Abstract
Objective
To use functional neuroimaging to probe the affective circuitry dysfunctions underlying disturbances in emotion processing and emotional reactivity in pediatric bipolar disorder (PBD).
Method
Equal numbers of controls (HC) and unmedicated patients with euthymia and PBD were matched for age, sex, race, socioeconomic status, and IQ (n = 10 per group; mean age 14.2 years [SD 2.0 years]). The task consisted of a “directed” emotion processing condition where subjects judged whether emotion in facial expression was positive or negative and an “incidental” condition where subjects judged whether faces expressing similar affect were older or younger than 35 years.
Results
Relative to the directed condition, the incidental condition elicited greater activation in the right amygdala and the right insula, the left middle frontal gyrus, and the left posterior cingulate cortex in patients with PBD, in contrast to the HC that showed greater activation in the right superior frontal gyrus. In both incidental and directed conditions, relative to visual fixation, patients with PBD showed less activation in the right prefrontal cortex (superior, middle, and inferior frontal gyri) and the pregenual anterior cingulate cortex and greater activation in the posterior visual and face-processing regions (i.e., right precuneus/cuneus, fusiform gyrus).
Conclusions
Increased amygdala activation observed in patients with PBD elicited by incidental emotional processing relative to directed emotional processing may indicate more intense automatic emotional reactivity. Furthermore, the right prefrontal systems that are believed to modulate affect seem to be less engaged in patients with PBD regardless of whether the emotion processing is incidental or directed, which may signify reduced top-down control of emotional reactivity in PBD.
Keywords: functional magnetic resonance imaging, facial emotion, emotion, cognition, bipolar disorder
Problems of emotion processing are core aspects of pediatric bipolar disorder (PBD).1 To understand the pathophysiology underlying these alterations of emotion processing, studies have examined affective responses to emotional stimuli to delineate functional abnormalities in frontolimbic circuitry. Facial stimuli have been used for recruiting activation in brain areas crucial for emotion processing, such as the prefrontal cortex (PFC) and the amygdala.2 For example, a functional magnetic resonance imaging (fMRI) study conducted by Rich et al.3 reported greater ventrolateral PFC (VLPFC) and amygdala activity while subjects tried to evaluate hostility in emotionally neutral faces in patients with PBD compared with the controls (HC).3 In the same study, when attention was directed to nonemotional aspects (estimating the nose width in neutral faces), there were no differences between patients with PBD and HC. Another fMRI study found that passive viewing of happy and angry faces induced greater amygdala activity and reduced VLPFC activity in subjects with PBD relative to HC.4 In a variant of an affective Stroop task, patients with PBD showed greater amygdala activation and decreased PFC activation (at the junction of the VLPFC and the dorsolateral PFC [DLPFC]) for negative words compared with neutral words and also relative to negative words in HC.5 Furthermore, similar to previous findings with patients with PBD,3,4 facial affect recognition in adult euthymic bipolar patients has been associated with reduced activation in the right VLPFC and increased activation in the left amygdala.6 Therefore, there is growing evidence for excessive amygdala activation in PBD in response to emotional stimuli, which is consistent with their clinical pattern of increased emotional reactivity. With regard to prefrontal cortical regions that provide modulatory input to limbic systems, findings have been inconsistent because studies have reported increased3 as well as decreased4–6 VLPFC activation in patients with PBD, perhaps based on differences in behavioral paradigms, clinical state, or treatment status of patient groups.
In addition to the functional neuroimaging abnormalities in the PFC and the amygdala, structural neuro-imaging studies indicate smaller amygdala volumes in patients with PBD relative to HC,7–10 which contrasts with adult studies that have reported larger11,12 or normal13,14 amygdala volumes. Larger amygdala in adult studies11,12,15,16 has in fact been hypothesized to result from hypertrophy due to chronic and excessive activation in manic patients.16 Although these findings of altered size, be it larger or smaller, do not necessarily imply intrinsic primary abnormalities in the amygdala, they may correlate with functional abnormalities such as increased activation in response to emotional stimuli, regardless of any dysfunction in PFC input to this limbic structure. Given its central role in assessing emotional valence and arousal to emotional stimuli,17–19 its role in emotion-cognition interface,20 and the morphological and functional abnormalities reported in bipolar disorder, examining alterations in amygdala function in PBD may be essential for understanding neural mechanisms of altered emotion processing in this disorder.
To probe the functional integrity of the PFC and the amygdala, studies of emotion have typically used two different emotional processing paradigms. “Directed” emotional processing involves making deliberate judgments about the intensity or type of affect conveyed in a stimulus. This deliberate evaluation process is believed to directly engage the DLPFC (a center for higher order cognitive control) and the VLPFC (a neocortical area providing higher order control of affective responses) that are believed to exert regulatory control over limbic structures such as the amygdala.17 In contrast, “incidental” processing tasks typically focus conscious attention toward nonaffective stimulus features, such as requiring a judgment about the sex or age of an emotional face. In this context, the DLPFC is often engaged in making cognitive decisions, but performing these paradigms does not seem to engage the VLPFC in evaluating emotional cues or regulating emotional responses in the limbic system. Nonbipolar adults,18,19 adult bipolar patients,21 and adult phobic patients22 showed greater amygdala activation, predominantly on the right side, during incidental than directed processing. The greater activation of the amygdala during incidental emotion processing may represent a neural correlate of automatic emotional responsivity when the VLPFC is not engaged in modulating emotion.18,19
In patients with PBD, as previously mentioned, Rich et al.3 examined the neural activity of emotion processing while focusing attention on emotional evaluation (directed processing) versus nonemotional judgments about neutral faces and reported increased VLPFC and left amygdala activation. The current study builds on this earlier study by directly examining incidental versus directed emotional processing of emotional rather than neutral faces. The block design paradigm consisted of a directed condition with deliberate processing of emotional faces (i.e., deciding whether an emotional face is happy or angry) and an incidental condition with implicit processing of emotional faces (i.e., deciding whether the emotional face is younger or older than 35 years). We included an equal number of angry and happy faces, known to have similar affective arousal levels from previous normative work,23 for both the incidental and directed conditions.
