaluation of sick patients (14). Unfortunately, blood culture contamination is a common occurrence and may lead to confusion regarding the significance of a positive blood culture. The most common contaminants are coagulase-negative staphylococcus species which are also becoming more prevalent as a primary pathogen in immunocompromised patients and in patients with indwelling intravascular devices (9, 15). The uncertain clinical significance of potential contaminants leads to longer hospital stays, unnecessary antibiotic therapy, and additional laboratory testing; as a result, the cost incurred by a hospital is many times that incurred by the laboratory (2).
Many factors influence the yield of blood cultures, but the single most important factor is blood volume. Several studies have shown that the rate of isolation of pathogens from blood cultures increases with the quantity of blood submitted (12). Hence, a blood culture may be falsely negative from an inadequate-volume blood culture (6). Furthermore, the blood culture contamination rate inversely correlates with the volume of blood (3). The site and method of blood collection have also been known to influence the rate of contamination of blood cultures (8). Vascular-access devices, such as arterial and central venous catheters, pass through the skin and are susceptible to bacterial colonization. Hence, it is easy for these bacteria to colonize and multiply in and around these ports, and they can be pulled into blood specimens drawn from those sites. Hence, the primary aims of this study were to determine the volume of blood obtained for culture in routine clinical practice and to evaluate if inadequate blood volumes lead to an increased incidence of contaminants. Finally, this study also assessed whether the site of blood draw was related to an increased frequency of contaminated cultures.
MATERIALS AND METHODS
This is a retrospective study of all blood cultures from infants and children up to 18 years of age who had at least one positive blood culture during the year 2006 at the Children's Hospital of Omaha, Omaha, NE. We obtained the information from the Sunrise Clinical Management system used by the Children's Hospital of Omaha. The following details were recorded for each sample: date of blood culture, volume of blood drawn, patient's date of birth, patient's weight on admission, source of blood draw, time to positivity of blood culture, and final culture result. The source of blood collection was classified as central if it came from a central venous catheter, arterial if it came from an arterial line, or peripheral if the blood was drawn via peripheral venipuncture. Blood cultures were ordered as a set which contained one aerobic bottle and one anaerobic bottle. The total blood volume collected was to be distributed equally between the two bottles present in each blood culture set. It is common to order two blood culture sets when ordering blood cultures for a patient. Only nonpediatric bottles were used for blood draws, and broth medium was used to grow these cultures. The cultures were placed into an automated continuous blood culture monitoring system, BacT/Alert (Bio Merieux, Durham, NC). Patients weighing more than 8 kg had two sets of blood cultures drawn from different sites. Blood culture sets were deemed adequate collections if they contained an appropriate weight-related volume of blood as per Table 1. This table represents the total blood volume that needs to be collected for two blood culture sets that are being used by the microbiology laboratory at the Children's Hospital of Omaha.
TABLE 1.
Volume of blood required for an adequate blood culture based on patient's weight
| Patient wt (kg) | Vol needed (ml) | Vol (ml) drawna |
|||
|---|---|---|---|---|---|
| 1st blood culture set |
2nd blood culture set |
||||
| 1st bottle | 2nd bottle | 1st bottle | 2nd bottle | ||
| <3.9 | 1 | 0.5 | 0.5 | ||
| 4-7.9 | 3 | 1.5 | 1.5 | ||
| 8-13.9 | 6 | 1.5 | 1.5 | 1.5 | 1.5 |
| 14-18.9 | 12 | 3 | 3 | 3 | 3 |
| 19-25.9 | 16 | 4 | 4 | 4 | 4 |
| 26-39.9 | 20 | 5 | 5 | 5 | 5 |
| 40-53.9 | 32 | 8 | 8 | 8 | 8 |
| >54 | 40 | 10 | 10 | 10 | 10 |
The first bottle in each blood culture set was aerobic, and the second was anaerobic.
For this analysis, single culture isolates growing diphtheroid species, micrococcus species, and nonpneumococcal alpha-hemolytic streptococci were classified as contaminants. Also, coagulase-negative staphylococci were classified as contaminants if they grew out of only one blood culture with an extended time to positivity (>24 h) and the absence of any clinical signs and symptoms such as febrile status, poor feeding, and lethargy (17). However, they were considered to represent true bacteremia if the same organism grew on multiple consecutive cultures with or without obvious clinical signs of bacteremic status in the patient. This was confirmed by reviewing the medical records during that admission via the chart review. These provisions had to be made since there were multiple true bacteremia episodes of coagulase-negative staphylococci due to a significant sample size of immunocompromised patients and patients with long-standing indwelling vascular catheters. Finally, statistical analysis of our results was undertaken by using the chi-square test, with a two-sided test and a significance of 0.05. The computer software used to run this statistical analysis was SAS 9.1 (SAS Inc., Cary, NC).
