SUMMARY
The adult Drosophila hindgut was recently reported to contain active, tissue-replenishing stem cells, like those of the midgut, but located within an anterior ring so as to comprise a single giant crypt. In contrast to this view, we observed no active stem cells and little cell turnover in adult hindgut tissue based on clonal marking and BrdU incorporation studies. Again contradicting the previous proposal, we showed that the adult hindgut is not generated by anterior stem cells during larval/pupal development. However, severe tissue damage within the hindgut elicits cell proliferation within a ring of putative quiescent stem cells at the anterior of the pylorus. Thus, the hindgut does not provide a model of tissue maintenance by constitutively active stem cells, but has great potential to illuminate mechanisms of stress-induced tissue repair.
INTRODUCTION
Mammalian and invertebrate tissues are frequently maintained by highly active stem cells as well as by damage-induced cell division (reviewed in Morrison and Spradling, 2008). Many examples of active stem cells have been studied within the ovary, testis and posterior midgut of Drosophila adults (Fuller and Spradling, 2007; Ohlstein and Spradling, 2006; Micchelli and Perrimon, 2006) but cells mediating inducible repair have not been described. Recently, another type of active stem cell was reported in the adult Drosophila hindgut (Takashima et al., 2008).
The adult hindgut comprises the posterior section of the alimentary canal (Fig. 1). Beginning at the end of the midgut near the junction with the Malpighian tubules, the hindgut is divided into three main sections: the pylorus, the ileum and the rectum. The pylorus functions as a valve that controls the passage of gut contents by constricting associated sphincter muscles (Snodgrass, 1935). The ileum mediates the absorption and excretion of water and ions. Takashima et al. (2008) reported that a small population of stem cells in the anterior region of the pylorus maintains this tissue during adulthood. The daughters of these stem cells are proposed to migrate posteriorly along the length of the pylorus and ileum, generating a regular flow of cells that was likened to cell behavior within a crypt of the mammalian intestine.
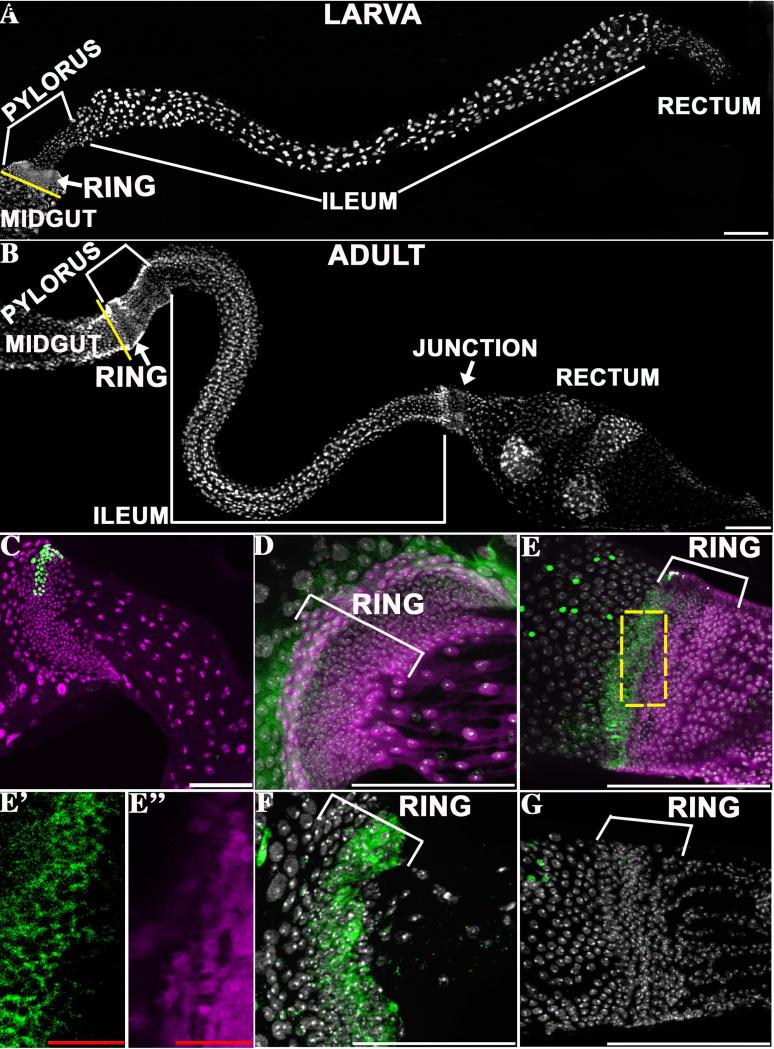
Figure 1. Similar larval and adult hindgut anatomy.