Based on the earlier findings illustrating frontolimbic dysfunction in PBD, and findings from adult bipolar studies using incidental versus directed emotion processing,21 we were primarily interested in the specific contribution of PFC and amygdala disturbances during emotion processing in PBD. The activation patterns in unmedicated patients with PBD and euthymia were compared with those in HC in this study. We hypothesized that the PBD group would have reduced control of automatic emotional responses in incidental processing condition relative to directed processing resulting in greater amygdala activation. Second, we predicted that the higher order systems that modulate emotional responses in the VLPFC24 would show decreased functioning in PBD during directed processing. The directed processing condition therefore served as a probe to test for a loss of top-down control of emotions. Elucidating the degree of relative disturbance in these incidental and directed processes can provide important information about the pathophysiology of emotion processing in PBD and potentially guide treatment development.
METHOD
Subjects
Subjects were recruited from the University of Illinois at Chicago pediatric mood disorders clinic and the surrounding community. Of the initial group of 16 subjects with PBD, fMRI data from 6 subjects were discarded because of motion artifacts. The remaining 10 patients (5 female subjects) had a mean age of 16.2 years (SD 1.3 years). Neuroimaging data were collected on 14 HC. Data from 4 of these subjects were unusable because of excessive head motion during scans. The remaining 10 subjects, who were age-, sex- and ethnically matched to the patient group, were included in the final analysis (mean age 15.0 years [SD 2.4 years]; 5 female subjects; Table 1). For minors, informed consent was obtained from at least one parent, and assent was obtained from each subject. For adolescents older than 15 years, informed consent was directly obtained from them in addition to the consent of their parents. The study was approved by the institutional review board at the University of Illinois at Chicago.
TABLE 1.
Demographic and Clinical Characteristics of HC and Subjects With PBD
| HC |
PBD |
Analysis |
|
|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | F (p) |
| Age, y | 14.3 (2.1) | 15.2 (2.0) | 0.96 (0.34) |
| WASI-FSIQa | 109.7 (12.1) | 106.9 (10.7) | 0.30 (0.59) |
| WRAT-3, Reading Subtest | 104.0 (11.4) | 104.6 (10.9) | 0.01 (0.91) |
| Socioeconomic statusb | 1.5 (0.73) | 1.7 (0.95) | .14 (0.71) |
| YMRS | 0.4 (1.3) | 7.0 (4.67) | 19.4 (0.01) |
| CDRS-R | 18.0 (0.00) | 19.7 (3.7) | 2.2 (0.16) |
| n (%) | n (%) | Fisher p | |
|---|---|---|---|
| Sex | |||
| Male | 5 (50) | 5 (50) | 1.0 |
| Female | 5 (50) | 5 (50) | |
| Race | |||
| White | 4 (40) | 6 (60) | 0.66 |
| Other | 6 (60) | 4 (40) |
Note: CDRS-R = Child Depression Rating Scale-Revised; HC = healthy controls; PBD = pediatric bipolar disorder; WASI-FSIQ = Wechsler Abbreviated Scale of Intelligence-Full Scale Intelligence Quotient; WRAT-3 = Wide Range Achievement Test-Third Edition; YMRS = Young Mania Rating Scale.
Matrix Reasoning and Vocabulary Subtests.
Mean revised Hollingshead socioeconomic status.10a
Inclusion criteria for the subjects with PBD were 12 to 18 years of age, diagnosis of bipolar type I disorder in euthymic phase, and agreement to be scanned in a medication-free state during the study period. Euthymia was defined by the subject not meeting DSM-IV criteria for major depression, dysthymia, mania, or hypomania at the time of assessment. Presence of a comorbid DSM-IV diagnosis was an exclusion criterion, with the exception of attention-deficit/hyperactivity disorder (ADHD). Each child and a parent were interviewed using the Washington University Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS).25 Interrater reliability among the research interviewers was 0.98 by Cohen κ for the diagnosis on the WASH-U-KSADS for a group of patients with PBD and subjects without PBD. Monthly reliability checks were used throughout the study to maintain high interrater reliability on all clinical measures. Consensus current and lifetime DSM-IV diagnosis was made based on information from clinical interviews, other available clinical data, and ratings on the WASH-U-KSADS. The patients were medication-free by choice of the patient or their parents or because they were participating in a medication-free trial under medical supervision for clinical reasons, during a low stress period when the patients were not in school and were being treated in our pediatric mood disorders program. All patients were previously on either mood stabilizers and/or second-generation antipsychotics, with or without stimulants. They were not receiving CNS-active medications for at least seven days before the scan; none were on fluoxetine or aripiprazole that warrants a longer washout period. For previously treated patients, medication dosages were reduced gradually over a 3-week period before the start of the drug-free period. The last medication type to be withdrawn was stimulants at least 8 days before the scan. The patients with PBD were euthymic for a minimum of 4 months before testing; the HC were interviewed using the WASH-U-KSADS to ensure the absence of any mental disorder.
