Summary
Background
The treatment of ischaemic stroke with neuroprotective drugs has been unsuccessful, and whether these compounds can be used to reduce disability after recurrent stroke is unknown. The putative neuroprotective effects of antiplatelet compounds and the angiotensin II receptor antagonist telmisartan were investigated in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial.
Methods
Patients who had had an ischaemic stroke were randomly assigned in a two by two factorial design to receive either 25 mg aspirin (ASA) and 200 mg extended-release dipyridamole (ER-DP) twice a day or 75 mg clopidogrel once a day, and either 80 mg telmisartan or placebo once per day. The predefined endpoints for this substudy were disability after a recurrent stroke, assessed with the modified Rankin scale (mRS) and Barthel index at 3 months, and cognitive function, assessed with the mini-mental state examination (MMSE) score at 4 weeks after randomisation and at the penultimate visit. Analysis was by intention to treat. The study was registered with ClinicalTrials.gov, number NTC00153062.
Findings
20 332 patients (mean age 66 years) were randomised and followed-up for a median of 2·4 years. Recurrent strokes occurred in 916 (9%) patients randomly assigned to ASA with ER-DP and 898 (9%) patients randomly assigned to clopidogrel; 880 (9%) patients randomly assigned to telmisartan and 934 (9%) patients given placebo had recurrent strokes. mRS scores were not statistically different in patients with recurrent stroke who were treated with ASA and ER-DP versus clopidogrel (p=0·38), or with telmisartan versus placebo (p=0·61). There was no significant difference in the proportion of patients with recurrent stroke with a good outcome, as measured with the Barthel index, across all treatment groups. Additionally, there was no significant difference in the median MMSE scores, the percentage of patients with an MMSE score of 24 points or less, the percentage of patients with a drop in MMSE score of 3 points or more between 1 month and the penultimate visit, and the number of patients with dementia among the treatment groups. There were no significant differences in the proportion of patients with cognitive impairment or dementia among the treatment groups.
Interpretation
Disability due to recurrent stroke and cognitive decline in patients with ischaemic stroke were not different between the two antiplatelet regimens and were not affected by the preventive use of telmisartan.
Funding
Boehringer Ingelheim; Bayer-Schering Pharma (in selected countries); GlaxoSmithKline (in selected countries).
Introduction
The treatment of ischaemic stroke with neuroprotective drugs has been unsuccessful,1-3 possibly because of the delay in giving these drugs after stroke. Whether neuroprotective drugs are effective when they are given before a recurrent stroke has not been investigated in a large sample. There are conflicting data between experiments in animals4,5 and in human beings6 that aspirin (acetylsalicylic acid, ASA) might be neuroprotective in acute ischaemic stroke. Dipyridamole used with aspirin had neuroprotective properties in cell cultures of neurons7,8 and in models of embolic stroke in rats9 but not in a secondary prevention study in human beings.10 There is no evidence that clopidogrel, another antiplatelet drug used to prevent stroke, has neuroprotective properties.11 Therefore, we compared the efficacy of prophylactic treatment with ASA and extended-release dipyridamole (ER-DP) with treatment with clopidogrel in the reduction of disability after recurrent strokes in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial.
Hypertension is the most important risk factor for stroke and cerebral small vessel disease, and antihypertensive treatment lowers the risk of first and recurrent strokes.12 Angiotensin II receptor antagonists, such as candesartan, telmisartan, losartan, and olmesartan, can reduce the rate of stroke in hypertensive rats and are neuroprotective in other animal models of stroke.13-18 Therefore, we hypothesised that telmisartan, in contrast with placebo, would reduce the disability due to recurrent strokes.
Small vessel disease is common in patients who have had a stroke, is associated with decreased cognition, and is a marker of incipient dementia. Vascular changes and vascular risk factors also have a role in Alzheimer's disease and vascular dementia.19-24 A meta-analysis of the results from four placebo-controlled studies found a non-significant 20% reduction in risk of dementia in favour of antihypertensive therapy.25-29 This was confirmed by the HYVET-COG trial in elderly patients.30 Therefore, we also collected data on cognitive performance, which enabled us to test the hypothesis that telmisartan could prevent or delay cognitive decline in this population.
Methods
Patients
From September, 2003, to July, 2006, patients aged 55 years or older who had had an ischaemic stroke in the previous 90 days were recruited to the PRoFESS trial. In addition, patients aged between 50 and 54 years or patients who presented 90 to 120 days after the qualifying stroke were also included, provided the patient had two of the following additional risk factors: diabetes mellitus, hypertension, was a smoker at the time of the qualifying stroke, obesity (BMI ≥30), previous vascular disease (stroke, MI, or peripheral arterial disease), end-organ damage (retinopathy, left-ventricular hypertrophy, or microalbuminuria), or hyperlipidaemia. Before randomisation, patients had to be clinically and neurologically stable (ie, they were not deteriorating or did not have a progressive stroke or other condition). Exclusion criteria and further details of the study design are reported elsewhere.31
All patients who were randomised provided written informed consent, and the confidentiality of their data is protected in accordance with the International Conference on Harmonisation guidelines.
Procedures
We used a two by two factorial design to randomly assign patients via a central telephone randomisation to the fixed combination of low-dose (25 mg) ASA and 200 mg ER-DP given twice daily or 75 mg clopidogrel given once daily in patients with recent ischaemic stroke. In a second, simultaneous randomisation, 80 mg telmisartan given once daily was compared with placebo in the presence of background antihypertensive treatment in patients who had recent ischaemic stroke. Patients were evaluated at discharge from hospital at 1 week, and then at 1, 3, and 6 months, and every 6 months thereafter. The minimum expected follow-up was 18 months. The trial was initially designed to compare clopidogrel and ASA with ER-DP and ASA. The design was modified after 2027 patients had been enrolled to compare clopidogrel only with ER-DP and ASA, after publication of the Management of Atherothrombosis with Clopidogrel in High-Risk Patients with Recent Transient Ischaemic Attack or Ischaemic Stroke (MATCH) trial results. The MATCH investigators showed that the addition of ASA to clopidogrel in patients who presented with transient ischaemic attack or minor stroke provided little additional benefit but significantly increased the risk of bleeding.32 The primary endpoint of both arms of the PRoFESS trial was time to any type of recurrent stroke. The most important secondary outcome was time to first occurrence of a composite of vascular events (stroke, myocardial infarction [MI], and vascular death). The results of these endpoints are reported elsewhere.33,34
Functional outcome was evaluated with the modified Rankin scale (mRS)35 3 months after a recurrent stroke. The Barthel index36 score was recorded 3 months after recurrent stroke. The mRS is a global disability scale, ranging from 0 (no disability) to 5 (patients are bed bound and require constant care) and 6 (dead). The Barthel index measures activities of daily living, with scores ranging from 0 (complete dependence) to 100 (independence). When patients were unable to attend a follow-up visit, scores were assigned by telephone assessment. For patients with severe aphasia, responses were obtained through a proxy or the treating physician.
Cognitive function was evaluated in all patients with the mini-mental state examination (MMSE).37 Scores range from 0 to 30, with lower scores indicative of a greater degree of cognitive impairment. The mean MMSE score for cognitively intact patients is 27·6 points; a score of 24 points or lower indicates some degree of cognitive impairment.38 The MMSE has been validated in several languages, including most of the primary languages spoken in all the countries in which the PRoFESS trial was run. The MMSE was done 1 month after randomisation, at 2 years, and at the penultimate visit. For patients who were in the study for less than 2 years, the final examination was at the penultimate visit. We also analysed how many patients had a decrease in MMSE score of 3 points or more, how many patients were cognitively impaired (MMSE <24) at the end of the study, and how many patients developed dementia during the trial, as assessed by clinical impression.