The general structure of the larval hindgut (A) and the adult hindgut (B) is shown. The major hindgut subdivisions and the pyloric rings are indicated. The yellow line denotes the midgut/hindgut boundary. C) A representative lacZ clone (green) induced in the first instar and analyzed in L3. D, E) Wg protein (green, cytoplasmic) is highly expressed in 1-3 rows of anterior ring cells in the pylorus of both larvae (D) and adults (E). Prospero-positive enteroendocrine cells (green, nuclear in E) mark the midgut, which lies anterior to the ring. More posterior ring cells strongly express byn-Gal4::UAS-GFP (purple). The separate channels are shown for a section indicated by the dashed box in E’ (Wg, green) and E” (byn-GAL4::UAS-GFP, purple). F) Posterior larval ring cells express the Notch activation reporter GBE-Su(H) lacZ (green). G) The adult ring, in contrast, does not express GBE-Su(H) lacZ (green). All panels- anterior to left, DAPI= nuclei (white). Scale bars- white=100μm, red=12.5μm.
The general origin of the Drosophila adult hindgut during pupal development from two distinct sources has been well characterized (Robertson, 1936). During metamorphosis the anterior hindgut arises from dense cells (the “larval imaginal ring”) that comprise most of the larval pylorus (Fig. 1A). There is no contribution from the larval ileum, which degenerates. Cells located within the genital disc separately generate the rectum, and the anterior and posterior hindgut anlage reconnect prior to eclosion.
Here we have re-investigated the cellular basis of hindgut development and maintenance using lineage labeling (Fox et al., 2009). In contrast to the claims of Takashima et al. (2008), we find no evidence for active adult stem cells, distal cell migration or for significant cell turnover in unstressed adult hindgut tissue. Thus, cellular homeostasis within the Drosophila hindgut does not conform to the crypt model. However, we discovered that Wg-positive cells within the anterior pylorus proliferate in response to serious tissue injury, and that uninjured cells respond preferentially. Thus, the Drosophila hindgut may be a valuable model of organs whose normally quiescent cells can be induced to divide and repair damage.
RESULTS
The adult pylorus contains a region similar to the larval imaginal ring
First, we analyzed the number, ploidy, and proliferation of cells in the pyloric and ileal regions of larval and adult animals to understand how each part of the hindgut develops and is maintained (see Experimental Procedures). The larval ring (Fig. 1A) has long been recognized as a dense diploid cell zone slightly posterior to the junction with the midgut and Malpighian tubules. Cell counts showed that the number of cells in the ring increases continuously throughout larval life, reaching about 600 cells at the end of the third instar (Fig. S1A). A narrower region of about 60 pyloric cells posterior to the ring remains unchanged in cell number throughout larval development (Fig. S1A). The adult pylorus (Fig. 1B) also contains an anterior ring of about 600 small cells (which we denote “the adult ring”) where the gut diameter is expanded (Fig. S1B). However, the distal pylorus is larger and consists of about 1,000 rather than 60 cells (Fig. S1B).
We confirmed that ring cells proliferate throughout larval development using lineage marking. Two different cell marking methods, the lacZ system (Harrison and Perrimon, 1993) and the MARCM system (Lee and Luo, 2001, see Experimental Procedures), were employed. No cell labeling was observed without heat-shock using the lacZ system, but a low level of spontaneous marking occurs using the MARCM system (Table S1). Using the lacZ system, we found that marked clones induced during the first instar (or the second instar) were confined to the ring and grew to about 20 cells (or 5 cells, respectively) in size by the end of the third instar (Fig. S1C). Interestingly, the clones consistently were elongated transversely to the proximal-distal axis of the gut, which may explain the expanded diameter of the larval ring (Fig.1C). Neither BrdU incorporation, nor mitotic markers were spatially restricted, indicating that all the cells in the larval ring are cycling (not shown). If all ring cells cycle at the same rate, the clonal data indicate they would have an average doubling time of about 16 hours (see Experimental Procedures).
Molecular expression studies revealed both similarities and differences between the larval and adult pyloric rings. We confirmed previous reports (Lengyel and Iwaki, 2002; Takashima et al., 2008) that the 2-3 anterior-most rows of ring cells in both larvae and adults express Wingless (Wg, Fig. 1D, E). The hindgut transcription factor brachyenteron (byn), is absent or weak in these cells, suggesting that they are distinct from other hindgut cells and may be undifferentiated (Fig. 1D, E). Strong expression of byn begins immediately posterior to the Wg zone, in both larvae and adults. However, expression of the Notch reporter GBE-Su(H)-lacZ (Fig. 1F,G), a possible indicator of ongoing cell differentiation, differs before and after metamorphosis. In larvae, GBE-Su(H)-lacZ is expressed in posterior ring cells, showing that these adult precursors receive a Notch signal (Fig. 1F). In contrast, no expression of GBE-Su(H)-lacZ was observed in the corresponding region of adults (Fig. 1G).