Exclusion criteria for all of the subjects were as follows: the presence of a neurological or medical condition; history of head trauma with loss of consciousness for more than 10 minutes; disorders requiring use of medication that alter cerebral blood flow (such as medications for migraine and blood pressure); IQ of less than 80; and the presence of metallic implants, retractors, or braces. IQ was estimated using Wechsler Abbreviated Scale of Intelligence.26
Incidental and Directed Emotion Processing Task
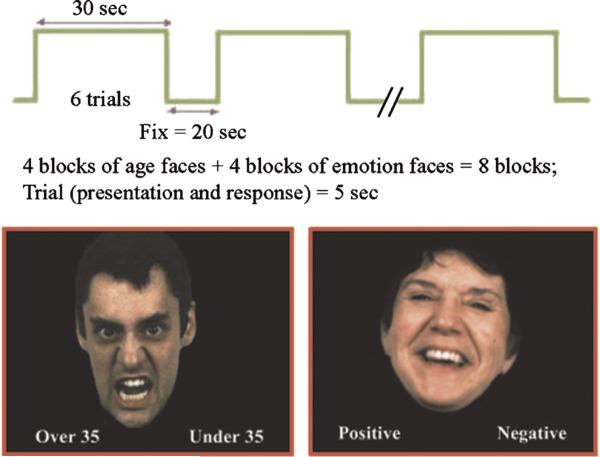
The fMRI task was a block design paradigm performed for 7 minutes (Fig. 1). It consisted of eight 30-second condition blocks, four involving incidental emotion processing (I), and four involving directed emotion processing (D; illustrated in Fig. 1). Condition blocks were separated by a 20-second fixation period (F) with the last 3 seconds of this period displaying what the upcoming block would be, that is, “age” for incidental condition and “emotion” for directed condition. We avoided any elaborate written instructions beyond these cues in lieu of training video that preceded the actual scanning, teaching the subjects how to perform the task. For all of the subjects, the sequence of I and D block presentation was pseudorandomized: (F) I (F) D (F) D (F) I (F) I (F) D (F) I (F) D (F). There were 6 trials in each condition block, with each trial consisting of a 5-second face presentation, for a total of 48 trials in the entire task.
Fig. 1.
Incidental and directed emotion processing task.
While building the paradigm, faces were selected with regard to age, sex, valence, and race from a database of 200 Gur faces with affective characteristics having established reliability and validity.23 During the incidental face processing condition, the participants judged whether the presented face was older or younger than 35 years. During the directed face processing condition, the participants judged whether the facial affect was positive/happy or negative/angry. Responses were by button press, with the correct response randomized and equated between right and left key presses. The emotional stimuli consisted of 24 happy and 24 angry faces23 that were matched for age, sex, and race and are counterbalanced across the blocks and conditions. The level of happy and angry emotionality is same in the incidental and directed conditions based on the normative data provided by Gur et al.23 Three angry (A) and three happy (H) face trials were presented in a pseudorandom order in each block of both conditions in an “AHHAAHAHHAAH” sequence (A = angry, H = happy). We used the rule that the same trial type (e.g., happy) could not occur more than twice in a row. Faces were not repeated during the task to avoid effects of repetition and familiarity. All of the faces were modified to optimize the display of the images. Specifically, the background was changed to black, and necks and shoulders were removed, but hair was left in the images.
Image Acquisition
Magnetic resonance imaging studies were performed using a 3.0 Tesla whole-body scanner (Signa, General Electric Medical System, Milwaukee). Functional images were acquired using gradient echo planar imaging, which is sensitive to regional alterations in blood flow via blood oxygenation level–dependent contrast effects. Twenty-five axial slices were acquired. Parameters for functional scans were as follows: echo time = 25 milliseconds; flip angle = 90°; field of view = 20 × 20 cm2; acquisition matrix = 64 × 64; repetition time = 2.5 seconds; 5-mm slice thickness, with 1-mm×gap. Anatomic images were acquired in the axial plane from all of the subjects (three-dimensional spoiled gradient recalled, 1.5-mm thick contiguous axial slices) for coregistration with the functional data.
Image Processing and Data Analysis
Functional Imaging Analysis Software–Computational Olio software27 was used to estimate and correct the functional neuroimaging data for head motion and low-frequency signal drift. Functional Imaging Analysis Software–Computational Olio provides diagnostics for identifying images with artifacts such as high shot noise and displacement that cannot be readily corrected by motion correction algorithms. This can improve sensitivity to blood oxygenation level–dependent effects across groups and reduce the risk of finding differential activation in patient groups because of greater head movement.
Individual volumes from the time series were excluded from the analysis if head displacement from the median head position in the time series was greater than 1.5 mm or if head rotation from the median head position was greater than 0.5°. The number of volumes retained after discarding those with motion artifact did not significantly differ across groups. To evaluate subject-wise effects, voxelwise effect size (r) maps were calculated for each subject for each pairwise condition contrast (incidental condition versus fixation, directed condition versus fixation, incidental versus directed condition). Then, a Fisher z transform was applied to normalize the data (zr).28
Analysis of Functional Neuroimages (AFNI) software29 was used to transform individual subjects’ zr maps (effect size) and spoiled gradient recalled anatomic images into Talairach space using AFNI's automated Talairach procedure.30 Functional maps were resampled to an isotropic 3 × 3 × 3–mm grid to provide a voxel dimension similar to that of the in-plane resolution of the acquired data. We used AFNI to carry out voxelwise statistical analyses on the zr maps.
The primary analysis for the study was a repeated-measure analysis of variance (ANOVA) with the between-subjects factor of group (PBD, HC) and the within-subjects factor of condition (incidental, directed, fixation). Significant clusters of activation were identified using a contiguity threshold (minimum cluster volume of 270 mm3) applied to the entire brain, which maintained an experiment-wise type I error rate of p < .025, based on AFNI's AlphaSim Monte Carlo simulations that were restricted to in-brain voxels. Significant effects were followed by step-down comparisons to clarify significant group and task differences.
Finally, given the importance of the amygdala for our hypotheses, we also performed a region-of-interest (ROI) analysis on the left and right amygdala because activation in this small region may not survive a contiguity threshold determined for the whole brain analysis. For the amygdala ROI, individual zr maps were resampled to a 1 × 1 × 1 grid, and the number of voxels in individual zr maps with activation above a p < .025 threshold was counted. Voxel counts were then subjected to ANOVA comparing ROI activation in the two groups and for the two conditions. This region in AFNI format as well as the rationale for ROI definition is available at http://ccm.psych.uic.edu/Research/NormalBrain/ROI_rules.htm and http://ccm.psych.uic.edu/Research/ResearchProgram/NormalBrain/ROIaffect_rules.aspx.