Statistical analysis
To compare antiplatelet drugs, we used a superiority approach, with a planned test of non-inferiority as a precursor; to compare telmisartan with placebo, a straightforward superiority assumption was used. All endpoints reported here were prespecified, with the exception of the statistical test for the 3-month post-stroke mRS, which was a post-hoc analysis. The mRS scores were analysed by treatment group with a non-parametric analog of the one-way ANOVA with arbitrary scores.39 Actual mRS scores were used in the test. We also dichotomised the mRS scores into 0–2 points and 3–5 points; the latter group was dependent and had poor outcome in functional capacity. The Barthel index was also dichotomised: 0–90 points (poor outcome) and 95–100 points (good outcome). The number of patients who had recurrent stroke (fatal or non-fatal) was used as the denominator to calculate percentages for mRS and Barthel index categories. Patients who died were assigned an mRS score of 6 points and a Barthel index score of 0. Cognitive impairment was defined as an MMSE score of 24 points or less. The denominator used to calculate the proportion of patients with cognitive impairment was the number of non-missing values at each assessment time window. All analyses that compared the treatment groups (ASA and ER-DP vs clopidogrel and telmisartan vs placebo) with respect to these dichotomised outcomes were done with the χ2 test.
On the basis of the data from the main study, a prognostic model for recurrent stroke was developed from the combination of the baseline characteristics for five selected risk scores (Framingham, SPI-II, Essen, NOMASS-7, NOMAS global vascular risk) in a Cox regression model. The final model included all statistically significant predictors of stroke: age, sex, physical activity, baseline systolic blood pressure, history of hypertension, diabetes, previous myocardial infarction, atrial fibrillation, peripheral arterial disease, and stroke before the qualifying event. The risk score for PRoFESS was calculated as the sum of the linear combination of the above 10 variables. The weight of each variable is the corresponding coefficient: (age–48)×0·2 (eg, add 0·2 for each year older than 48)+sex male×2+physical activity classified as sedentary×2+(baseline systolic blood pressure–90)×0·05 (eg, add 0·05 for each SBP unit >90 mm Hg)+history of hypertension×2+diabetes mellitus×4+previous MI×2+atrial fibrillation×3+peripheral artery disease×3+stroke in addition to qualifying event×6.
The protocol and other study documents, such as amendments, informed consent forms, patient information sheets, and safety reporting procedures were approved by the regulatory authority in each participating country or region, as required, and by the appropriate independent ethics committees or institutional review boards at the national and local site levels.
The trial is registered as ClinicalTrials.gov, number NCT00153062.
Role of funding source
The sponsor was involved in study design, collection, analysis, and interpretation of the data. The sponsor was not involved in the decision to submit the paper for publication. All authors had full access to all of the data and analyses and vouch for the accuracy and completeness of the data reported. All authors were involved in the final decision to submit the manuscript.
Results
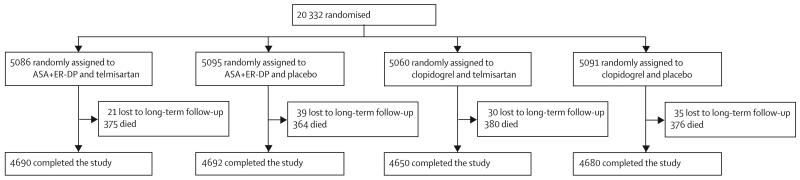
Investigators at 695 sites in 35 countries or regions randomised 20 332 patients who had had ischaemic stroke. The baseline characteristics of the study population of the PRoFESS trial are reported elsewhere.40 Mean age was 66·1 years (SD 8·6) and 36% (7319) of the patients were women. The median time from the qualifying event to randomisation was 15 days, with 40% (8133) of patients randomised within 10 days. The median time of follow-up was 2·44 years. In accordance with the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria,41 29% (5805) of the qualifying strokes were due to large vessel disease, 52% (10 578) small vessel disease, 2% (369) cardioembolism, 2% (416) to other aetiology, and 16% (3148) were of undetermined aetiology. Concomitant vascular disease and risk factors were not different in the various treatment groups. The most commonly used concomitant medications at randomisation were statins (9614 [47%]), angiotensinconverting enzyme inhibitors (7519 [37%]), calciumchannel blockers (4960 [24%]), and beta blockers (4231 [21%]). Mean systolic blood pressure at randomisation was 144 mm Hg (SD 17) and mean diastolic blood pressure at randomisation was 84 mm Hg (SD 11); the mean reduction in systolic BP with telmisartan was 8·3 mm Hg at 1 month compared with 2·9 mm Hg in the placebo group (difference −5·4 mm Hg). The mean BMI was 26·8 (SD 5). The median MMSE score at 30 days after randomisation was 28 points (IQR 26–30 points). Figure 1 shows the trial profile. There was no significant difference in baseline risk factors, TOAST criteria, mRS score, or MMSE score between patients who received clopidogrel, ASA with ER-DP, telmisartan, or placebo (table 1). 1814 patients had recurrent stroke: 916 patients (9%) on ASA with ER-DP and 898 patients (9%) on clopidogrel (hazard ratio [HR] 1·01, 95% CI 0·92–1·11; p=0·78 for superiority test). 880 patients on telmisartan and 934 patients on placebo had a recurrent stroke (HR 0·95, 0·86–1·04; p=0·23). 13% (246) of patients with recurrent stroke had more than one investigator-determined stroke, with no difference between treatment groups. 48% (693) of mRS assessments were done within 2 weeks of the 90-day cut-off after the first confirmed recurrent stroke, and 80% (1150) were done within 6 months of the first confirmed recurrent stroke. Cognitive outcome was not prospectively assessed immediately post-stroke; rather, it was assessed at 1 month, 2 years, and at the end of the trial. 25% (500) of patients had some study medication stopped after recurrent stroke, and 17% (309) were offall medication at the time of recurrent stroke. There was no significant difference in primary and secondary endpoints between patients on ASA with ER-DP or patients on clopidogrel and those on telmisartan and placebo.32,33 The mean stroke-risk score at baseline for patients with recurrent stroke was 13·9 (SD 4·4) points for ASA with ER-DP and telmisartan; 14·1 (4·7) points for ASA with ER-DP and placebo; 14·2 (4·6) points for clopidogrel and telmisartan; and 14·2 (4·7) points for clopidogrel and placebo. Table 2 shows the adverse events that occurred with a frequency of greater than 1%. The number and type of adverse events were not different among the four treatment arms.
Figure 1. Trial profile.
ASA=acetylsalicylic acid. ER-DP=extended-release dipyridamole.
Table 1.
Baseline characteristics
| ASA+ER-DP (n=10181) |
Clopidogrel (n=10151) |
Telmisartan (n=10146) |
Placebo (n=10186) |
|
|---|---|---|---|---|
| Age (years) | 66·1 (8·6) | 66·2 (8·5) | 66·1 (8·6) | 66·2 (8·6) |
| Women | 3653 (36%) | 3657 (36%) | 3619 (36%) | 3691 (36%) |
| Previous stroke | 1841 (18%) | 1865 (18%) | 1837 (18%) | 1869 (18%) |
| Median time from qualifying stroke (days) | 15 | 15 | 15 | 15 |
| Time to randomisation | ||||
| ≤10 days | 4029 (40%) | 4058 (40%) | 4021 (40%) | 4066 (40%) |
| 11–30 days | 2967 (29%) | 2920 (29%) | 2959 (29%) | 2928 (28%) |
| 31–90 days | 2825 (28%) | 2791 (27%) | 2785 (27%) | 2831 (28%) |
| >90 days | 340 (3%) | 358 (4%) | 360 (4%) | 338 (3%) |
| TOAST classification | ||||
| Large-artery atherosclerosis | 2928 (29%) | 2877 (28%) | 2907 (29%) | 2898 (29%) |
| Cardioembolism | 184 (2%) | 185 (2%) | 187 (2%) | 182 (2%) |
| Small-artery occlusion (lacunae) | 5292 (52%) | 5286 (52%) | 5283 (52%) | 5295 (52%) |
| Acute stroke of other determined aetiology | 202 (2%) | 214 (2%) | 202 (2%) | 214 (2%) |
| Stroke of undetermined aetiology | 1568 (15%) | 1580 (16%) | 1560 (15%) | 1588 (16%) |
| Modified Rankin scale score | ||||
| 0 | 1418 (14%) | 1435 (14%) | 1420 (14%) | 1433 (14%) |
| 1 | 3796 (37%) | 3784 (37%) | 3732 (37%) | 3848 (38%) |
| 2 | 2536 (25%) | 2545 (25%) | 2555 (25%) | 2526 (25%) |
| 3–5 | 2431 (24%) | 2387 (24%) | 2439 (24%) | 2379 (23%) |
| MMSE (at 1 month) | 27·0 (28, 4·1) |
27·0 (28, 4·1) |
26·9 (28, 4·2) |
27·0 (28, 4·0) |
| MMSE categories at 1 month | ||||
| 30 | 2738 (29%) | 2731 (29%) | 2720 (29%) | 2749 (29%) |
| 29 | 1765 (19%) | 1818 (19%) | 1782 (19%) | 1801 (19%) |
| 28 | 1227 (13%) | 1279 (14%) | 1266 (13%) | 1240 (13%) |
| 25–27 | 2011 (21%) | 1980 (21%) | 1992 (21%) | 1999 (21%) |
| ≤24 | 1663 (18%) | 1667 (18%) | 1666 (18%) | 1664 (18%) |
Data are number (%), number (SD), or mean (median, SD), unless otherwise stated. Missing values were counted as part of the percentage calculations for time from qualifying stroke (n=44), TOAST classification (n=16), but not for MMSE at 1 month (n=1453). ASA+ER-DP=acetylsalicylic acid plus extended-release dipyridamole. TOAST=Trial of Org 10172 in Acute Stroke Treatment. MMSE=mini-mental state examination
Table 2.
