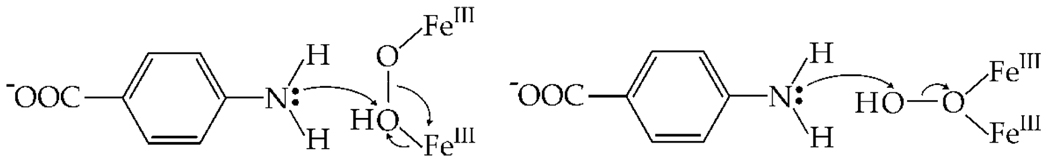Abstract
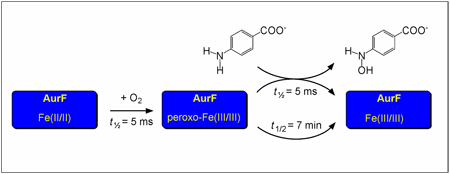
The amine oxygenase AurF from Streptomyces thioluteus catalyzes the six-electron oxidation of p-aminobenzoate (pABA) to p-nitrobenzoate (pNBA). In this work, we have studied the reaction of its reduced Fe2(II/II) cofactor with O2, which results in generation of a peroxo-Fe2(III/III) intermediate. In the absence of substrate, this intermediate is unusually stable (t1/2 = 7 min at 20°C), allowing for its accumulation to almost stoichiometric amounts. Its decay is accelerated ~105-fold by the substrate, pABA, implying that it is the complex that effects the two-electron oxidation of the amine to the hydroxylamine. The nearly quantitative conversion of pABA to pNBA by solutions containing an excess of the intermediate suggests that it may also be competent for the two subsequent two-electron oxidations leading to the product.
AurF from Streptomyces thioluteus utilizes O2 as oxidant to effect the six-electron oxidation of p-aminobenzoate (pABA) to p-nitrobenzoate (pNBA), a building block of the antibiotic aureothin.1 This conversion entails three successive two-electron oxidations via p-hydroxylaminobenzoate (pHABA) and p-nitrosobenzoate intermediates.2,3 Sequence analysis and the crystal structure of AurF showed that it has a carboxylate-bridged dinuclear metal center, which was presumed to activate O2 for the oxidation reactions. Following initial controversy as to the identities of the metal ions in the functional form(s),2–7 Zhao and co-workers recently established that the diiron form is competent to convert pABA to pNBA in the presence of O2 and a reducing system.8 Here we demonstrate that the Fe2II/II cluster in AurF reacts with O2 in the absence of substrate to form a remarkably stable (t1/2 ~ 7 min at 20 °C) adduct with spectroscopic properties characteristic of a peroxo-Fe2III/III complex. The intermediate complex decays rapidly (t1/2 ~ 0.005 s at 20 °C) when mixed with stoichiometric pABA, implying that it is competent for at least the first oxidation in the three-step sequence. The nearly quantitative (> 80%) conversion of pABA to pNBA upon addition of < 0.3 equiv of the amine substrate to a solution of the intermediate suggests that the complex might effect all three steps in the sequence. The results establish a new reactivity of non-heme diiron-peroxide intermediates: arylamine oxygenation.