RESULTS
Clinical and Demographic Data
On the Young Mania Rating Scale and Child Depression Rating Scale-Revised, the PBD group's rating was 7.0 (SD 4.7) and 19.7 (SD 3.7), respectively. The patients with PBD were rated significantly higher on the Young Mania Rating Scale than the HC subjects (F1,18 = 19.4; p < .01), but the groups did not differ on Child Depression Rating Scale-Revised ratings. Demographic and clinical data are summarized in Table 1. One subject with PBD had comorbid ADHD by the virtue of meeting criteria for ADHD before the age of 7 years.
Behavioral Data
Response time (RT) and performance accuracy during fMRI studies were analyzed in separate repeated-measure ANOVAs with group (PBD, HC) as a between-subjects factor and condition (incidental, directed) as a within-subjects factor. For RT, we included only correct responses for the incidental and directed conditions in the analyses. Accuracy rates (percentage of trials performed correctly) and median RT were calculated for each subject and condition (Table 2). For RT data, a significant main effect of condition (F1,18 = 13.06; p = .002) indicated that median RT was slower for the incidental than the directed condition (Table 2). There was no significant group difference in RT data (F1,18 = 0.005; p > .05), nor was there a significant group by condition interaction (F1,18 = 0.055; p > .05). Similarly, for the response accuracy data, a significant main effect of condition (F1,18 = 21.81; p = .002) revealed lower accuracy for incidental than directed processing across groups. Again, there was no significant effect of group (F1,18 = 0.004; p > .05) or group by condition interaction (F1,18 = 0.06; p > .05). Thus, response accuracy and latency, for the incidental and directed processing conditions, were similar in PBD and HD groups.
TABLE 2.
Response Time and Accuracy Measures for HC and Subjects With PBD on the Incidental and the Directed Emotion Processing Conditions
| HC |
PBD |
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Response time, ms | ||
| Incidental condition | 1609.8 (372.8) | 1618.4 (571.9) |
| Directed condition | 1320.3 (358.8) | 1288.5 (338.6) |
| n (%) | n (%) | |
|---|---|---|
| Accurate responses | ||
| Incidental condition | 18.00 (75) | 18.00 (75) |
| Directed condition | 20.00 (83) | 20.00 (83) |
Note: HC = healthy controls; PBD = pediatric bipolar disorder.
fMRI Data
Across groups, the incidental condition yielded greater activation than the directed condition in the PFC (bilateral superior and middle frontal gyrus); in the posterior visual processing regions; and in the right caudate, thalamus, and bilateral parahippocampal gyrus. Greater activation for the directed than the incidental condition was present in the left inferior frontal gyrus and left pregenual anterior cingulate cortex (ACC), as well as in temporoparietal regions. Regardless of task condition, the PBD group showed less activation than the HC in the right orbitofrontal PFC, the right middle frontal gyrus (DLPFC) and inferior frontal gyrus (VLPFC), the right superior frontal gyrus, the left pregenual ACC, and the right insula. The PBD group exhibited greater activation than the HC in the right fusiform gyrus and precuneus. Details about these group and condition effects are available at http://ccm.psych.uic.edu/Research/ResearchProgram/MoodDisorder/AdditionalResults.aspx.
Incidental Versus Directed Condition: Between-Group Effects
Table 3 summarizes findings from the comparison of activation for the incidental versus directed condition in the PBD group relative to the HC. Right amygdala activation was greater in the PBD group compared with that in the HC for the incidental condition relative to the directed condition (Fig. 2; Table 3). In addition, the PBD group showed greater activation in the incidental condition relative to the directed condition than the HC in the left middle frontal gyrus/DLPFC, the left posterior cingulate cortex, and the right cuneus, and less activation than the HC in the right superior frontal gyrus.
TABLE 3.
Responses to Incidental vs. Directed Processing: Comparing Subjects With PBD and HC
| Talairach Coordinates for Peak Activation | Areas (BA) | Volume, mm3 | t Values for Peak Activation | |
|---|---|---|---|---|
| PBD > HC: incidental vs. directed | ||||
| Right cuneus | 14, –73, 14 | BA 18 | 351 | 3.08 |
| Right amygdala | 20, –10, –13 | 621 | 3.87 | |
| Right insula | 35, –19, 14 | BA 13 | 270 | 3.11 |
| Left posterior cingulate cortex | –16, –58, 8 | BA 30 | 297 | 3.62 |
| Left middle frontal gyrus (DLPFC) | –19, 35, 35 | BA 9 | 378 | 3.57 |
| HC > PBD: incidental vs. directed | ||||
| Right superior frontal gyrus | 23, 50, –4 | BA 10 | 270 | 2.67 |
Note: This table shows Talairach coordinates and t values for peak activation in significant clusters (p < .025 with contiguity threshold) representing greater activation in one group vs. the other, and vice versa, for the incidental vs. directed condition. BA = Brodmann area; DLPFC = dorsolateral prefrontal cortex; HC = healthy controls; PBD = pediatric bipolar disorder.
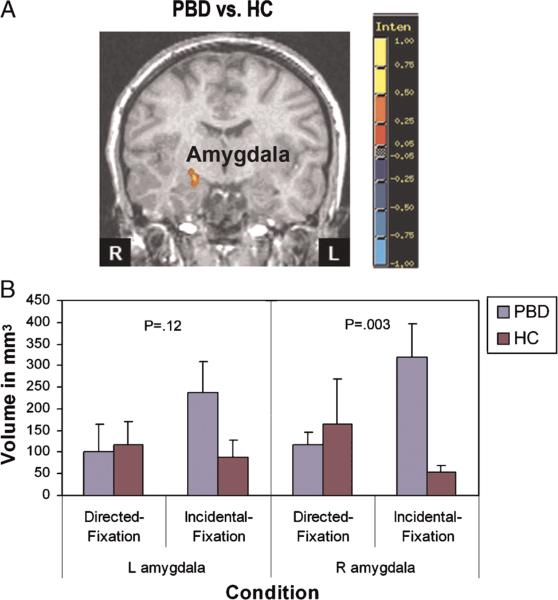
Fig. 2.