Frequency of the most commonly reported serious adverse events
| ASA+ER-DP (n=10181) |
Clopidogrel (n=10151) |
Telmisartan (n=10146) |
Placebo (n=10186) |
|
|---|---|---|---|---|
| Total patients with serious adverse events | 2749 (27%) | 2724 (27%) | 2812 (28%) | 2661 (26%) |
| Cardiac disorders | 470 (5%) | 463 (5%) | 485 (5%) | 448 (4%) |
| Unstable angina | 119 (1%) | 89 (1%) | 131 (1%) | 109 (1%) |
| Atrial fibrillation | 110 (1%) | 130 (1%) | 107 (1%) | 101 (1%) |
| Gastrointestinal disorders | 390 (4%) | 344 (3%) | 380 (4%) | 354 (4%) |
| General disorders and administration-site conditions |
265 (3%) | 222 (2%) | 262 (3%) | 225 (2%) |
| Chest pain | 107 (1%) | 76 (1%) | 100 (1%) | 83 (1%) |
| Hepatobiliary disorders | 86 (1%) | 87 (1%) | 76 (1%) | 97 (1%) |
| Infections | 584 (6%) | 634 (6%) | 615 (6%) | 603 (6%) |
| Pneumonia | 202 (2%) | 202 (2%) | 204 (2%) | 200 (2%) |
| Urinary tract infection | 86 (1%) | 114 (1%) | 102 (1%) | 98 (1%) |
| Injury, poisoning, and procedural complications | 384 (4%) | 392 (4%) | 391 (4%) | 385 (4%) |
| Fall | 121 (1%) | 116 (1%) | 101 (1%) | 136 (1%) |
| Metabolism and nutrition disorders |
228 (2%) | 200 (2%) | 222 (2%) | 206 (2%) |
| Musculoskeletal and connective tissue disorders |
216 (2%) | 255 (3%) | 247 (2%) | 224 (2%) |
| Benign, malignant, and unspecified neoplasms (including cysts and polyps) |
358 (4%) | 368 (4%) | 358 (4%) | 368 (4%) |
| Nervous system disorders | 537 (5%) | 514 (5%) | 561 (6%) | 490 (5%) |
| Psychiatric disorders | 142 (1%) | 101 (1%) | 128 (1%) | 115 (1%) |
| Renal and urinary disorders | 194 (2%) | 182 (2%) | 205 (2%) | 171 (2%) |
| Respiratory, thoracic, and mediastinal disorders |
226 (2%) | 202 (2%) | 228 (2%) | 200 (2%) |
| Vascular disorders | 185 (2%) | 195 (2%) | 202 (2%) | 178 (2%) |
Adverse events per system, organ, or class reported by at least 1% of patients in any group. ASA+ER-DP=acetylsalicylic acid plus extended-release dipyridamole.
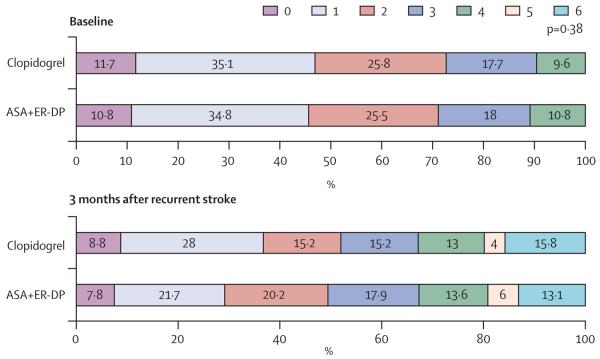
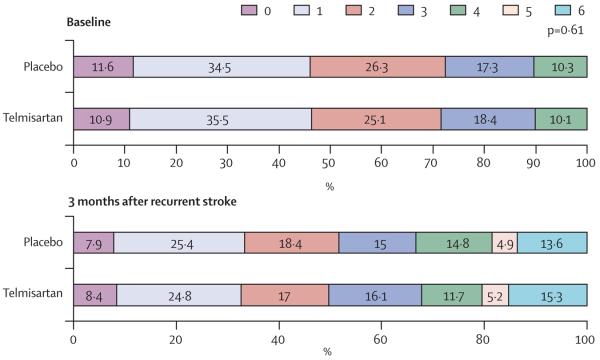
There was no statistically significant difference in mRS score for the 816 patients who had recurrent stroke while they were treated with ASA and ER-DP compared with the 817 patients who had a recurrent stroke while on clopidogrel (p=0·38). These results are shown in table 3 and figure 2. Similarly, the mRS scores were not different between stroke recurrences while on telmisartan versus placebo (p=0·61), which is shown in table 3 and figure 3. These findings are consistent whether the mRS score was compared as an ordinal scale or if it was dichotomised (2 points or less vs 3 points or more).
Table 3.
Modified Rankin scale (mRS) scores at 3 months after recurrent stroke
| ASA+ER-DP (n=816) |
Clopidogrel (n=817) |
χ2 for linear trend ANOVA p value |
Telmisartan (n=795) |
Placebo (n=838) |
χ2 for linear trend ANOVA p value |
|
|---|---|---|---|---|---|---|
| 0 | 61 (7%) | 72 (9%) | 0·38 | 67 (8%) | 66 (8%) | 0·61 |
| 1 | 177 (22%) | 229 (28%) | 193 (24%) | 213 (25%) | ||
| 2 | 165 (20%) | 124 (15%) | 135 (17%) | 154 (18%) | ||
| 3 | 146 (18%) | 124 (15%) | 144 (18%) | 126 (15%) | ||
| 4 | 111 (14%) | 106 (13%) | 93 (12%) | 124 (15%) | ||
| 5 | 49 (6%) | 33 (4%) | 41 (5%) | 41 (5%) | ||
| 6 (died) | 107 (13%) | 129 (16%) | 122 (15%) | 114 (14%) |
Data are number (%). If a patient died within 3 months of the recurrent stroke the mRS was coded as 6. 1633 of 1814 patients had an mRS assessment after recurrent stroke. Missing values were not included in the analysis. A worst-case analysis with missing values imputed as mRS=5 was done with similar results. ASA+ER-DP=acetylsalicylic acid plus extended-release dipyridamole.
Figure 2. Comparison of modified Rankin scale scores at baseline and 3 months for antiplatelet treatments.