The ileum adjoins the pylorus and consists of polyploid absorptive enterocytes in both larvae and adults (Fig. 1A, B). Counts revealed that the larval ileum contains fewer enterocytes than the adult ileum (280 vs 550, Fig. S1B) but their ploidy is higher (64C vs. 8C). These studies revealed a paradox. If adult pyloric cells comprise stem cells and their undifferentiated daughters as proposed (Takashima et al., 2008), then according to our counts the adult hindgut would contain many times more undifferentiated cells than the total differentiated population of the ileum. In most stem cell-based tissues, stem cells and differentiating daughters are relatively rare. Even in the adult posterior midgut, which has a high density of stem cells, undifferentiated cells comprise only about 20% of all cells (Ohlstein and Spradling, 2006).
The adult pylorus and ileum are generated from distinct larval precursors rather than from a common stem cell in the “proliferation zone”
We investigated the role of the larval ring during pupal development by using the lacZ system to mark cells at specific times during larval and pupal development (Fig. 2A). When the marked clones were analyzed in adults, we immediately noticed evidence of cellular stability in the adult hindgut: clone size was virtually the same regardless of how long adults were aged prior to analysis (Fig 2B). Experimentally, this meant we could analyze larval and pupal clones at any time after eclosion, but in practice we used 4-7 day old adults to maximize precision.
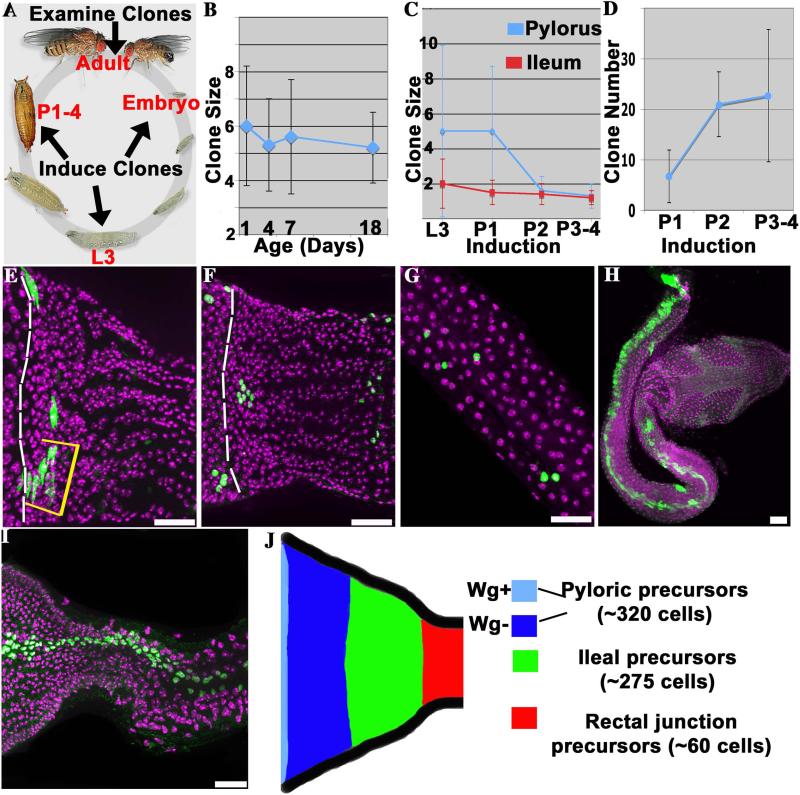
Figure 2. Separate progenitors produce the adult pylorus and ileum.
A) The clone induction scheme used to study adult hindgut progenitors. B) The size of pyloric clones induced during pupal development does not change significantly during adulthood. C) Pyloric (blue) and ileal (red) clones induced during late larval development (L3) are small, and decline further in size when induced after pupal day 1 (P1). D) The number of pyloric clones per gut induced by a standard heat shock increases during pupal development. E-G) Examples of clones induced in late larvae or pupae and analyzed in adults. E) Adult pylorus showing an L3 pyloric clone (yellow bracket) in contact with the posterior boundary of the Wg ring (dashed line). F) Adult pylorus showing 6 P1 pyloric clones, 4 of which do not contact the Wg ring (boundary = dashed line). G) P1 ileal clones, showing small size (1-2 cells). H) Rare large spontaneous clone spanning pylorus and ileum. I) Example of large lacZ clone induced during embryogenesis. J) Proposed fate map of the larval pylorus. All images- anterior to the left. DAPI (purple), clones (green) were labeled with membrane GFP (E,H) or nuclear lacZ (F,G,I). N > 70 clones/time point. Scale bars = 25μm. Lifecycle image adapted from http://flymove.uni-muenster.de/
Comparing the size of clones labeled just before or during pupal development revealed that larval progenitors of the adult hindgut undergo relatively little further proliferation prior to differentiation. Clones induced in third-instar larvae (L3) or in 1-day old pupae (P1) contain an average of only 5 cells in the adult pylorus (Fig. 2C, E, F). Thus, progenitors present in late larvae and early pupae divide on average only about twice prior to constructing the adult pylorus. Consistent with this interpretation, clones induced on P2 produced clones of only about two adult cells and on P3-4 produced clones of little more than one adult cell (Fig. 2C). As expected, the number of clones per gut was higher when clones were induced later, reflecting the increase in the precursor pool (Fig. 2D). Spatially, clones were found in all regions of the adult pylorus, and frequently did not include any Wg-positive cells from the anterior ring (Fig. 2E, F). Given the presence of about 1600 adult pyloric cells, these data imply that about 320 adult pyloric precursors (i.e. 1600 cells / 5 cells per precursor) are present in the late larval pylorus.