RESULTS
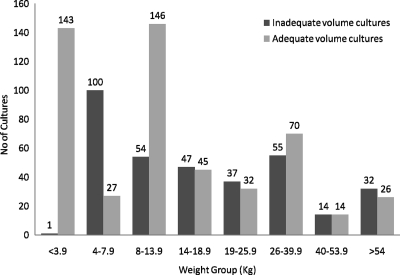
During the study period from 1 January 2006 until 31 December 2006, 5,023 total blood cultures were drawn at the Children's Hospital of Omaha. Of these total cultures, 1,231 blood cultures were collected from patients who had at least one positive blood culture. However, of the 1,231 aforementioned cultures, only 843 cultures were analyzed in our study after excluding 388 cultures due to the lack of blood volume data. Of these 843 blood cultures, 340 (40%) had inadequate volume compared to the remaining 503 (60%) blood cultures. The adequacy of blood culture volume across the different weight groups is shown in Fig. 1.
FIG. 1.
Blood culture volumes delineated as adequate versus inadequate based on the patient's weight. n = 843.
There were 274 organisms isolated from the 262 episodes of bacteremia/fungemia (12 corresponded to polymicrobial cases). The numbers and percentages of specific organisms isolated are presented in Table 2.
TABLE 2.
Numbers and percentages of specific organisms isolated from 262 patients with episodes of positive blood cultures
| Microorganism | No. (%) of cases | No. (%) of contaminants |
|---|---|---|
| Gram-positive bacteria | 216 (78.8) | 95 (44) |
| Staphylococcus aureus | 13 (4.7) | |
| Methicillin-resistant S. aureus | 12 (4.4) | |
| Coagulase-negative staphylococcus | 123 (44.9) | 83 (67) |
| Enterococcus sp. | 9 (3.3) | |
| Streptococcus pneumoniae | 7 (2.6) | |
| Group B Streptococcus | 4 (1.5) | |
| Other Streptococcus spp. | 18 (6.6) | 2 (11) |
| Corynebacterium sp. | 6 (2.2) | 4 (67) |
| Bacillus sp. | 20 (7.3) | 2 (10) |
| Micrococcus sp. | 4 (1.5) | 4 (100) |
| Gram-negative bacteria | 45 (16.4) | |
| Escherichia coli | 12 (4.4) | |
| Klebsiella sp. | 7 (2.6) | |
| Pseudomonas aeruginosa | 4 (1.5) | |
| Enterobacter sp. | 8 (2.9) | |
| Salmonella sp. | 3 (1.1) | |
| Citrobacter sp. | 2 (0.7) | |
| Haemophilus sp. | 2 (0.7) | |
| Roseomonas sp. | 2 (0.7) | |
| Other | 5 (1.8) | |
| Anaerobes | 1 (0.4) | |
| Clostridium sp. | 1 (0.4) | |
| Fungi | 12 (4.4) | |
| Candida sp. | 12 (4.4) | 3 (25) |
| Total | 274 | 98 |
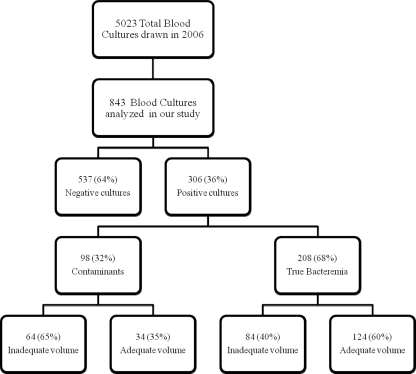
Of the 843 blood cultures that were analyzed in our study, 537 (64%) cultures were negative, whereas 306 (36%) cultures were positive (Fig. 2). Moreover, of the 306 positive cultures, 98 (32%) grew contaminants and 208 (68%) cultures were reported to have significant pathogens. Of the blood cultures that grew contaminants, 64 (65%) had inadequate volumes and 34 (35%) had adequate volumes. Similarly, of the cultures with true bacteremia, 84 (40%) had inadequate volumes and 124 (60%) had adequate volumes. There was a significant statistical difference between the 35% adequate-volume blood cultures in the contaminant group and the 60% adequate-volume blood cultures in the true bacteremia group, with a P value of <0.001.