Numbers for baseline modified Rankin scale scores (ASA+ER-DP/clopidogrel) are: score 0 points=99/105; score 1 point=319/315; score 2 points=234/233; score 3 points=165/159; score 4 points=99/86; scores 5 and 6 points=0/0. 3-month poststroke data are given in table 3. ASA+ER-DP=acetylsalicylic acid with extended-release dipyridamole. p value calculated from one-way ANOVA test.
Figure 3. Comparison of modified Rankin scale scores at baseline and 3 months for telmisartan versus placebo.
Numbers for baseline modified Rankin scale scores (telmisartan/placebo) are score 0 points=96/108; score 1 point=312/322; score 2 points=221/246 points; score 3 points=162/162; score 4 points=89/96; score 5 and 6 points=0/0. 3-month poststroke data are given in table 2. p value calculated from one-way ANOVA test.
Analogous analyses for the dichotomised Barthel index score (<95 vs ≥95) did not show any difference in patients who had recurrent stroke by allocation to antiplatelet treatment or telmisartan versus placebo (table 4). These results indicate that there is no difference between ASA plus ER-DP and clopidogrel with respect to recovery from recurrent stroke. Similarly telmisartan (alone or in addition to standard antihypertensive therapy) did not reduce functional deficit due to recurrent stroke.
Table 4.
Barthel index scores 3 months after the first recurrent stroke
| ASA+ER-DP (n=821) | Clopidogrel (n=820) | Relative risk | p | Telmisartan (n=798) | Placebo (n=843) | Relative risk | p | |
|---|---|---|---|---|---|---|---|---|
| Barthel index score 0–90* | 472 (57%) | 446 (54%) | 1·06, 0·97–0·15 | 0·21 | 450 (56%) | 468 (56%) | 1·02, 0·93–1·11 | 0·72 |
Data are number (%) or relative risk, 95% CI. Numbers include the patients with a 3-month, poststroke follow-up and the patients who died within 3 months of recurrent stroke.
Includes patients who died. 1641 of 1814 patients with recurrent stroke had a Barthel assessment after recurrent stroke. Missing values were not included in the analysis. ASA+ER-DP=acetylsalicylic acid plus extended-release dipyridamole.
There were no statistically significant differences in MMSE scores over time between the two antiplatelet regimens or between telmisartan and placebo. At 1 month after randomisation, the mean MMSE score was 27·0 points for patients randomised to ASA and ER-DP or to clopidogrel. The median MMSE score was 28·0 points at 1 month for both groups and 29 points during the follow-up period. The corresponding scores were the same for telmisartan versus placebo. Among the patients who had a recurrent stroke, the percentage of individuals who were cognitively impaired (ie, MMSE score ≤24 points) at the penultimate visit was 28% (144 of 515) in the ASA with ER-DP group and 23% of patients in the clopidogrel group (p=0·06). The proportions were 25% (127 of 500) in the telmisartan group and 25% (141 of 554) in the placebo group, respectively (p=0·98). We have not presented the 2-year timepoint data separately because fewer than 8000 patients (<40%) were evaluated for MMSE at 2 years, and for many patients the 2-year time window coincided with the penultimate visit.
Table 5 shows the treatment comparison for patients with an MMSE score of 24 points or less, and table 6 shows the proportion of patients with a decrease in MMSE score of 3 points or more from the first evaluation (this parameter was used in the Perindopril Protection Against Recurrent Stroke Study [PROGRESS] trial).42
Table 5.
Distribution of patients with MMSE score ≤24 points over time
| ASA+ER-DP | Clopidogrel | Relative risk | Telmisartan | Placebo | Relative risk | |
|---|---|---|---|---|---|---|
| Month 1 | 1663 (18% [9404]) | 1667 (18% [9475]) | 1·01, 0·95–1·07 | 1666 (18% [9426]) | 1664 (18% [9453]) | 1·00, 0·94–1·07 |
| Penultimate visit | 1165 (15% [7637]) | 1175 (15% [7828]) | 1·02, 0·94–1·10 | 1178 (15·2 [7739]) | 1162 (15·0 [7726]) | 1·01, 0·94–1·09 |
Data are number (% [total number of patients]) or relative risk, 95% CI. ASA+ER-DP=acetylsalicylic acid plus extended-release dipyridamole.
Table 6.
Number of patients with a decrease of 3 points or more in MMSE score from 1 month to penultimate visit
| ASA+ER-DP | Clopidogrel | Relative risk | Telmisartan | Placebo | Relative risk | |
|---|---|---|---|---|---|---|
| Penultimate visit | 764 (10% [7427]) | 863 (11% [7622]) | 0·91, 0·83–1·00 | 795 (11% [7531]) | 832 (11% [7518]) | 0·95, 0·87–1·05 |
Data are number (% [total number of patients]) or relative risk, 95% CI.
The percentage of patients deemed by the investigator to have dementia at the penultimate visit was 426 of 8607 (5%) in the ASA with ER-DP group versus 391 of 8663 (5%) for the clopidogrel group (p=0·18), 408 of 8624 (5%) for the telmisartan group, and 409 of 8646 (5%) for the placebo group (p=0·99). In the subgroup of patients who had recurrent stroke, the proportions who were deemed to have dementia at the penultimate visit were 12% (76 of 645) and 11% (68 of 645) for the ASA with ER-DP and clopidogrel groups, respectively, and 13% (77 of 615) and 10% (67 of 675) for the telmisartan and placebo groups, respectively. The differences between the groups were not statistically significant (p=0·48 and p=0·14, respectively).
Discussion
PRoFESS is the largest trial so far to investigate in a prespecified manner whether treatment with antiplatelet drugs or angiotensin II receptor antagonists (such as telmisartan) are neuroprotective in patients who have had recurrent stroke. The degree of functional impairment at 3 months poststroke was similar across treatment arms. The most probable explanation is that neither ASA with ER-DP nor clopidogrel are neuroprotective,43 despite indications from experiments in animals that this might be the case. About half of recurrent strokes were due to small vessel disease, which is less likely to be influenced by neuroprotective therapy. The less probable explanation would be that both treatments are equally neuroprotective, and no difference in functional impairment after recurrent stroke is apparent.
Our hypothesis that telmisartan would influence functional impairment after recurrent stroke was not substantiated. In contrast with the results of most other clinical trials on neuroprotective therapy in acute stroke, telmisartan was present in the brain when the patients had a stroke; therefore, concerns about the therapeutic time window were not relevant. This finding reinforces the message that caution should be used when extrapolating data from animal experiments to human stroke. The doses of angiotensin II receptor antagonist used in animal experiments might have been much higher than the doses used to treat hypertension in human beings. Another problem might be that the study drug was withdrawn or temporarily discontinued in most patients after an acute stroke, which limited the length of exposure. The half life of ASA is 2–3 h, dipyridamole has a half life of 13 h, and telmisartan has a half life of 20 h. Therefore, the length of exposure to telmisartan should have been enough to be neuroprotective, even after the drug was stopped. One shortcoming of the analysis of functional impairment after recurrent stroke is that patients were not randomised to treatment with a presumed neuroprotective substance at the time of the recurrent stroke.
Comparisons of a subset of patients who had recurrent stroke should be done with caution because this would not be a true comparison of randomised patients. Because the frequency of recurrent strokes was similar on the two treatments, our analysis is unlikely to have been affected by differences in the incidence of stroke. Of the 1715 projected patients with recurrent stroke, the trial was adequately powered (90%) to show a clinically meaningful difference of 8% in binary endpoints (eg, difference between 50% of patients with disabling strokes as determined by an mRS score of ≥3 points in one treatment group vs 58% in the other treatment group). For the full sample of patients, the trial was vastly overpowered (>99%) to show a clinically meaningful difference of 2 points in MMSE score.
Similarly, unlike in animal experiments,44,45 we were not able to show any influence of the different treatments on cognitive decline, as measured with the MMSE. This was also true in the subgroup of patients who had recurrent stroke. Although the MMSE is a crude index and can not detect minor changes in cognitive function, it is validated in many languages, and investigators do not need extensive training to apply it. PRoFESS had a cognitive substudy that used more sophisticated neuropsychological assessments in a subgroup of patients, the results of which will be reported later.