The progenitors of the adult ileum were found to be distinct from the pyloric precursors and to undergo even less proliferation during pupal development prior to differentiation. At all induction times adult ileal clones were dispersed and small-averaging 2 cells when induced in L3 larvae (Fig. 2C,G). Since the adult ileum contains about 550 cells, these data indicate that a separate population comprising about 275 precursor cells generates the adult ileum (550 cells / 2 cells per precursor). No common pyloric and ileal precursors exist at the time of clone induction, because among more than 3,200 clones examined with the lacZ system, none contained both pyloric and ileal cells. The number of pyloric and ileal progenitors revealed by our clonal studies account for all of the approximately 600 cells of the larval ring. Adult ileal cells cannot arise from the larval ileum, which contains only polyploid cells and is known to degenerate during the pupal period (Robertson, 1936). Consequently, a simple model of the distribution of adult progenitors in the late larval pylorus, which minimizes the need for cell migration, is presented in Fig. 2J. Based on their location with respect to the pyloric and ileal precursors, we propose that the 60 distal cells that lie outside the imaginal ring give rise to the junction zone with the rectum (see Fig. 1B).
Both the absence of a common pyloric and ileal precursor in late larvae, and the stability of labeled clones during adulthood are inconsistent with the proposal that active hindgut stem cells in a proliferation zone produce and maintain the adult hindgut. Very rarely with the MARCM system we observed large clones that did overlap the pylorus and ileum, identical to those cited (Takashima et al., 2008) as hindgut stem cell clones (Fig. 2H). However, such clones were observed at the same low frequency without induction (Table S1), suggesting they arise from spontaneous induction early in development. We confirmed this conclusion by inducing clones throughout development using both clonal systems. Large clones spanning the pylorus and ileum were observed only when induction occurred during embryogenesis (Fig.2I, Table S1). Thus, while pyloric and ileal cells share a common progenitor in the embryo, these lineages become separated before or during larval development.
The adult hindgut is not maintained by active stem cells
The presence of active stem cells and ongoing tissue turnover can be readily detected by BrdU labeling. For example, in a tissue such as the midgut that contains many stem cells and turns over weekly, a large fraction of tissue cells are expected to label following daily pulses of BrdU. Consequently, to look for hindgut stem cell activity we fed 4-day old adults BrdU continuously for one week and examined their hindguts the next day (Fig. 3A). Only a small fraction of hindgut cells were labeled in these experiments (Fig. 3B, C). In contrast, a high fraction of midgut cells showed strong BrdU incorporation, which served as a positive control (Fig. 3B). These included many polyploid cells as well as diploid cells (Fig. 3D). Labeling of polyploid cells is expected, since midgut intestinal stem cells continually produce daughters that polyploidize and differentiate to replace enterocytes lost to turnover (Ohlstein and Spradling, 2006). In contrast, BrdU incorporation in the hindgut was absent from polyploid ileal enterocytes (Fig. 3C), indicating that there is no ongoing adult production of ileal enterocytes.
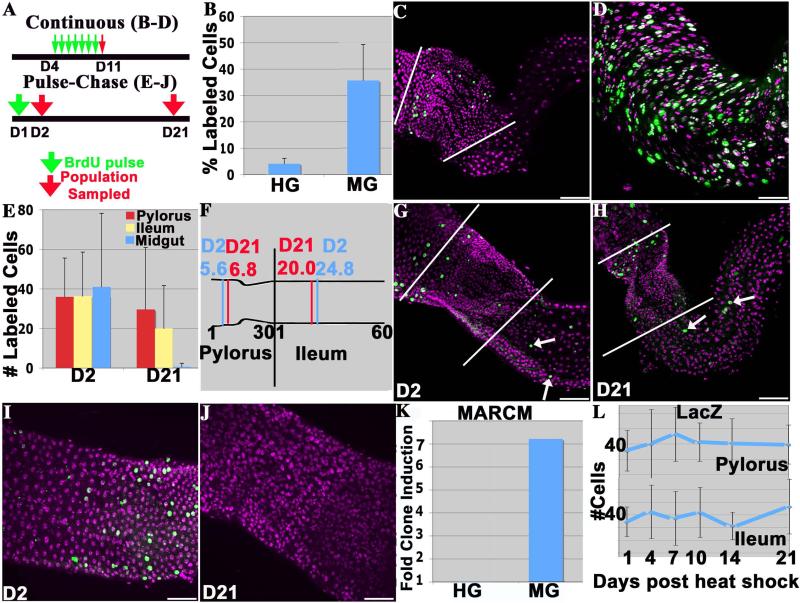
Figure 3. The adult hindgut is not maintained by active stem cells.