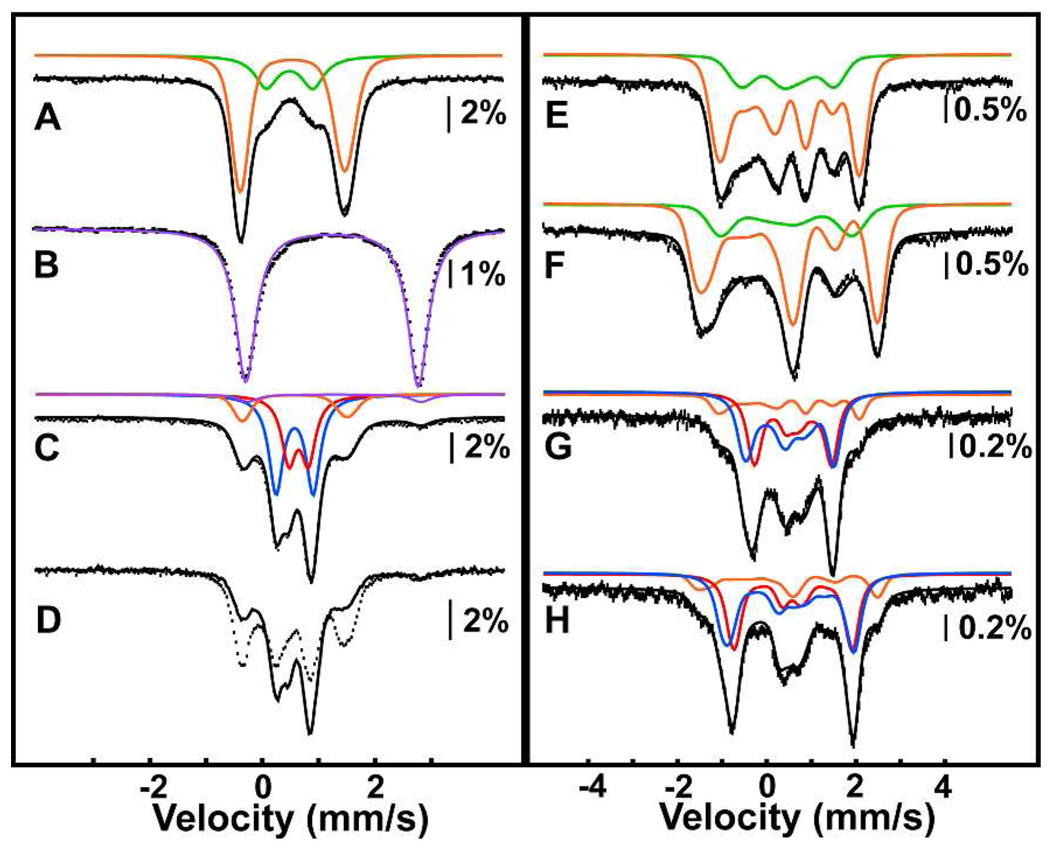
Diiron AurF was isolated from over-producing Escherichia coli cells as described in the Supporting Information. The UV/visible absorption spectrum of the protein (Fig. 1, blue trace) has a band at ~360 nm similar to those exhibited by other proteins with µ-oxo-Fe2III/III clusters, wherein it has been assigned to an oxo-to-iron charge transfer transition.9 The 4.2-K/53-mT Mössbauer spectrum of AurF isolated from cultures supplemented with 57FeSO4 (> 95% 57Fe) can be simulated as two quadrupole doublets with parameters typical of high-spin FeIII [δ1 = 0.54 mm/s, ΔEQ,1 = −1.86 mm/s; δ2 = 0.48 mm/s ΔEQ,2 = 0.80 mm/s] and relative contributions to the experimental spectrum of 78% (orange line) and 22% (green line), respectively (Fig. 2A). The large |ΔEQ,1| of 1.86 mm/s is typical of µ-oxo-Fe2III/III clusters, consistent with the absorption spectrum and the crystal structure.8 The complex with smaller ΔEQ could have one or more µ-hydroxo bridge(s). Mössbauer spectra collected in externally applied fields of 5T and 8T (Fig. 2E and 2F) can be simulated well with the same parameters and the assumption of a diamagnetic ground state. The S = 0 ground state arises from antiferromagnetic coupling between the two high-spin FeIII sites.
Figure 1.
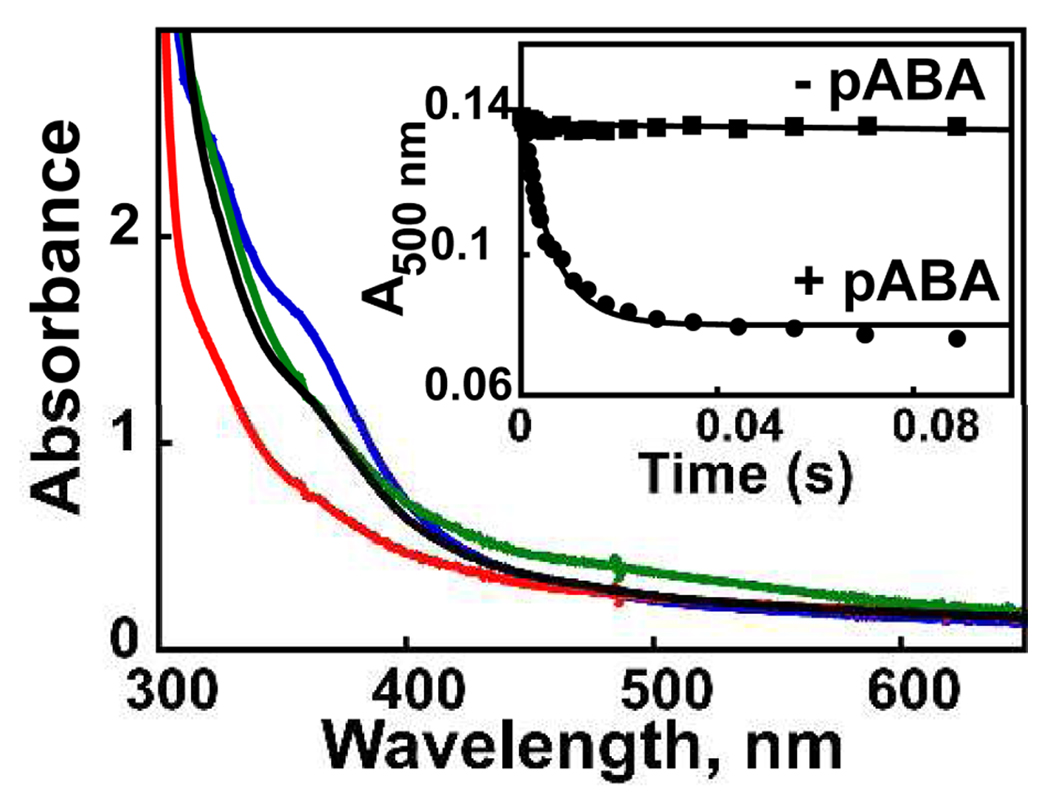
Absorption spectra of a sample of AurF subjected to DT reduction and O2 re-oxidation: as-isolated protein (0.40 mM in 50 mM HEPES pH 7.5 and 10% glycerol buffer; blue line); after treatment with 1 equiv DT for 10 min at 20 °C (red line); after addition of 0.2 volumes of cold oxygenated buffer ([O2]final = 0.38 mM, green line), and after addition of pABA (0.92 mM final; black line). Inset: Kinetic traces after mixing DT-reduced AurF (0.64 mM) at 21 °C with an equal volume of O2-saturated buffer, allowing the reaction to proceed for 0.1 s, and then mixing the resultant solution with an equal volume of anaerobic buffer either lacking pABA (squares) or containing 1 equiv pABA (circles). The solid lines are simulations with parameters described in the text.
Figure 2.
4.2-K Mössbauer spectra (hashed marks) of as-isolated AurF (A, E, F); DT-reduced AurF (B); DT-reduced AurF exposed to 2 atm O2 for 2 min, and then either directly frozen for Mössbauer analysis (C) or further treated with 0.3 equiv pABA prior to being frozen for analysis (D), and DT-reduced AurF treated with a slight excess of O2 from an O2-saturated buffer solution (G, H). Spectra were collected in external fields of 53 mT (A–D), 5T (E, G), or 8T (F, H). The solid lines in A – C are simulations with parameters quoted in the text. The solid line in D is the experimental spectrum from C re-plotted to illustrate the spectral changes. The solid lines in E – H are spin Hamiltonian simulations with parameters in the text, asymmetry parameters η = -0.4 (orange), η = 1 (green), η = 1.4 (red), η = 0.3 (blue), and the assumption of S = 0 ground states for all species.
Treatment of the protein with 1 equiv sodium dithionite (DT) bleaches the band at 360 nm in the absorption spectrum (Fig. 1, red trace) and produces a new quadrupole doublet with parameters (δ = 1.24 mm/s, ΔEQ = 3.06 mm/s) typical of high-spin FeII (Fig. 2B). These observations indicate that the Fe2III/III protein is reduced to the Fe2II/II form by DT treatment.
Reaction of Fe2II/II AurF with O2 produces a new broad absorption band centered at ~500 nm (ε500 ~ 0.5 mM−1cm−1; Fig. 1, green trace). Formation of the 500-nm-absorbing species is complete within 0.01 s at ~ 0.6 mM O2 and 20 °C (Fig. S1). The species does not decay noticeably on the 100-s time scale of the SF absorption experiment. The 4.2-K/53-mT Mössbauer spectrum of the protein following vigorous stirring of the Fe2II/II complex for ~ 2 min under ~ 2 atm O2 (Fig. 2C) shows that the diiron cluster is almost quantitatively oxidized. In addition to small amounts of the sub-spectra of Fe2II/II (5%, purple line) and µ-oxo-Fe2III/III (18%, orange line) complexes, the spectrum reveals several new sharp peaks, which can be simulated as two quadrupole doublets with similar parameters. The parameters [δ1 = 0.54 mm/s, ΔEQ,1 = −0.66 mm/s (49 %, blue line) and δ2 = 0.61 mm/s, ΔEQ,2 = 0.35 mm/s (33 %, red line)] are typical of high-spin Fe(III).10 Spectra collected in external fields of 5T and 8T (Fig. 2G and 2H)11 confirm that the new state has S = 0 ground state(s), due to antiferromagnetic coupling of two high-spin FeIII sites. Because it is an Fe2III/III cluster derived from the Fe2II/II enzyme by treatment with O2, we assign this state as peroxo-Fe2III/III. The ~1.5:1 intensity ratio of the two quadrupole doublets suggests the presence of two or more isomeric complexes, perhaps in equilibrium (as has been observed in the reactive intermediate states of other iron enzymes).12–14 Mössbauer spectra of samples of the peroxide state frozen after subsequent incubation at 20 °C show that it is stable for minutes in the absence of substrate (t1/2 ~ 7 min) and decays to the µ-oxo-Fe2III/III species (Fig. S2).