Most importantly, the average length of follow-up (2·5 years) might have been too short to show the effects of the medication on cognitive function. Trials of antihypertensive drugs with favourable results had a longer follow-up (eg, 3·9 years for the PROGRESS trial).41 In the Syst-Eur trial,27 the reduction in blood pressure was greater than the reduction in the present trial. Cognitive decline was reduced by candesartan versus placebo in the Study on Cognition and Prognosis in the Elderly (SCOPE) trial, which had a mean follow-up of 3·5 years and a much larger difference in blood pressure reduction (systolic=20 mm Hg; diastolic=10 mm Hg) than the reduction in the PRoFESS trial.46
The PRoFESS results are valid internally because biases and random errors were minimised by the study design and conduct, outcome events were adjudicated in accordance with standardised criteria and blinded to the treatment allocation, and the results for recurrent stroke and other major vascular events are consistent with those of older studies, such as CAPRIE,47 ESPS-2,48 and ESPRIT.49 Although the measures of disability (modified Rankin score and Barthel index at 3 months after stroke) and, particularly, the measure of cognitive function (MMSE at 4 weeks after randomisation and at the penultimate visit) might not be optimal, they are reasonably valid, reliable, sensitive to important changes, and sufficiently simple and familiar to be measured pragmatically in a large multicentre and multinational trial of this kind. The MMSE is an appropriate measure in the context of this study. The results might not be valid externally for patients younger than 50 years who have had a stroke due to cardiac embolism and who are clinically unstable, because none of these types of patient were enrolled in the trial.
In conclusion, there is no improvement in functional or cognitive outcome with ASA and ER-DP versus clopidogrel or telmisartan versus placebo.
Acknowledgment
We acknowledge administrative support by Vicky Hinstridge, a medical writer whose services were funded by Boehringer Ingelheim.
The PRoFESS study group
H-C Diener (co-chair)*, R Sacco (co-chair)*, S Yusuf (co-chair)*, B Blank†, D Cotton*, V Gu*, K Hermansson*, L Hilbrich†, M Humphreys†, W A Lawton*, T Machnig, S Ôunpuu*, C VanderMaelen*, T Voigt*, Y Wu†, G Albers, P Bath, N Bornstein, B Chan, S-T Chen, L Cunha, B Dahlöf, J DeKeyser, G Donnan, C Estol, P Gorelick, M Kaste, C Lv, P Pais, R Roberts, V Skvortsova, P Teal, D Toni, M Weber, B-W Yoon. Data-monitoring committee: P Wolf (chair), M Fisher, B Norrving, Y Palesch (independent statistician), P Sleight, A Turpie. Adjudication committee: T Buck, J Chong, G Dagenais, R delaPaz, A Diehl, M DiTullio, D Easton, C Ehrenfeld, M Elkind, J Fiebach, M Forsting, E Gizewski, D Gohs, J Halperin, M Haude, C Herz, S Homma, O Kastrup, J Krakauer, A Magun, M Maschke, J P Mohr, C Möller-Hartmann, P Mummel, J Pile-Spellman, C Rodriguez, S Sack, J Schlaak, A Schmermund, R von Kummer, I Wanke, C Weimar, H Wieneke, T Zoepf. Publication committee: H-C Diener (chair), P Bath, G Donnan, D Cotton, C Estol, L Hilbrich†, M Humphreys† , S Ôunpuu, R Roberts, P Teal, D Toni, R Sacco, S Yusuf. Substudy subcommittee: P Bath (co-chair), R Sacco (co-chair), D Cotton, B Dahlöf, H-C Diener, P Gorelick, R Roberts, M Weber, S Yusuf, L Hilbrich†. Principal investigators by country (NC=national coordinator): Argentina—C Estol (NC), S Ameriso, D Baumann, J J Cirio, T S Coleman, M M Esnaola y-Rojas, H Fraiman, H Gabrielli, J Garrote, M Garrote, P Loli, J L Ferreiro, S Lepera, M F Pardal, R Rey, A Ruiz, G Saredo, C Simonsini, A Zinnerman. Australia—G Donnan (NC), C Bladin, D Crimmins, S Davis, J Frayne, P Hand, R Henderson, N Ingham, C Levi, M Parsons, S Read, D Schultz, A Slattery, M Williams, J A Zavala. Austria—S Horner (NC), F Aichner, M Brainin, U Baumhackl, T Brücke, W Doppler, V Dorda, H-P Haring, A Seiser, H W Wege, G Wille, J Willeit. Belgium—S Blecic (NC)†, V. Thijs (NC), B Bruneel, J Caekebeke, N De Klippel, A De Windt, P P De Deyn, P Desfontaines, S Dethy, M Dupuis, A Görner, S Jeangette, P Laloux, M Pandolfo, A Peeters, R Sheorajpanday, W Van Landegem, P Vermylen, G Vanhooren, C Willems. Brazil—A R Massaro (NC), C André, A C F Almeida, R Brondani, J J Carvalho, F Cendes, G R de Freitas, S R F Fábio, M A G Friedrich, M Martins, S C O Martins, A B Maulaz, C Minelli, C H C Moro, J Oliveira-Filho, M S Rocha, J I Siqueira-Neto, R Valiente, V H F Zétola. Canada—P Teal (NC), N Amir, B A Anderson, R Arts, P Bailey, N Bayer, M Beaudry, L Berger, J-M Boulanger, D Brunet, T Collier, R Côté, V Daniels, A Demchuk, H Desai, A M Fontaine, M Gawel, D Gladstone, W Goldstein, V Hachinski, F Herbert, K Hesser, H Hink, K Ho, D Howse, K Kastelic, P Kostyrko, M Lapierre, L-H Lebrun, A Mackey, M Maharaj, L Miners, J Minuk, G Moddel, R Mosewich, D Novak, A Penn, Y Pesant, P Pikl, H Rabinovitch, A Rabinovitch, D Sahlas, C Schanz, J Scott, D Selchen, M Sharma, A Shuaib, J Silva, F L Silver, D Spence, M Stefanelli, S Stoger, T Stokes, J Teitelbaum, F Veloso, S Verreault, C Voll, C Walker, S Walker, R Wiegner, T Winder, M Winger, A Woolfenden. China—C Lv(NC), D W Chen, H B Chen, S D Chen, Y Cheng, Z F Chi, L Y Cui, Q Di, F Deng, M P Ding, S J Ding, X S Ding, D S Fan, W Fan, X B Fan, H L Feng, J Gong, T Gong, Z Hong, X J Hou, X Q Hu, Y N Huang, C Y Li, L Li, T Li, W Li, Z Y Li, H Lin, Q Y Lin, C F Liu, J R Liu, J R Liu, M Liu, R Liu, X F Liu, Y Liu, G G Luo, G Q Luo, J T Miao, L Miao, Z Y Pang, W Qiu, B Shao, X J Sun, D X Wang, F Wang, L J Wang, S Z Wang, W Wang, Y J Wang, J Wu, J Xia, E Xu, H Q Xu, S B Xu, X Xu, Q D Yang, X Yi, P M Yu, G L Zeng, J S Zeng, Y Zhai, B Zhang, C Y Zhang, H Zhang, S H Zhang, W W Zhang, Y B Zhang, Y Zhang, Y Zhang, G