FIG. 2.
Distribution of blood cultures based on results and volumes.
Of the 843 cultures used in this study, 41 (5%) were drawn from an arterial line, 371 (44%) from a central venous catheter, and 431 (51%) via peripheral venipuncture. Of the 41 arterial blood specimens, 24 (59%) were negative and 17 (41%) were positive cultures. Of these 17 positive cultures, 9 (53%) had adequate volumes and 8 (47%) had inadequate volumes. Furthermore, out of those 9 adequate-volume cultures, 1 (11%) was a contaminant and 8 (89%) were reported as true bacteremia.
Similarly, of the 371 cultures drawn via a central venous access, 237 (64%) were negative and 134 (36%) were positive. Of the 134 positive cultures, 65 (41%) cultures had adequate volume and 69 (59%) had inadequate volume. Finally, of the 65 adequate-volume cultures, 10 (15%) were contaminants and 55 (85%) were true pathogens.
There were 431 blood cultures drawn from peripheral sites. Of these, 276 (64%) were negative and 155 (36%) were positive. Of the 155 positive cultures, 84 (54%) had adequate volume of blood, whereas 71 (46%) had an inadequate volume of blood. Finally, of the 84 adequate-volume cultures, 23 (27%) were contaminants and 61 (73%) had significant pathogens. However, there was no significant statistical difference in the rate of contamination between either of the sites, with a P value of 0.155.
DISCUSSION
For physicians, contamination of blood cultures complicates decision making during patient care. In a febrile patient with leukocytosis, if one of the two blood cultures grows gram-positive cocci, empirical vancomycin therapy may be initiated. When coagulase-negative staphyloccus is identified and the second culture does not grow any organisms, it may be considered as a contaminant, resulting in unnecessary antibiotic use, risk of a drug reaction, drug resistance, and additional cost of hospitalization (4). On further examination via chart review, we found that 19 (20%) of the 98 blood cultures that grew contaminants led to the use of inappropriate empirical treatment with antibiotics, adding to unnecessary costs and potential development of antibiotic resistance. As per our data, we calculated a contamination rate of about 1.95% of the total 5,023 blood cultures drawn in the year 2006. The acceptable contamination rate in microbiology laboratories nationwide as per the American Society of Microbiology should not exceed 3% (16).
The optimal volume of blood that should be drawn from infants and children has not been defined with absolute certainty, but past data indicate a direct relationship between the volume of blood cultured and the ability to detect bloodstream infections (1). Using blood culture volume criteria used at our Children's Hospital, our analysis found that a considerable amount of blood cultures had inadequate volumes and only 503 (60%) of the 843 total blood cultures contained adequate volumes. This was higher than the result obtained by Connell et al. in their study at the Children's Hospital in Melbourne, Australia, where they examined 1,067 blood cultures, of which 491 (46%) contained an adequate volume of blood (6). However, our criteria for blood volumes were based on the patient's weight rather than age, which was used in their study.
We noticed an increase in the ratio of inadequate-volume cultures to adequate-volume cultures as the weight of patients increased (Fig. 1). This could be due to the increasing volume requirements needed for larger-weight groups. In a previous study by Bekeris et al., the blood culture contamination rate was inversely correlated with the volume of blood (3). Similarly, our data indicated that the true bacteremia group had a higher percentage of adequate volume cultures than the contaminant group, which was statistically significant (60% versus 35%; P < 0.001). We are unsure as to why inadequate-volume cultures are prone to grow contaminants. We hypothesize that with low blood volumes, the contaminant organisms may have higher concentrations than adequate-blood-volume cultures. Furthermore, low-blood-volume cultures possibly could have been drawn from patients with poor peripheral venous accessibility to begin with and hence causing a decreased ability to maintain a sterile technique during the blood draw.
When we examined the site of blood draw, we did not find any significant statistical difference between the rates of contamination among the various sites of blood draw. However, the sample size of the arterial group comprised only 5% (41/843) of all blood draws and hence was not large enough to contribute to statistical significance. Moreover, these results conflict with data from past studies which indicate that blood cultures obtained from vascular catheters are more likely to be contaminated than percutaneously obtained cultures, due to difficulty in sterilizing catheters (7, 13). Past studies on blood draws from arterial lines as a source for blood cultures indicate that their results are not associated with higher diagnostic yields than venous blood cultures and are not recommended (11).