Effect size map showing amygdala activation during incidental versus directed emotion processing. The graph shows mean volume of tissue with significant task-related activation. A, Coronal slice y = 2; red indicates greater activation in the PBD group compared with the HC; blue indicates reduced activation in the PBD group compared with the HC. HC = healthy controls; L = left; PBD = pediatric bipolar disorder; R = right.
Incidental or Directed Condition Versus Visual Fixation Between-Group Effects
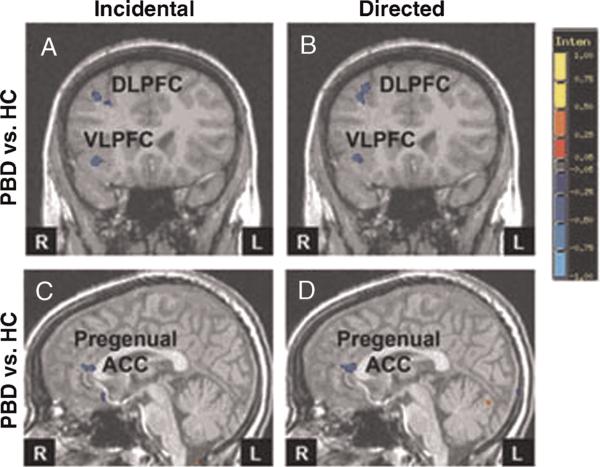
The PBD group, relative to the HC, displayed decreased PFC activation, mainly in the right hemisphere, in both the incidental and directed conditions (Table 4; Fig. 3A, B). For the incidental condition relative to visual fixation, the PBD group showed less activation than the HC in the right inferior frontal gyrus/VLPFC, the middle frontal gyrus/DLPFC, the superior frontal gyrus, the subgenual ACC, and the left pregenual ACC (Fig. 3A, C). The PBD group showed greater activation than the HC in the right visual and face-processing regions (precuneus and fusi-form gyrus).
TABLE 4.
Incidental or Directed Condition vs. Visual Fixation: Comparison Between Subjects With PBD vs. HC
| Talairach Coordinates for Peak Activation | Areas (BA) | Volume, mm3 | t Values for Peak Activation | |
|---|---|---|---|---|
| PBD > HC: incidental vs. fixation | ||||
| Right precuneus | 26, –76, 26 | BA 31 | 270 | 2.38 |
| Right fusiform gyrus | 35, –43, –13 | BA 37 | 648 | 4.07 |
| HC > PBD: incidental vs. fixation | ||||
| Right inferior frontal gyrus (VLPFC) | 35, 26, –19 | BA 47 | 297 | 3.23 |
| Right middle frontal gyrus (DLPFC) | 32, 23, 38 | BA 9 | 378 | 3.73 |
| Right subgenual ACC | 5, 20, –16 | BA 25 | 270 | 3.62 |
| Right superior frontal gyrus | 26, 47, 8 | BA 10 | 756 | 2.54 |
| Left pregenual ACC | –1, 35, 11 | BA 24/32 | 297 | 2.9 |
| Right insula | 38, 17, –1 | BA 13 | 351 | 2.82 |
| PBD > HC: directed vs. fixation | ||||
| Right cuneus | 29, –79, 26 | BA 19 | 540 | 2.69 |
| Right fusiform gyrus | 32, –43, –13 | BA 37 | 621 | 3.92 |
| Left insula | –43, –19, –7 | BA 13 | 297 | 3.91 |
| HC > PBD: directed vs. fixation | ||||
| Right middle frontal gyrus (DLPFC) | 32, 23, 38 | BA 9 | 486 | 3.03 |
| Right superior frontal gyrus | 23, 47, 2 | BA 10 | 324 | 2.94 |
| Right inferior frontal gyrus (VLPFC) | 47, 32, –13 | BA 47 | 270 | 3.74 |
| Left pregenual ACC | –1, 32, 11 | BA 24/32 | 405 | 3.88 |
Note: This table shows Talairach coordinates and t values for peak activation in significant clusters (p < .025 with contiguity threshold) representing greater activation in one group relative to the other for each condition versus fixation. ACC = anterior cingulate cortex; BA = Brodmann area; DLPFC = dorsolateral prefrontal cortex; HC = healthy controls; PBD = pediatric bipolar disorder; VLPFC = ventrolateral prefrontal cortex.
Fig. 3.
Effect size maps showing reduced prefrontal activation in PBD versus HC. A and B, Coronal slice y = –9. C and D, Sagittal slice x = –1; Red indicates greater activation in the PBD group compared with the HC; blue indicates reduced activation in the PBD group relative to the HC. ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; HC = healthy controls; L = left; PBD = pediatric bipolar disorder; R = right.
Similar to findings for the incidental condition, in the directed condition relative to visual fixation, the PBD group exhibited less activation than the HC in the right inferior frontal gyrus/VLPFC, the middle frontal gyrus/DLPFC, and the left pregenual ACC (Fig. 3B, D), and greater activation than the HC in the posterior right visual and face-processing regions (cuneus, fusiform gyrus).
Incidental Versus Directed Condition: PBD Group
Table 5 lists significant findings for the incidental versus directed condition in the PBD group. A central finding was that the PBD group exhibited greater activation in the right amygdala and PFC (right inferior frontal gyrus/VLPFC and bilateral middle frontal gyrus/DLPFC) and in the right dorsal ACC for the incidental than for the directed condition (Table 5). The PBD group also exhibited greater activation for the incidental condition in the right posterior cingulate cortex and in the visual and face-processing regions (bilateral middle occipital gyrus, cuneus, fusiform gyrus, and right middle temporal gyrus). The PBD group also showed less activation for the incidental than the directed condition in the temporal cortex (right superior temporal gyrus, left middle temporal gyrus), the left precuneus, and the bilateral pregenual ACC.