Zhao, J H Zhao, J Zhao, X Q Zhao, G X Zhou, H D Zhou, G M Zhu, Y C Zhu. Denmark—P Petersen (NC), G Andersen, P Arlien-Soeborg, I C Bach, M Binzer, G Boysen, A Heick, A-M Homburg, S Husted, H Iversen, L-H Krarup, T S Olsen, K Overgaard, P Von Weitzel-Mudersbach. Finland—M Kaste (NC), M Hillbom, K Koivisto, M Männikkö, S Mustanoja, H Numminen, J Nuutinen, J Sivenius. France—X Ducrocq(NC), D Leys, C Lucas, F Macian, L Milandre, J-P Neau, D Saudeau, H Vespignani, M Voicu, M Zuber. Germany—O Busse (NC), A Ahlers, J Allendoerfer, R Benecke, S Boy, A Dethlefs, M Dichgans, H-C Diener, M Eicke, F Erbguth, G Gahn, S Gass, J Glahn, M Görtler, A Grau, B Griewing, M Grond, K-H Grotemeyer, J Haan, G Hamann, L Harms, S Harscher, A Hetzel, A Hoferichter, H B Huelsboemer, G Ickenstein, M Kaps, M Kirchner, H Kunte, J Liepert, R Malessa, Y Mewald, A Müller-Jensen, D Nabavi, M Nedelmann, M Nueckel, H Poppert, K Rabe, J Regula, P Ringleb, M Rosenkranz, W-R Schaebitz, I Schaberger, F Schlachetzki, D Schneider, J Schrader, U Sliwka, J Sobesky, H Soda, W Steinke, H G Thomalla, H Topka, J Treib, M Vry, T Warnecke, R Weber, C Weiller, K Wessel, H Wiethölter, O Witte, H Wuttig. Greece—I Iliopoulos (NC), I Ellul, S Giannopoulos, C Karageorgiou, P Papathanasopoulos, D Vassilopoulos, S Voyaki. Hong Kong—P W Ng (NC), B H Fung, K F Hui, T Leung, V Mok, K S Wong. India—D Xavier (NC), A Agarwal, R R Agrawal, A M Anandan, V Anandhi, G K Babu, S Bandishti, A Bhargava, N Bhargava, A Bharani, A Bhatt, N Chidambaram, Y Dewan, M Dinaker, R Joshi, S Joshi, A Kalanidhi, S P Kalantri, S Kothari, A Kumar, P Kumar, V Jain, M M Mehndiratta, S Mijar, V Mishra, S Murali, R S Muralidharan, J M K Murthy, R Nair, J T Narayanan, R B Panwar, P Patel, F Poncha, V V R Prasad, A Rath, B C S Reddy, A Rohatgi, A K Roy, S Sadanandham, A Salam, G R K Sarma, H Singh, Y Singh, S Shanmugasundaram, S Sharma, S Sivakumar, R Sundararajan, T Sundararajan, U Tukaram, R Umarani, S Varma, C U Velmurugendran, A Venkitachalam, R Verghese, K P Vinayan, A Vyas, R S Wadia. Ireland—R Galvin (NC), P Kelly, D O'Mahony. Israel—N Bornstein (NC), B Assa-Meirov, B Gross, Y Lampl, A Mahagney, O Merzlyak, R Milo, M Rabey, L Shopin, J Streifler, D Tanne, G Telman, L Turiansky, B Weller, D Yarnitsky. Italy—L Provinciali (NC), M Arnaboldi, P Bassi, B Bergamasco, A Carolei, G Cascone, F Chiodo Grandi, G Comi, D Consoli, F Corea, P Dudine, F A De Falco, C Gandolfo, E Giaccaglini, B Gobbi, D Inzitari, G Lembo, M Melis, R Mutani, E Natalé, G Neri, M Rasura, M L Sacchetti, A Semplicini, M Stornello, M Stramba-Badiale, R Sterzi, G Torcasio, V Toso. Japan—S Uchiyama (NC), T Yamaguchi (NC), K Chiba, Y Fujino, F Hattori, K Hattori, O Hirai, A Imamura, K Ishii, T Ishihara, M Isobe, K Ito, T Jinnouchi, M Kaido, T Kawamoto, M Kawanishi, I Kim, K Kitazawa, M Kotera, Y Kujiraoka, S Kurokawa, Y Maeda, K Matsumoto, M Matsumoto, S Matsumoto, F Nakagawa, Y Nakajima, T Obata, H Ohnishi, N Sato, T Seguchi, T Seki, Y Shibagaki, M Shitamichi, A Tabuchi, K Takahashi, M Takekawa, Y Takimoto, H Tanabe, H Taniguchi, Y Tatsuoka, K Toda, T Toriyama, M Yamazaki, H Yoshida. Malaysia—K S Tan (NC), T Hassan, H T Chong, K S Tan, J K J Tharakan. Mexico—A Arauz (NC), J Aguayo, C Cantu, C Espinoza, J Fernández-Vera, J Guzman, C León, A Leyva, M López, M López-Ruiz, S Reyes, J Ruiz, R Vazquez, J Villarreal. Netherlands—J De Keysern (NC), M Aramideh, J Boiten, P Brouwers, B de Bruijn, C Franke, J den Heijer, P Dellemijn, J Hagemans, K Keizer, S Kok, P de Kort, J de Kruijk, J Kuipers-Lo Dico, J van Leusden, H van Leusen, B Jansen, W M Mulleners, K ten Napel, J F de Rijk-van Andel, J Van Remmen, W Rutgers, W J Schonewille, T Simons, T J Tacke, E Vries, J Wessel, M van Zagten. Norway—B Indredavik (NC), H Næss, G Rohweder, Ø Røsjø. Portugal—L Cunha (NC), J Campillo, J M Ferro, G Lopes, A A Pinto, V Salgado. Russia—V Skvortsova (NC), V Alifirova, A Amintaeva, O Antukhova, G Avakyan, A Belkin, A Belova, A Boiko, A Boiko, V Bulgakov, M Cherman, N Dokuchaeva, B Doronin, M Evzelman, A Fedin, N Fedorova, O Gileva, M Glezer, K Golikov, N Halo, E Isakova, A Ivleva, L Kabanova, S Kotov, A Kovalenko, G Kozin, T Lokshtanova, M Lutsky, R Magzhanov, M Martynov, T Mirsaev, D Mulyarov, V Narodova, M Odinak, A Orlov, V Parfenov, N Pizova, J Popova, E Poroshina, S Pronina, N Pryanikova, E Pynchuk, L Renzhina, A Savchenko, E Shirokov, V Shmyrev, I Sholomov, N Shmidt, V Simanenkov, A Skoromets V Sorokoumov, N Spirin, L Stakhovskaya, A Stepanchenko, E Strachunskaya, V Stulin, Z Suslina, S Timerbaeva, Y Trinitatsky, Y Varakin, O Voskresenskaya, E Yacupov, N Yahno, S Yanishevsky, G Yudina, Y Yudelson, J Zhitkova. Singapore—B P L Chan (NC), H M Chang, C C Chen, W Cheong, D A De Silva, R N Gan, C Meng, A B H Seah, V K Sharma, C S P Soon, N Venketasubramanian, Y Wai, M C Wong, C W Yip. South Africa—J Smuts (NC), P Francis, J Gardiner, W Guldenpfennig, D Lurie, A Mochan, C Retief, W Van Niekerk. South Korea—B-W Yoon (NC), O-Y Bang, K-H Cho, S-W Han, J-H Heo, D-W Kang, H-A Kim, H-M Kwon, J-H Kwon, S-U Kwon, B-C Lee, H-S Nam, M-S Park, J-H Rha, K-H Yu. Spain—A Gil-Nunez (NC), J Alvarez-Sabín, C Antón, J A Egido, J Gállego, F Gracia, J M Moltó, R Navarro, F Rubio. Sweden—N-G Wahlgren (NC), B Andersson, M von Arbin, A Berglund, E Bertholds, L Bokemark, C Carlstroem, A-C ElgÅsen, J P Eriksson, P-O Hansson, T-B Käll, A Lindgren, J Lökk, J Malm, I Markström, M Milovanovic, S-Å Nilsson, B Persson, Ö Skogar, M Stenstam, J Teichert, S Karlsson-Tivenius, M Von Euler, T Wallén. Taiwan—S-T Chen (NC), K-C Chang, M-H Chang, Y-J Chang, C-H Chen, W-H Chen, Y-T Chuang, C-Y Hsu, H-H Hu, Y-C Huang W-J Hwang, J-S Jeng, J-T Lee, L-M Lien, R -T Lin, Y-J Lin, C-H Liu, G- S Peng, H L Po, T-Y Tan, S-C Tsai, S-F Wang, W-J Wong. Thailand—S