The reactivity of the peroxo-Fe2III/III state was examined by stopped-flow experiments in which the Fe2II/II protein was allowed to react with O2 for 100 ms and the resultant peroxo-Fe2III/III state was then mixed with the substrate, pABA, or (as a control) buffer. The 500 nm band of the peroxo-Fe2III/III state, stable in the absence of pABA (inset to Figure 1, squares), decays rapidly (kobs = 150 ± 20 s−1) upon exposure to one equiv of the substrate (inset to Figure 1, circles).
The 4.2-K/53-mT Mössbauer spectra of samples prepared by oxygenation of Fe2II/II AurF (Figure 2C, replotted in Figure 2D as solid line for clarity) and subsequent treatment of the oxygenated sample with 0.3 equiv pABA for 1 min (Figure 2D, hashed marks) confirm the reactivity of the peroxo-Fe2III/III state toward the substrate. The features of the intermediate complex(es) decay, and those of the µ-oxo-Fe2III/III cluster develop. Analysis of the small-molecule components of reactions performed similarly (described in Supporting Information) showed that, at these low pABA/AurF ratios of ≤ 0.3, the substrate is converted to pNBA with a yield of > 80%.15
The combined absorption and Mössbauer spectroscopic results thus establish that addition of O2 to the Fe2II/II cluster of AurF produces a long-lived state that has properties consistent with a peroxo-Fe2III/III formulation. The stopped-flow data show that the intermediate state reacts rapidly with pABA, implying that it is competent for at least the first oxidation in the three step AurF sequence. The possibility that it is competent for all three oxidations seems likely (on the basis of the efficient conversion of limiting pABA to pNBA) but requires more rigorous evaluation.
The spectroscopic properties of the AurF state are different from those of peroxo-Fe2III/III complexes detected in related diiron oxidases and oxygenases such as ribonucleotide reductase subunit R2,16 stearoyl acyl carrier protein Δ9 desaturase,17 and soluble methane monooxygenase hydroxylase (sMMOH).18 The intermediates in these proteins have µ-1,2-peroxo19 (or perhaps µ-(η2,η2)-peroxo, for the case of sMMOH20) diiron cores. The properties of the AurF complex are more similar to those of a peroxo-Fe2III/III intermediate recently detected in toluene/o-xylene monooxygenase (ToMO).21 In this enzyme, the peroxide complex is thought to be attacked as an electrophile by the π-system of the substrate. A similar mechanism for the first step in the AurF reaction, involving nucleophilic attack of the lone pair of the neutral arylamine on the peroxo electrophile (Scheme 1), seems plausible. An attractive possibility is that the peroxo complexes in ToMO and AurF differ structurally from the canonical µ-1,2-peroxides in ways that activate them as electrophiles. Protonation of the peroxide unit, perhaps with rearrangement to a µ-1,1-bridging geometry, seems likely to have this effect.
Scheme 1.
Proposed mechanism for hydroxylation of pABA by a putative µ-1,2 (left) or µ-1,1 (right) hydroperoxo-Fe2III/III intermediate.
The remarkable stability of the AurF intermediate, which permits its preparation in concentration, purity, and physical form (e.g., a transmitting glass) appropriate for most spectroscopic methods, should be a considerable asset in ongoing efforts to define the structure of the complex by a combination of experiments and density functional theory calculations.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (GM-55365 to JMB and CK), the Dreyfus Foundation (Teacher-Scholar Award to CK), and the Pennsylvania State University.