TABLE 5.
Responses to Incidental vs. Directed Processing Within the PBD Group and the HC
| Talairach Coordinates for Peak Activation | Areas (BA) | Volume of Activation, mm3 | t Values for Peak Activation | |
|---|---|---|---|---|
| PBD: incidental > directed | ||||
| Left cuneus | –16, –88, 8 | BA 17 | 1998 | 3.82 |
| Right cuneus | 29, –73, 32 | BA 19 | 8370 | 5.77 |
| Left posterior cingulate cortex | –10, –49, 5 | BA 29 | 567 | 5.58 |
| Right amygdala | 23, –13, –10 | 1215 | 4.26 | |
| Right middle frontal gyrus (DLPFC) | 38, 29, 23 | BA 9 | 2727 | 4.71 |
| Left middle frontal gyrus (DLPFC) | –31, 50, 20 | BA 10 | 1566 | 2.9 |
| Right inferior frontal gyrus (VLPFC) | 32, 26, 5 | BA 45 | 837 | 3.89 |
| Left insula | –31, 17, 5 | BA 13 | 675 | 2.85 |
| Right middle occipital gyrus | 38, –73, 7 | BA 19 | 6858 | 3.74 |
| Left middle occipital gyrus | –28, –73, 17 | BA 19 | 324 | 3.64 |
| Left inferior occipital gyrus | –37, –76, –4 | BA 19 | 1512 | 5.3 |
| Right fusiform gyrus | 32, –34, –16 | BA 20 | 378 | 3.61 |
| Left fusiform gyrus | –43, –58, –13 | BA 37 | 729 | 4.36 |
| PBD: directed > incidental | ||||
| Right pregenual ACC | 14, 38, –4 | BA 24/32 | 540 | 2.7 |
| Left pregenual ACC | –10, 35, –1 | BA 24/32 | 1404 | 3.73 |
| Right superior temporal gyrus | 44, –49, 14 | BA 39 | 540 | 4.93 |
| Left middle temporal gyrus | –58, –52, 2 | BA 21 | 540 | 2.73 |
| HC: incidental > directed | ||||
| Right superior frontal gyrus | 32, 56, 14 | BA 10 | 1512 | 3.08 |
| Right middle frontal gyrus (DLPFC) | 44, 38, 20 | BA 46 | 1782 | 3.5 |
| Right inferior frontal gyrus (VLPFC) | 26, 32, –7 | BA 47 | 486 | 4.29 |
| HC: directed > incidental | ||||
| Right superior temporal gyrus | 59, –43, 8 | BA 22 | 810 | 3.88 |
| Left superior temporal gyrus | –64, –28, 11 | BA 42 | 729 | 5.23 |
| Right insula | 35, –25, 17 | BA 13 | 1080 | 3.19 |
| Left insula | –34, –22, 23 | BA 13 | 513 | 4.86 |
Note: This table shows Talairach coordinates and t values for peak activation in significant clusters (p < .025 with contiguity threshold) representing greater activation in each group for one condition relative to the other. ACC = anterior cingulate cortex; BA = Brodmann area; DLPFC = dorsolateral prefrontal cortex; HC = controls; PBD = pediatric bipolar disorder; VLPFC = ventrolateral prefrontal cortex.
Incidental Versus Directed Condition: HC
The HC exhibited greater activation for the incidental than the directed condition in the right inferior frontal gyrus/VLPFC, the right middle frontal gyrus/DLPFC, and the right superior frontal gyrus (Table 5). Greater activation for the directed than the incidental condition was present in the HC in the bilateral superior temporal gyrus and insula. Unlike the PBD group, the HC had no significant difference in amygdala activation between the two task conditions.
Amygdala: ROI Analysis
We conducted the ROI analysis for the amygdala, where the activation may not survive the use of a spatial contiguity threshold because of its smaller size. In addition, also by the virtue of its smaller size, there is a greater risk for type II error, given the anatomic variability in whole brain analyses. Figure 2B depicts significant volumes of the right and the left amygdala activation in the HC and the PBD group for the incidental and directed conditions compared with fixation. Separate ANOVAs were conducted for the left and the right amygdala ROI, with group as a between-subjects factor and condition as a within-subject factor. With regard to the right amygdala, there was a significant interaction of group by condition (F1,18 = 4.50; p < .048). Planned comparisons revealed that only for the incidental versus fixation comparison did the PBD group show significantly greater right amygdala activation than the HC (F1,180 = 11.33; p < .003). The effect size (Cohen d = 1.82) was large, indicating a robust group difference. Similar to the right amygdala, the left amygdala exhibited higher activation in the PBD group than in the HC for the incidental versus fixation condition, although the interaction did not reach significance (p = .12; effect size [Cohen d = 0.84]). There were no significant (F < 1) group differences for the directed versus fixation condition (Fig. 2B). Moreover, only for the PBD group, there was a marginally significant trend (F91,180 = 3.74; p < .06) in that, right amygdala activation was greater for the incidental versus fixation condition than for the directed versus fixation condition. With regard to the left amygdala, the group by condition interaction was not significant (p = .12), and no other significant results were found (Fig. 2B). Amygdala activation in the HC did not differ across the conditions (p = .30; Fig. 2).
DISCUSSION
Our study identified abnormalities in the neural substrate of incidental and directed emotion processing in PBD. Emotion processing is a quintessential step in the course of affect regulation, which is a defining clinical characteristic of PBD. Careful evaluation of these processes can offer mechanistic understanding of alterations in the neural substrate of emotion processing abnormalities in PBD. The experimental paradigm included positive and negative emotions within a block-design fMRI study. A major strength of this study is the examination of unmedicated youths with euthymia and PBD to avoid the potential confounds of acute symptoms and medications. One central finding is the increased right amygdala activity in the PBD group compared with the HC during the incidental processing relative to directed processing. This finding confirmed our first hypothesis and suggests greater automatic emotional reactivity in the PBD group relative to the HC. No increased amygdala activation was found in the directed emotional processing condition.