Chankrachang, Y Chinvarun, A Chutinet, S Muengtaweepongsa, Y Nilanont, N Poungvarin, P Sithinamsuwan, N Suwanwela, S Tanprawate. Turkey—S Bahar (NC), S Aktan, M Bakar, S Balkan, T Dalkara, K Kutluk, G Ozdemir. Ukraine—S Moskovko (NC), O Balyskyy, V Bitensky, O Dubenko, L Dzyak, A Goloborodko, G Grebenyuk, A Koselkin, V Kulgeyko, S Kuznetzova, V Lebedynets, V Maly, S Medvedkova, E Melnyk, T Mishchenko, G Moskovko, V Orzheshkovskyy, N Ovsyannikova, V Yavorska, I Yurdanova, V Zushkha. UK—P Bath (NC), L Brawn, M Brown, L Campbell, D Cohen, R Curless, J Davis, G Durward, G Ford, C Gray, F L Hammonds, T Hendra, M James, L Kalra, R Kumar, S Jackson, D Jenkinson, K Lees, G Lip, R MacWalter, K Muir, P Murphy, J Okwera, E Orugun, P Passmore, J Potter, A Sharma, J Sharma, M Sterling, S Ragab, T Robinson, C Roffe, A Rowe, J Turton. USA—G Albers (NC), P Gorelick (NC), R Sacco (NC), F Abbott, J Absher, A Acharya, H Adams, P Akins, E Albakri, M Alberts, R Alonso, I Altafullah, E Anderson, J Andrefsky, R Armstrong, G Arnold, A Arora, R Atkinson, S Azhar, S Bansil, K Becker, R Bell, L Benardo, G Bernardini, A Bernstein, P Blachman, J Boiser, B Boop, C Boutwell, D Bressler, C Brooks, W Brooks, R Calhoun, F Campanella, S Carlson, D Carpenter, J Castaldo, R Castaldo, M Cauli, K Chan, S Chaturvedi, S Cherian, M Chesser, D Chiu, W Clark, B Cleeremans, S Cohen, T Coin, L Collins, M Concha, G Cooper, J Couch, B Coull, E Crisostomo, A Cruz, P Cullis, R Dafer, S Dash, D Davis, P Davis, J DeMatteis, B Diamond, A Dick, D Dietrich, R Dunnigan, D Duong, L Edelsohn, H Ehrenfeld, J Elkins, M Englert, S Erlemeier, G Eubank, P Fayad, R Felberg, W Felton III, R Ferguson, S Flitman, P Fonzetti, K Furie, M Garcia, G Gardziola, J Gebel, J Glass, L Goldstein, A Goldszmidt, S Goli, G Graham, D Graybeal, B Grayum, E Green, J Green, P Green, D Greer, T Gropen, J Gross, J Grotta, R Gunwardane, B Haake, T Habiger, J Halsey, D Hanley, J Hanna, C Hansen, S Hanson, G Harpold, J Harris, M Harris, R Hart, K Hedges, B Hendin, J Hinchey, J Ho, M Hoffman, J Hollander, W Holt, K Holzmacher, G Howell, S Howell, D Huang, R Hughes, R Hull, T Hwang, M Jacobson, M Jacoby, A Jayam-Trouth, C Jackson, K John, B Johnson-Finley, M Johnson, S Johnston, A Kamal, P Karanjia, C Kase, S Kasner, L Katz, P Katz, T Kent, R Kelley, C Kidwell, A Khanna, H Kirshner, D Kleindorfer, C Knox, J Kramer, L Labiche, R Lada, E F LaFranchise, M LaMonte, M Lee, P Lee-Kwen, D Leifer, E Leira, K Levin, R Libman, D Liebeskind, K Lindholm, JN Livingstone II, G Locke, W Logan, J Luciano, H Lutsep, A Majid, V Mangeshkumar, S Markind, L Mate, J McCain IV, WA McElveen, B McKown, J Merino, B Meyer, T Mikesell, T Mirsen, L Montoya, S Moon, M Moonis, K Moore, J Nasrallah, A Nassief, M Nelson, F Nichols, J Osborne, F Oser, B Ovbiagele, D Palestrant, S Panezai, N Papamitsakis, G Parry, M Perez, C Perkins, J Porter, W Preston, H Rabiee, M Raikhel, R Reichwein, K Remmel, B Richardson, R Ringel, D Rosenbaum, D Ross, M Rubin, K Ruffing, H Sachdev, M Sauter, J Saver, J Schafer, R Schiftan, J Schim, A K Schleining, M Schneck, M Selim, S Sen, S Shafer, B Shafran, S Sharfstein, D Sherman, C Sila, S Silliman, B Silver, B Silverman, I Silverman, R Singh, N Skillings, A Slivka, D Smith, R Soto, S Sparr, R Stephens, H Sullivan, G Sung, J Sutherland, A Syed, M Tabbaa, D Tamulonis, R Taylor, D Tesfaye, T Thomas, G Tietjen, A Todorov, M Torbey, B Tolge, M Tremwell, W Truax, D Unwin, A Vaishnav, N Varma, Y S Venkatesh, P Verro, R Verson, T Vidic, P Vrooman, R Wallis, D Wang, J Wang, M Waters, L Wechsler, J Weinberger, J Weissman, G Wheatley, R Whitehead, A Willhite, H Willis, E Wilson, R Wilson, J Wilterdink, E Wirkowski, M Wozniak, C Wright, M Young, S Zachariah, S Zuckerman, R Zweifler.
Footnotes
Current members of the trial management committee.
Previous members.
Conflicts of interest
H-CD has received honoraria, consulting, and lecture fees from Abbott, AstraZeneca, Bayer Vital, Bristol Myers Squibb, Boehringer Ingelheim, D-Pharm, Fresenius, GlaxoSmithKline, Janssen Cilag, Merck Sharpe & Dohme, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Sankyo, Servier, Solvay, Thrombogenics, Wyeth, and Yamaguchi, and grant support from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Novartis, Janssen-Cilag, and Sanofi-Aventis. RLS has received honoraria and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, and Sanofi-Aventis. SY has received honoraria, consulting fees, and grant support from Boehringer Ingelheim, Bristol Myers Squibb, Servier, Sanofi-Aventis, AstraZeneca, and GlaxoSmithKline. GWA has received consulting, lecture fees, and grant support from Boehringer Ingelheim. PB has received consulting and lecture fees from Boehringer Ingelheim. S-TC, JDK, PG, and PP have received consulting fees from Boehringer Ingelheim. BD has received consulting and lecture fees from Novartis, Boehringer Ingelheim, and Merck, and lecture fees from Pfizer. GAD has received consulting fees from Boehringer Ingelheim, Sanofi-Aventis, Servier Australia, Phoenix BioPharm, and Johnson & Johnson. MK has received consulting and lecture fees from Boehringer Ingelheim. PT has received honoraria and consulting fees from Boehringer Ingelheim, Sanofi-Aventis, and Bristol Myers Squibb. DT has received consulting fees from Boehringer Ingelheim and lecture fees from Boehringer Ingelheim, Sanofi-Aventis, and Novo Nordisk. MW has received consulting and lecture fees from Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Forest Pharmaceuticals, Novartis, and Sanofi-Aventis, consulting fees from Gilead and Takeda Pharmaceuticals, and lecture fees from GlaxoSmithKline. DC, VG, KH, LH, WAL, TM, SÔ, CV, TV are employees of Boeheringer Ingelheim. YP and RHM have received consulting fees from Boehringer Ingelheim.