A) Experimental plan for continuous or pulse chase BrdU labeling studies; green arrow = BrdU addition, red arrow = tissue analysis. B) Percentage of BrdU-positive cells (average +/− standard deviation) in hindgut (HG) and midgut (MG) after seven days of BrdU exposure. C) BrdU incorporation following continuous labeling in the hindgut appears sporadic and is limited to the pylorus. D) Widespread BrdU incorporation in the midguts of the same animals. E) Slow turnover of BrdU-labeled cells in hindguts compared to the midguts of the same animals following the pulse-chase protocol. N >23 guts/time point. F) Absence of significant posterior cell movement over a 20-day period. The mean cell position of BrdU positive cells along the proximal-distal axes of the pylorus (30 cell diameters long) or the ileum (60 cell diameters long) at day 2 vs day 21. N > 200 pyloric and >100 ileal cells/time point. G) A hindgut 1d after the BrdU pulse (D2), or H) 20d post-pulse (D21). Arrows- ileal labeling; white lines= pyloric boundary. I) A midgut 1d after the BrdU pulse (D2), or J) 20d post-pulse (D21). K) The increase in the number of MARCM clones 21d after adult heat-shock, normalized to the number of clones in non-heat shocked controls. No increase is seen in the hindgut, unlike the stem cell-based midgut where the number of clones is strongly increased. L) The number of lacZ clones induced in 4-day-old adults per gut is stable during adulthood. 12-34 animals were scored at each time point. All images- anterior to the left. DAPI (purple), BrdU (green), Scale bar= 50μm.
As a second test, we analyzed the stability of BrdU-labeled pyloric and ileal cells during adulthood (Fig. 3A, “pulse-chase”). A few pyloric and ileal cells could still be labeled in newly eclosed adults, which reflects our observation that the final stages of adult hindgut development continue for 1-2 days after eclosion. One day old adults were labeled with BrdU, and when examined at day 2 (D2) both the pylorus and ileum contained a small number of labeled cells in a sporadic distribution (Fig. 3E,G). Contrary to expectation if the adult hindgut turns over and is replenished by stem cells, the average number of these labeled hindgut cells was virtually unchanged 20 days later (D21, Fig. 3E, H). This cellular stability contrasted sharply with the behavior of BrdU-labeled cells within the midguts of these same flies. All the midgut cells labeled on day 1 were gone within 20 days (Fig. 3E, I, J). These observations verify that the pulse-chase conditions were effective.
We also determined the mean location of the labeled cells at both time points to look for cell movement in a posterior direction. Contrary to the prediction of the crypt model (Takashima et al., 2008), the mean position of labeled ileal cells was actually more anterior at 20 days post pulse than at one day (Fig. 3F). In the pylorus we observed an anterior bias in BrdU labeling at day 2, and a small posterior shift of approximately one cell diameter, on average, by day 21. Thus, a small amount of posterior cell movement may occur over long periods of time under the conditions of our experiment, but only in the pylorus.
As a third test, we examined the frequency and stability of induced clones in the adult hindgut. Using the MARCM system, we measured the average number of clones present in 4-day old adult animals subjected to a heat shock, compared to control animals that were not heat shocked. In the hindgut, heat shock did not increase the frequency of clones above background, indicating an absence of ongoing cell division (Fig. 3K, Table S1). In contrast, the frequency of midgut clones increased more than sevenfold in these same animals (Fig. 3K). Taken together, the three lines of evidence presented above demonstrate that the adult hindgut, in contrast to the midgut, is not an actively cycling tissue and is not maintained by active stem cells.
Infrequent lacZ clonal labeling of adult hindgut cells
Although the BrdU and MARCM clone experiments demonstrated that the adult hindgut undergoes very little cell division, a small number of labeled clones were detected using the lacZ system. No labeled cells were found in controls, but following clone induction an average of 36 (of 1,600) individual pyloric hindgut cells expressed the lacZ marker (Fig. 3L, Table S1). The sporadic distribution of the labeled pyloric cells was reminiscent of the sporadic cells that incorporate BrdU in 4-day old adults. Like the BrdU-labeled cells, neither the number, size nor the position of these “clones” changed when the treated flies were analyzed over a subsequent three-week period (Fig. 3L, Fig. S2). We concluded that consistent with our previous studies, adult hindgut cells are not undergoing rapid turnover or replacement by the progeny of stem cells. However, both the BrdU data and the clone induction data might be explained if adult pyloric cells rarely undergo one further division sometime during adulthood.