There was also increased DLPFC activation during the incidental processing compared with directed processing in the PBD group relative to the HC. This suggests relatively greater allocation of cognitive/modulatory resources by the patients with PBD during incidental processing. Within-group analyses showed that there was an overall reduction in the VLPFC and DLPFC activation in the PBD group during both incidental and the directed emotion processing conditions. The finding of decreased PFC function in PBD in directed emotion processing, both relative to incidental processing (DLPFC) and to visual fixation (DLPFC and VLPFC), supports our second hypothesis that there may be intrinsic abnormality in top-down regulation of emotion processing in PBD as well. In summary, these findings indicate that PBD is associated with increased automatic emotional responsivity, as well as a dysfunction in higher-order cognitive and affective control systems that provide top-down regulation of the limbic system. The two groups did not show any significant behavioral differences for either condition, and yet, the patients with PBD and the HC engaged brain systems differently when processing facial emotions.
Increased Amygdala Activation With Incidental Processing
In the present study, incidental processing of emotional stimuli, compared with directed processing, elicited greater right amygdala activation in patients with PBD relative to the HC. This observation is in agreement with our previous report of increased left amygdala response during passive viewing of emotional faces (i.e., where subjects were asked to view the faces without necessarily judging emotions as in the current study)4 and during the directed processing of hostility in neutral faces in the study by Rich et al.3 In a comparable study, adult manic bipolar patients exhibited increased activation of frontolimbic circuitry, including the right amygdala, during incidental emotional processing.21 Alterations during incidental processing, as seen in the current study, may in part result from the direct transfer of implicit emotional stimuli17–19,21,31,32 from visual association cortex to the amygdala32,33 via the occipitotemporal inferior longitudinal fasciculus.34 This is in contrast to the left-sided activation of the amygdala seen in previous studies of explicit processing,3,4 illustrating that, perhaps, the type of emotional processing determines the laterality of amygdala activation. The increased right amygdala activation is similar to that of Straube et al.22 in incidental emotion processing. In fact, the amygdala is found to play a key role in the interaction of emotion and cognition such as emotion's influence on attention and perception.20 It has been shown that emotional qualities of stimuli are processed automatically,35,36 and this early automatic response to emotional stimuli is an important factor in the amount of attention directed to stimuli.37 In line with this thinking, brain imaging studies have shown enhanced occipital cortex activation to arousing stimuli that correlated with amygdala activation.38 Our results showed that automatic arousal was heightened as reflected in greater right amygdala activation, accompanied by increased activation in visual regions suggesting greater attentional investment, during incidental processing in the PBD group. However, the greater right amygdala activation relative to the left, in response to incidental emotional stimuli, remains preliminary until this finding can be replicated in a larger sample than that in this study. During directed processing, amygdala activation was reduced relative to incidental condition. This may reflect the fact that the PFC (DLPFC and VLPFC) is engaged in cognitive control and emotional modulation when making conscious evaluations of affective aspects of emotional faces.
In addition, apart from the increased amygdala activation, greater activation in visual and cognitive regions was also found in patients with PBD versus the HC during incidental processing relative to directed processing. Discerning the age of the adult faces may require more attention with fine-grained processing of perceptual detail of faces than making judgments about emotions, and this could be responsible for the greater activation in visual processing regions and the increased activation in the cognitive regions including the DLPFC and posterior cingulate cortex.39 Interestingly, we also found that left insula was more active in incidental processing compared with directed processing within the PBD group. There is evidence for the involvement of the insula in processing of negative emotions,40–42 positive emotions,43–47 and in the general processing of facial emotions as part of the extended network.19
Cortical Neural Correlates of the Incidental and Directed Emotion Processing
Decreased activation of the right VLPFC in patients with PBD relative to the HC with both directed and incidental processing, relative to fixation, is similar to the decreased right VLPFC activation with passive viewing of “emotional” faces shown in a previous study from our group.4 This finding is in contrast with that of increased right VLPFC activation during direct processing of emotions and no alteration during the incidental processing of emotions noted by Rich et al.3 As mentioned earlier, there are important differences in the paradigms used by the two studies that may explain this difference in results. In the study by Rich et al.,3 subjects explicitly judged hostility during directed processing or nose width during incidental processing while looking at emotionally neutral faces, whereas in the present study, we examined the incidental and directed processing of emotionally valent stimuli. For example, in the study by Rich et al.,3 despite the incidental nature of the processing, the evaluation of nose width in neutral faces may not have evoked emotional reactivity in PBD as opposed to that in our study in which the age of emotional faces was judged. In addition to the decreased right VLPFC activation in the PBD group relative to the HC, in response to both types of emotion processing in the present study, we also demonstrated decreased activation in the left pregenual ACC, the affective subregion of the ACC, similar to our previous study.5 Activity in the pregenual ACC, with its strong connectivity to the amygdala,48,49 has been shown to be reliably associated with emotional appraisal, experience, and expression in animals48,50 and in adult humans.51,52 Based on models of the functional anatomy of the PFC proposed by Goldman-Rakic53 and Petrides and Pandya,39 PFC regions with specifically assigned functions (i.e., DLPFC that is involved in judging types of emotion, medial PFC that ascertains emotional significance,54 VLPFC and pregenual ACC that modulate emotional responses to faces) all showed decreased activation in patients with PBD relative to the HC in both conditions. This pattern suggests impairment in the integration of the cognitive and affective higher cortical regions as elicited by the incidental and the directed emotion processing conditions.