Possible limitations to our study include the fact that this was a retrospective analysis. Since there are no set criteria regarding adequacy of blood culture volumes, we used criteria applied at a teaching children's hospital. Moreover, there must be separate criteria for immunocompromised patients as they tend to have an increased bacterial load during infections and may not require as much blood for culture (5, 10). All of these factors make our classifications of the volumes used in our blood cultures subjective. Similarly, even though we were thorough in our chart reviews to distinguish between microorganisms grown as contaminants versus true bacteremia, some of them may have been misclassified. Finally, our study analyzed only a portion of the total blood cultures drawn during 2006 due to the lack of volume data, and hence, this may contribute to bias.
Nevertheless, despite these limitations, our study highlights the incidence of inadequate blood culture volume in a routine clinical practice. Moreover, we did notice a relationship between the rate of adequate-volume blood cultures and the rate of contamination. However, we did not find any significant difference in the rates of contamination between the different sites of blood draw.
Acknowledgments
We thank Jeanette Manley from the microbiology laboratory at Children's Hospital for providing a blood culture list for data collection.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Baron, E. J., M. P. Weinstein, W. M. Dunne, P. Yagupsky, D. F. Welch, and D. M. Wilson. 2005. Cumitech 1C, Blood cultures IV. Coordinating ed., E. J. Baron. American Society for Microbiology, Washington, DC.
- 2.Bates, D. W., and L. Goldman. 1991. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA 265:365-369. [PubMed] [Google Scholar]
- 3.Bekeris, L., J. Tworek, M. Walsh, and P. Valenstein. 2005. Trends in blood culture contamination: a College of American Pathologists Q-Tracks study of 356 institutions. Arch. Pathol. Lab. Med. 129:1222-1225. [DOI] [PubMed] [Google Scholar]
- 4.Bouza, E., D. Sousa, M. Rodriguez-Creixems, J. G. Lechuz, and P. Muñoz. 2007. Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections? J. Clin. Microbiol. 45:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. F., and R. E. Warren. 1990. Effect of sample volume on yield of positive blood cultures from adult patients with hematological malignancy. J. Clin. Pathol. 43:777-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell, T. G., M. Rele, D. Cowley, J. P. Buttery, and N. Curtis. 2007. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children's hospital. Pediatrics 119:891-896. [DOI] [PubMed] [Google Scholar]
- 7.Everts, R. J., E. N. Vinson, P. O. Adholla, and L. B. Reller. 2000. Contamination of catheter-drawn blood cultures. J. Clin. Microbiol. 39:3393-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, K. K., and J. A. Lyman. 2006. Updated review of blood culture contamination. Clin. Microbiol. Rev. 19:788-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huebner, J., and D. Goldman. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:223-236. [DOI] [PubMed] [Google Scholar]
- 10.Kellogg, J. A., J. P. Manzella, and D. A. Bankert. 2000. Frequency of low-level bacteremia in children from birth to fifteen years of age. J. Clin. Microbiol. 38:2181-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin, P. D., M. Hersch, B. Rudensky, and A. M. Yinnon. 2000. The use of the arterial line as a source for blood cultures. Intensive Care Med. 26:1350-1354. [DOI] [PubMed] [Google Scholar]
- 12.Mermel, L., and D. Maki. 1993. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann. Intern. Med. 119:270-272. [DOI] [PubMed] [Google Scholar]
- 13.Norberg, A., N. C. Christopher, M. L. Ramundo, J. R. Bower, and S. A. Berman. 2003. Contamination rates of blood cultures obtained by dedicated phlebotomy vs. intravenous catheter. JAMA 289:726-729. [DOI] [PubMed] [Google Scholar]
- 14.Shafazand, S., and A. Weinacker. 2002. Blood cultures in the critical care unit: improving utilization and yield. Chest 122:1727-1736. [DOI] [PubMed] [Google Scholar]
- 15.Von Eiff, C., G. Peters, and C. Heilmann. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677-685. [DOI] [PubMed] [Google Scholar]
- 16.Weinbaum, F. I., S. Lavie, M. Danek, D. Sixsmith, G. F. Heinrich, and S. S. Mills. 1997. Doing it right the first time. Quality improvement and the contaminant blood culture. J. Clin. Microbiol. 35:563-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein, M. P. 2003. Blood culture contamination: persisting problems and partial progress. J. Clin. Microbiol. 41:2275-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]