FLP-mediated recombination between precisely aligned interchromosomal FRT sites (as in the lacZ system) is normally limited to cycling diploid cells (Harrison and Perrimon, 1993), but we also observed 34 (of 550) labeled polyploid 8C ileal cells using the lacZ system in adults one day after clone induction (Fig. 3L, Table S1). The level of lacZ expression in these adult-labeled ileal cells was noticeably weaker than adult ileal cells labeled during larval or pupal development. Growing polyploid cells such as ovarian nurse cells do not become labeled using the lacZ system, but the observed labeling must have occurred in the polyploid state because potential diploid progenitors are absent from the ileum and 24 hours is insufficient time for 8C cells to have developed from such a precursor. Site-specific recombination can take place in postmitotic cells (Ahmad and Golic, 1996) or cycling polyploid cells (Hong et al. 2007) when FRT sites lie in close proximity on the same chromosome. Perhaps homologous chromatids pair more closely in adult ileal cells than in other polyploid cell types, facilitating a low level of recombination that generates the weak lacZ labeling we observed.
The hindgut responds to tissue injury
Since we found no evidence for constitutively active adult hindgut stem cells, we investigated whether this tissue possesses inducible proliferative capacity. We generated a severe injury specifically in the hindgut by inducing apoptosis using UAS-hid, UAS-rpr driven by byn-GAL4. To control the timing and extent of damage, flies also expressed a temperature-sensitive GAL80 repressor to block transgene expression at the permissive temperature. When 4-day old adults of this genotype were shifted to the non-permissive temperature and examined 1-2 days later, cells with a nuclear morphology characteristic of apoptotic cells were observed (Fig. 4A). All animals maintained at the non-permissive temperature in this manner died within 14 days, however, if shifted back to the permissive temperature after 2 days, 80% survived. We concluded that this system allows a severe but non-lethal injury to be targeted to the hindgut.
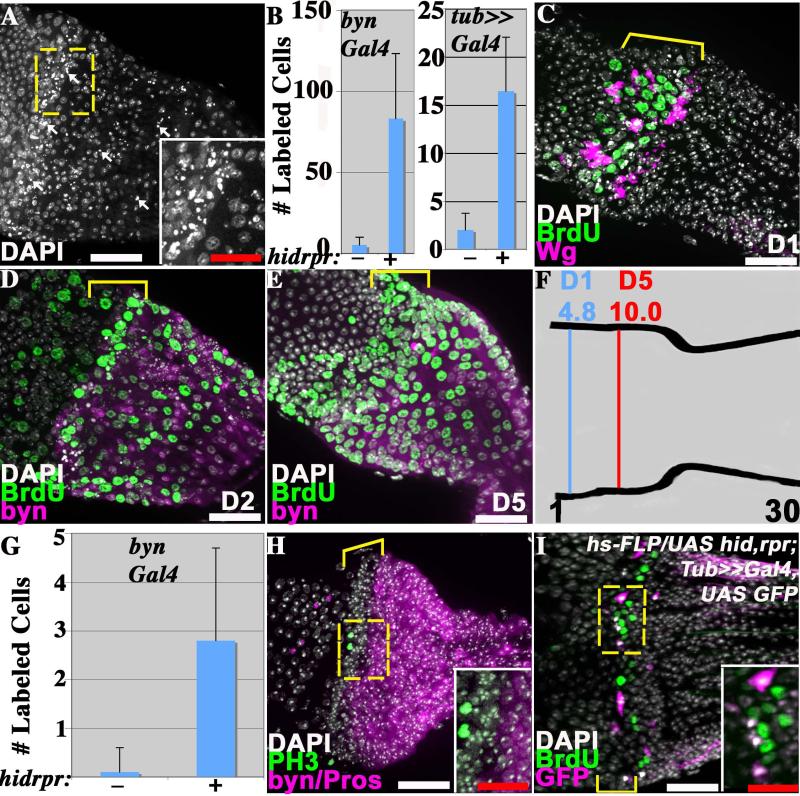
Figure 4. The pylorus proliferates in response to damage.
In A-H) adults of the genotype UAS hid rpr ; Gal80ts ; byn-GAL4 UAS-GFP were shifted to 30 °C to induce damage in the hindgut. A) Punctate nuclei of dying pyloric cells (arrows) 2 days after damage induction. B) The number of BrdU-labeled adult hindgut cells (average +/− standard deviation) is greatly increased following damage induction (+), relative to controls lacking hid and rpr expression (−). Note: A Tub-GAL4 FLP-out system was substituted for byn-GAL4 in one experiment (see I). C). BrdU incorporation occurs preferentially in the adult pyloric Wg ring (bracket) one day after damage induction. D) BrdU incorporation in the adult pylorus 2 days after damage induction is seen both in the Wg ring (bracket) and more posteriorly. E) Adult pyloric cells shifted to the non-permissive temperature and labeled with BrdU for one day (as in C) were examined after 4 additional days at 18 °C. Labeled cells are now seen throughout the pylorus. Bracket = Wg ring. F) The mean position of BrdU-labeled cells after 1 day of damage (D1, see panel C) and after 5 days (D5, see panel E) demonstrates cell movement. G) Cells positive for phosphohistone H3 (PH3) (average +/− standard deviation) are greatly increased in adult hindguts following damage induction (+), relative to controls lacking hid and rpr expression (−). H) Cell division marked by PH3 (green) two days after damage induction is confined to the Wg ring (bracket). I) BrdU incorporation 1 day after the induction of FLP-out clones marked with GFP (pink) and expressing hid and rpr (full genotype indicated on panel). BrdU incorporation (green) is observed within the Wg ring (bracket) in unrecombined cells adjacent to GFP-expressing cells (see inset). All images- anterior to the left. Dashed box = region shown in inset. In D, E and H, byn = GFP (purple); in I, GFP = UAS hid,rpr positive. Pros=Prospero, Wg = Wingless. Scale bars- white=25μm, red=12.5μm.