References
- 1.De Keyser J, Uyttenboogaart M, Koch MW, et al. Neuroprotection in acute ischemic stroke. Acta Neurol Belg. 2005;105:144–48. [PubMed] [Google Scholar]
- 2.Schabitz WR, Fisher M. Perspectives on neuroprotective stroke therapy. Biochem Soc Trans. 2006;34:1271–76. doi: 10.1042/BST0341271. [DOI] [PubMed] [Google Scholar]
- 3.Hill MD. Stroke: the dashed hopes of neuroprotection. Lancet Neurol. 2007;6:2–3. doi: 10.1016/S1474-4422(06)70658-5. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Z, Schwab S, Grau A, Berger C. Neuroprotection by early and delayed treatment of acute stroke with high dose aspirin. Brain. 2007;1186:275–80. doi: 10.1016/j.brainres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Gomes I. Aspirin: a neuroprotective agent at high doses? Natl Med J India. 1998;11:14–17. [PubMed] [Google Scholar]
- 6.Paciaroni M, Agnelli G, Caso V, et al. Prior use of antithrombotic agents and neurological functional outcome at discharge in patients with ischemic stroke. J Thromb Haemost. 2006;4:1957–61. doi: 10.1111/j.1538-7836.2006.02095.x. [DOI] [PubMed] [Google Scholar]
- 7.Blake AD. Diypridamole is neuroprotective for cultured rat embryonic cortical neurons. Biochem Biophys Res Comms. 2004;314:501–04. doi: 10.1016/j.bbrc.2003.12.115. [DOI] [PubMed] [Google Scholar]
- 8.Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18:5112–23. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldandashi S, Noor R, Wang CX, Uddin G, Shuaib A. Combination treatment with dipyridamole, aspirin, and tPA in an embolic model of stroke in rats. Exp Neurol. 2007;205:563–68. doi: 10.1016/j.expneurol.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Sivenius J, Cunha L, Diener HC, et al. Antiplatelet treatment does not reduce the severity of subsequent stroke. European Stroke Prevention Study 2 Working Group. Neurology. 1999;53:825–29. doi: 10.1212/wnl.53.4.825. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH. Lack of antiapoptotic effects of an antiplatelet drug, aspirin and clopidogrel, and antioxidant, MCI-186, against focal ischemic brain damage in rats. Neurol Res. 2005;27:483–92. doi: 10.1179/016164105X17134. [DOI] [PubMed] [Google Scholar]
- 12.Bosch J, Yusuf S, Pogue J, et al. for the HOPE Investigators Heart outcomes prevention evaluation. Use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002;324:699–702. doi: 10.1136/bmj.324.7339.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krikov M, Thone-Reineke C, Müller S, Villringer A, Unger T. Candesartan but not ramipril pretretment improves outcome after stroke and stimulates neutrophin BNDF/TrkB system in rats. J Hypertens. 2008;26:544–52. doi: 10.1097/HJH.0b013e3282f2dac9. [DOI] [PubMed] [Google Scholar]
- 14.Fagan SC, Kozak A, Hill WD, et al. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J Hypertens. 2006;24:535–39. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- 15.Faure S, Oudart N, Javellaud J, Fournier A, Warnock DG, Achard JM. Synergistic protective effects of erythropoietin and olmesartan on ischemic stroke survival and post-stroke memory dysfunctions in the gerbil. J Hypertens. 2006;24:2255–61. doi: 10.1097/01.hjh.0000249704.34607.4c. [DOI] [PubMed] [Google Scholar]
- 16.Engelhorn T, Doerfler A, Heusch G, Schulz R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neurosci Lett. 2006;406:92–96. doi: 10.1016/j.neulet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Groth W, Blume A, Gohlke P, Unger T, Culman J. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J Hypertens. 2003;21:2175–82. doi: 10.1097/00004872-200311000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Thone-Reineke C, Zimmermann M, Neumann C, et al. Are angiotensin receptor blockers neuroprotective? Curr Hypertens Rep. 2004;6:257–66. doi: 10.1007/s11906-004-0019-3. [DOI] [PubMed] [Google Scholar]
- 19.Roman GC. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20:91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 20.Erkinjuntti T, Roman G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke. 2004;35:1010–17. doi: 10.1161/01.STR.0000120731.88236.33. [DOI] [PubMed] [Google Scholar]
- 21.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–36. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 22.Kivipelto M, Solomon A. Alzheimer's disease—the ways of prevention. J Nutr Health Aging. 2008;12:89S–94S. doi: 10.1007/BF02982595. [DOI] [PubMed] [Google Scholar]
- 23.Desmond DW. Vascular dementia. Clin Neuroscience Res. 2004;3:437–48. [Google Scholar]
- 24.Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–54. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feigin V, Ratnasabapathy Y, Anderson C. Does blood pressure lowering treatment prevents dementia or cognitive decline in patients with cardiovascular and cerebrovascular disease? J Neurol Sci. 2005;229–230:151–55. doi: 10.1016/j.jns.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 26.The PROGRESS Collaborative Group Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–75. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 27.Forette F, Seux M, Staessen J, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–51. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 28.Lithell H, Hansson L, Skoog I, et al. for the SCOPE Study Group The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomised double-blind intervention trial. J Hypertens. 2003;21:875–86. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 29.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 30.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–89. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 31.Diener HC, Sacco R, Yusuf S, for the Steering Committee and PRoFESS Study Group Rationale, design and baseline data of a randomised, double-blind, controlled trial comparing two antithrombotic regimens and telmisartan vs. placebo in patients with strokes: the prevention regimen for effectively avoiding second strokes (PRoFESS) trial. Cerebrovasc Dis. 2007;23:368–80. doi: 10.1159/000100105. [DOI] [PubMed] [Google Scholar]
- 32.Diener H, Bogousslavsky J, Brass LM, et al. on behalf of the MATCH Investigators Management of atherothrombosis with clopidogrel in high-risk patients with recent transient ischaemic attack or ischaemic stroke (MATCH): Study design and baseline data. Cererbrovasc Dis. 2004;17:253–61. doi: 10.1159/000076962. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S, Diener HC, Sacco RL, et al. for the PRoFESS Study Group Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008 doi: 10.1056/NEJMoa0804593. published online August 27. DOI:10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacco R, Diener H, Yusuf S, et al. for the PRoFESS Study group Aspirin and extended-release dipyridamole versus copidogrel for recurrent stroke. N Engl J Med. 2008 doi: 10.1056/NEJMoa0805002. published online August 27. DOI:10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rankin J. Cerebral vascular accidents in patients over the age of 60, II: prognosis. Scot Med J. 1957;2:200–15. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 36.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 37.Folstein M, Folstein S, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Helkala E-L, Kivipelto M, Hallikainen M, et al. Usefulness of repeated presentation of mini-mental state examination as a diagnostic procedure - a population-based study. Acta Neurol Scand. 2002;106:341–46. doi: 10.1034/j.1600-0404.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 39.Gibbons JD. Nonparametric statistical inference. 2nd edition Marcel Dekker; New York: 1985. [Google Scholar]
- 40.Diener HC, Sacco R, Yusuf S. Rationale, design and baseline data of a randomised, double-blind, controlled trial comparing two antthrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS) Cerebrovasc Dis. 2007;23:368–80. doi: 10.1159/000100105. [DOI] [PubMed] [Google Scholar]
- 41.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 42.Tzourio C, Anderson C, Chapman N, et al. for the PROGRESS Collaborative Group Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–75. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 43.Paciaroni M, Agnelli G, Caso V, et al. Prior use of antithrombotic agents and neurological functional outcome at discharge in patients with ischemic stroke. J Thromb Haemost. 2006;4:1957–61. doi: 10.1111/j.1538-7836.2006.02095.x. [DOI] [PubMed] [Google Scholar]
- 44.Barnes NM, Champaneria S, Costall B, Kelly ME, Murphy DA, Naylor RJ. Cognitive enhancing actions of DuP 753 detected in a mouse habituation paradigm. Neuroreport. 1990;1:239–42. doi: 10.1097/00001756-199011000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Wright JW, Harding JW. Brain angiotensin receptor subtypes in the control of physiological and behavioral responses. Neurosci Biobehav Rev. 1994;18:21–53. doi: 10.1016/0149-7634(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 46.Skoog I, Lithell H, Hansson L, et al. for the SCOPE Study group Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on Cognition and Prognosis in the Elderly (SCOPE) Am J Hypertens. 2005;18:1052–59. doi: 10.1016/j.amjhyper.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 47.CAPRIE Steering Committee A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 48.Diener HC, Cuhna L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 49.The ESPRIT Study Group Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised, controlled trial. Lancet. 2006;367:1665–73. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]