Footnotes
Supporting Information Available: Procedures to produce AurF and quantify pABA and pNBA in reaction samples; stopped-flow absorption and Mössbauer spectra of samples from the reaction of Fe2II/II-AurF with O2 in the absence of substrate; Mössbauer analysis of the sample used for high-field studies of the peroxo-Fe2III/III state. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Hirata Y, Nakata H, Yamada K, Okuhara K, Naito T. Tetrahedron. 1961;14:252–274. [Google Scholar]
- 2.Winkler R, Hertweck C. Angew. Chem., Int. Ed. 2005;44:4083–4087. doi: 10.1002/anie.200500365. [DOI] [PubMed] [Google Scholar]
- 3.Simurdiak M, Lee J, Zhao H. ChemBioChem. 2006;7:1169–1172. doi: 10.1002/cbic.200600136. [DOI] [PubMed] [Google Scholar]
- 4.Winkler R, Richter MEA, Knüpfer U, Merten D, Hertweck C. Angew. Chem., Int. Ed. 2006;45:8016–8018. doi: 10.1002/anie.200603060. [DOI] [PubMed] [Google Scholar]
- 5.Zocher G, Winkler R, Hertweck C, Schulz GE. J. Mol. Biol. 2007;373:65–74. doi: 10.1016/j.jmb.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Winkler R, Zocher G, Richter I, Friedrich T, Schulz GE, Hertweck C. Angew. Chem., Int. Ed. 2007;46:8605–8608. doi: 10.1002/anie.200703089. [DOI] [PubMed] [Google Scholar]
- 7.Krebs C, Matthews ML, Jiang W, Bollinger JM., Jr Biochemistry. 2007;46:10413–10418. doi: 10.1021/bi701060g. [DOI] [PubMed] [Google Scholar]
- 8.Choi YS, Zhang H, Brunzelle JS, Nair SK, Zhao H. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6858–6863. doi: 10.1073/pnas.0712073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CA, Remar GJ, Musselman RL, Solomon EI. Inorg. Chem. 1995;34:688–717. [Google Scholar]
- 10.An alternative solution of similar quality yields: δ1 = 0.50 mm/s and ΔEQ,1 = 0.59 mm/s (49 %) and δ2 = 0.66 mm/s and ΔEQ,2 = 0.45 mm/s (33 %); both solutions indicate that the iron sites are high-spin Fe(III).
- 11.The preparation and analysis of the 4.2K–53mT Mössbauer spectrum of this sample is described in Supporting Information.
- 12.Saleh L, Krebs C, Ley BA, Naik S, Huynh BH, Bollinger JM., Jr Biochemistry. 2004;43:5953–5964. doi: 10.1021/bi036099e. [DOI] [PubMed] [Google Scholar]
- 13.Stone KL, Hoffart LM, Behan RK, Krebs C, Green MT. J. Am. Chem. Soc. 2006;128:6147–6153. doi: 10.1021/ja057876w. [DOI] [PubMed] [Google Scholar]
- 14.Galonić DP, Barr EW, Walsh CT, Bollinger JM, Jr, Krebs C. Nature Chem. Biol. 2007;3:113–116. doi: 10.1038/nchembio856. [DOI] [PubMed] [Google Scholar]
- 15.Experiments were performed both by addition of substrate to intermediate and by oxygenation of reduced enzyme in the presence of pABA. The results were not significantly different.
- 16.Bollinger JM, Jr, Krebs C, Vicol A, Chen S, Ley BA, Edmondson DE, Huynh BH. J. Am. Chem. Soc. 1998;120:1094–1095. [Google Scholar]
- 17.Broadwater JA, Ai J, Loehr TM, Sanders-Loehr J, Fox BG. Biochemistry. 1998;37:14664–14671. doi: 10.1021/bi981839i. [DOI] [PubMed] [Google Scholar]
- 18.Liu KE, Wang D, Huynh BH, Edmondson DE, Salifoglou A, Lippard SJ. J. Am. Chem. Soc. 1994;116:7465–7466. [Google Scholar]
- 19.Skulan AJ, Brunold TC, Baldwin J, Saleh L, Bollinger JM, Jr, Solomon EI. J. Am. Chem. Soc. 2004;126:8842–8855. doi: 10.1021/ja049106a. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldo D, Philipp DM, Lippard SJ, Friesner RA. J. Am. Chem. Soc. 2007;129:3135–3147. doi: 10.1021/ja0654074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray LJ, Naik SG, Ortillo DO, García-Serres R, Lee JK, Huynh BH, Lippard SJ. J. Am. Chem. Soc. 2007;129:14500–14510. doi: 10.1021/ja076121h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.