The hindgut might respond to tissue damage by activating quiescent stem cells or by stimulating differentiated tissue cells to divide. To look for induced cell cycling, we labeled hindguts with BrdU for 1 day beginning when 4-day old adults were shifted to the non-permissive temperature. The number of BrdU-positive cells increased more than tenfold compared to controls lacking UAS-hid UAS-rpr (Fig. 4B, C). The results did not depend on using UAS-hid UAS-rpr to induce damage because BrdU incorporation in adult hindguts also increased dramatically following hindgut-directed expression of the toxins DTI (Fig. S3A) or Ricin (Fig. S3B).
The spatial distribution of the cells responding to damage by BrdU incorporation was nonrandom. During the first day following a shift to the non-permissive temperature, adult hindgut cells located within the Wg-positive ring preferentially replicated (Fig. 4C) despite the fact that byn is only weakly expressed in these cells (Fig. 1E-E”). The same selective response of the Wg-positive cells was seen when damage was induced with DTI (Fig. S3A) or Ricin (Fig. S3B). BrdU fed during the second day after inducing apoptosis was incorporated in cells located throughout the pylorus (Fig. 4D). Thus, the ring of Wg-expressing cells responds first to damage, but BrdU-labeled cells are eventually found throughout the tissue. To examine whether the late-arising BrdU-labeled cells might originate in the Wg-region we labeled adults with BrdU for one day at the time of damage induction, and then shifted the flies to 18 °C for 4 days prior to analysis (Fig. 4E). BrdU-labeled cells were now found throughout the pylorus, and the mean position of labeled cells after the chase (D5) was 5.2 cell diameters more posterior than before the chase (D1) (Fig. 4F). These observations suggested that new cells preferentially arise in the Wg region before migrating throughout the rest of the pylorus.
We analyzed the number of phosphohistone H3 (PH3)-positive cells before and after the temperature shift to test whether the increased BrdU-incorporation was associated with cell proliferation or for some other reason such as a repair response. PH3-positive cells were virtually undetectable before the shift to the non-permissive temperature. However, one day after the shift, the number of such cells increased dramatically, and they were found only within the Wg zone where early BrdU incorporating cells are located (Fig. 4G, H). Even on day two, PH3-positive cells were only observed in the ring. These results provide further evidence that cellular damage induces both DNA replication and mitotic division in adult hindgut cells located within the Wg-positive ring.
Finally, we examined whether these cells respond to internal damage, or to external signals that may be sent by apoptotic cells or in response to altered tissue structure. The strategy was to induce apoptosis in a limited subset of hindgut cells by generating FLP-out clones (Struhl and Basler, 1993), and then assaying whether damaged, undamaged or both types of cells could respond. UAS-hid UAS-rpr in these experiments was driven in cells undergoing recombination by a tubulin promoter and hence damage was not limited to byn-expressing cells. Surprisingly, FLP-out clones expressing UAS-hid, UAS-rpr were only observed in the Wg-ring region (Fig. 4I, see also Fig. S3D). This suggests that Wg-positive pyloric cells are in a cell cycle state more susceptible to recombination than other diploid pyloric cells. One day after inducing clonal damage, BrdU incorporation had increased greatly in the hindgut (Fig. 4B). Strikingly, BrdU was not incorporated into the damaged cells, but into neighboring Wg-ring cells usually located only 1 or 2 cells away from a damaged cell (Fig. 4I). These observations show that the Drosophila hindgut contains a system of inducible damage response that causes normally quiescent cells within the Wg-ring to proliferate in response to local tissue damage.
DISCUSSION
The adult Drosophila hindgut lacks active stem cells
Our results call into question the recent report that the Drosophila adult hindgut is maintained by a “proliferation zone” corresponding to the Wg-positive ring and cells lying to its posterior within the adult pylorus (Takashima et al., 2008). The experiments reported here clearly demonstrate that there are no active stem cells, regular cell division or non-random cell movement in the Drosophila hindgut in the absence of damage. We also found that the most dramatic evidence favoring the proliferation zone model, namely the long clones spanning much of the adult hindgut from the pylorus through the ileum, arise due to low level spontaneous background recombination during embryogenesis when using the MARCM system, but not the lacZ system. Therefore, the Drosophila hindgut does not correspond to a giant intestinal crypt as previously suggested (Takashima et al., 2008; Pitsouli and Perrimon, 2008; Casali and Batlle, 2009).