Finally, our current results must be interpreted with the caveat that we used a block design with randomly intermixed trials with faces of varying positive and negative facial emotions in each block. Therefore, we could not contrast potentially different effects of positive or negative facial expressions in our tasks. Our paradigm, similar to that of Gur et al.,55 was developed to examine general differences in the emotional face processing circuitry between the PBD group and the HC rather than to test hypotheses about specific affective responses or to contrast activation effects during accurate and inaccurate judgments. Future efforts may beneficially examine incidental and directed emotion processing using event-related fMRI approaches for answering specific cognitive neuroscience questions of interest such as differences between positive and negative emotions and for examining the time course of limbic reactivity and its modulation by the PFC. Another limitation of the study is the small sample size, and therefore, results need to be replicated in larger samples. However, it is notable that unmedicated youths with euthymia were recruited for this study to reduce confounds of acute symptoms and medication effects that can add significant heterogeneity in measures of interest.
CONCLUSIONS
Scientific understanding of the neural correlates of affective responses is relevant to real-life clinical situations. Automatic emotional responsivity of limbic areas seems to be increased in PBD. Furthermore, cognitive regions such as the DLPFC and the affective control regions such as the VLPFC and the ACC may underfunction in PBD when patients are exposed to emotional stimuli, suggesting reduced top-down regulation of emotional arousal. This combination of alterations could contribute to exaggerated or more persistent emotional reactivity in patients with PBD. Knowledge of brain function supporting affect processing and regulation as shown in this study can therefore help explain some of the diverse emotional characteristics of PBD and, in the long term, potentially guide the development of interventions that can dampen affective reactivity or enhance top-down regulatory control.
Results from the present study highlight an increased activation of the right amygdala in response to incidental emotion processing in unmedicated patients with PBD and euthymia relative to directed emotion processing. These results illustrate a potential mechanism by which enhanced limbic activation by incidental stimuli may trigger increased emotional responsivity in patients with PBD. Our results also highlight similarities in the decreased PFC responses in patients with PBD relative to the HC in both incidental and directed emotional processing—suggesting an overall failure of higher cortical regulatory systems that may contribute to the overly intense and prolonged emotional responses that characterize PBD regardless of the type of emotion processing. Thus, findings from the present study indicate that both enhanced automatic emotional reactivity and reduced top-down emotion regulation may contribute to the emotional disturbances associated with PBD. Finally, posterior visual and face-processing regions such as the superior occipital, temporal, and fusiform gyri have shown greater activation in both incidental and directed emotion processing in patients relative to the HC, suggesting more effort is needed for emotional face processing in PBD.
Disclosure
Dr. Pavuluri has received research funding from NARSAD, NICHD, Dana Foundation, Colbeth Foundation, GlaxoSmithKline-NeuroHealth, Abbott Pharmaceuticals, and Johnson & Johnson. Dr. Sweeney receives support from the NIH, GlaxoSmithKline, Johnson & Johnson, AstraZeneca, and Eli Lilly. The other authors report no conflicts of interest.
This research was funded by NIH K23 RR018638, NARSAD, and the Dana Foundation.
REFERENCES
- 1.Schenkel LS, Pavuluri MN, Harral EM, Sweeney JA. Facial emotion processing in medicated and unmedicated patients with pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- 2.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 3.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2(3 Pt 2):237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 8.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 9.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 10.Frazier JA, Ahn MS, DeJong S, Bent EK, Breeze JL, Giuliano AJ. Magnetic resonance imaging studies in early-onset bipolar disorder: a critical review. Harv Rev Psychiatry. 2005;13:125–140. doi: 10.1080/10673220591003597. [DOI] [PubMed] [Google Scholar]
- 10a.Hollingshead AB. Two-Factor Index of Social Position. Yale University Press; New Haven, CT: 1975. [Google Scholar]
- 11.Altshuler L, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 12.Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 13.Swayze VW, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 14.Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4:80–88. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- 15.Brambilla P, Harenski K, Nicoletti M, et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 16.Altshuler LL, Bartzokis G, Grieder T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 17.Gorno-Tempini ML, Pradelli S, Serafini M, et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 18.Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41:585–596. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 20.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Ann Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Straube T, Mentzel HJ, Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 24.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 25.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 26.Psychological Corporation . Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Brace & Company; San Antonio: 1999. [Google Scholar]
- 27.Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Physica-Verlag; Heidelberg: 1996. pp. 39–49. [Google Scholar]
- 28.Rosenthal R. Meta-analytic Procedures for Social Research. Sage; Newbury Park: 1991. [Google Scholar]
- 29.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Thieme Medical Publishers; Stuttgart: 1988. [Google Scholar]
- 31.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. An anatomically constrained neural network model of fear conditioning. Behav Neurosci. 1995;109:246–257. doi: 10.1037//0735-7044.109.2.246. [DOI] [PubMed] [Google Scholar]
- 33.Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- 34.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 35.Whalen PJ, Bush G, McNally RJ, et al. The emotional counting stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 36.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 38.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 39.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 40.Phillips ML, Young AW, Scott SK, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia. 2007;45:163–173. doi: 10.1016/j.neuropsychologia.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Hess U, Blairy S. Facial mimicry and emotional contagion to dynamic emotional facial expressions and their influence on decoding accuracy. Int J Psychophysiol. 2001;40:129–141. doi: 10.1016/s0167-8760(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 45.Wild B, Erb M, Bartels M. Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: quality, quantity, time course and gender differences. Psychiatry Res. 2001;102:109–124. doi: 10.1016/s0165-1781(01)00225-6. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Stoleru S, Gregoire MC, Gerard D, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- 48.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 49.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 50.Damasio A, Van Hoesen G. Emotional disturbances associated with focal lesions of the frontal lobe. In: Heilman K, Satz P, editors. The Neuropsychology of Human Emotion. Guilford Press; New York: 1983. pp. 85–110. [Google Scholar]
- 51.Nelson EE, McClure EB, Monk CS, et al. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- 52.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 53.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 54.Kruger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 55.Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]