The larval anterior pylorus (“hindgut imaginal ring”) develops by uniform cell proliferation, not from a proliferation zone
We also find no evidence of an anterior proliferation zone during larval hindgut development. Clones induced during larval development are elongated transversely rather than along the anterior-posterior axis, and frequently do not extend to the anterior margin of the larval pylorus, as would be predicted. Furthermore, our clonal studies of larval and pupal hindgut development revealed no evidence that the precursors of the adult pylorus and ileum cells share a common progenitor after embryogenesis, and showed definitively that they do not derive from a common stem cell during or after the third instar.
Instead, our observations show that progenitors of the adult hindgut expand uniformly within the larval pylorus, like they would in an imaginal disc. All cells in the imaginal ring appear to proliferate at about the same rate, and by the end of the third instar the number of adult hindgut precursors is close to the total cell number in the imaginal ring, suggesting that most late larval pyloric cells are adult progenitors. Consequently, we favor a simple model in which the larval progenitors of the adult pylorus lie anterior to those of the adult ileum, while the 60 posterior-most larval pyloric cells contribute to the junction with the rectum. Consistent with this, cells in the posterior half of the imaginal ring express a Notch reporter, and these may correspond to the ileal precursor population. A similar population was not observed in the adult ring.
The hindgut may undergo inducible repair like several vertebrate endodermal tissues
What then, is the role of the larval and adult rings? Both contain Wg-positive zones of similar size (about 150 cells) and organization (2-3 rows), followed by byn-positive cells. Takashima et al. (2008) documented additional organized patterns of gene expression posterior to the Wg rings. Our experiments argue strongly that the adult ring participates in facultative tissue repair. When damage was induced throughout most of the adult hindgut using byn-GAL4, cells within the Wg-positive ring of the pylorus responded preferentially by incorporating BrdU and dividing mitotically. We propose that these cells function as repair stem cells. The Wg-positive cells in the larval pylorus may serve a similar function. The pylorus is thought to serve as a valve regulating the passage of materials into the hindgut. This function might subject the region to chemical damage and mechanical stress, hence a system of inducible repair may exist to preserve pyloric integrity throughout the normal lifespan.
In contrast to the cellular stability of the normal adult hindgut, our results suggest that pyloric cells migrate away from the Wg-positive zone after the hindgut is damaged. BrdU-labeled cells initially appear within the Wg-expressing zone of the adult pylorus, and PH3-positive cells were only seen in this region. Within 24 hours, however, BrdU-labeled cells can be found throughout the pylorus where they may begin to replace dying cells and repair damage caused by the apoptotic inducer. Detailed linage analysis will be required to determine if cells in the Wg-zone undergo self-renewing divisions or divide equally as part of their response to tissue damage.
Many vertebrate tissues do not normally undergo rapid cell turnover and replacement from active stem cells. However, these tissues nonetheless show considerable ability to repair and regenerate under special circumstances. For example, breast structures regenerate extensively during pregnancy (Vaillant et al., 2007) and lung stem cells support tissue repair following injury or viral infection (Rawlins and Hogan, 2006). Several endodermal tissues, including the pancreas and liver, exhibit low cell turnover (Teta et al., 2005; Fausto and Campbell, 2003) and potentially lack active stem cells (Overturf et al., 1999; Dor et al., 2004). However, following severe damage, these tissues elicit a proliferation response that may involve re-activation of developmental cues (Fausto and Campbell, 2003; Rawlins and Hogan, 2006; Xu et al., 2008). There are excellent Drosophila models of constitutively active stem cells, and the biology of some of their lineages changes under stress caused by ingestion of toxic molecules or pathogens (Amcheslavsky et al., 2009; Buchon et al., 2009). However, there is currently no tissue in which to model inducible repair activity and study its dependence on quiescent stem cells.
We propose that the Drosophila hindgut represents such a tissue. Intriguingly, the hindgut may respond differently to differing levels of damage, like the vertebrate liver (reviewed in Zaret and Grompe, 2008). Low levels of sporadic cell loss may be compensated by the ability of a neighboring differentiated adult pyloric cell to re-enter the cell cycle and divide to produce a replacement. Such ongoing replacement division might explain at least some of the sporadic BrdU incorporation and lacZ clonal labeling of single cells that we observed in adults. In contrast, when faced with severe damage, such as the widespread cell death induced in our experiments, quiescent stem cells in the Wg-ring may be activated leading to a much more robust repair capability. The experiments reported here show that the hindgut, although lacking in constitutively active stem cells, represents a promising model for understanding novel and critically important aspects of endodermal tissue maintenance.
EXPERIMENTAL PROCEDURES
Fly work
For all experiments with adults, flies were fed fresh yeast paste every 1-2 days. For BrdU labeling, the yeast paste was supplemented with 5mg/ml BrdU (Invitrogen) as indicated for each experiment. To enrich for Phospho-H3 labeling, flies were fed yeast paste with 0.2 mg/ml colchicine (Sigma) for 12 hours. Embryos and 1st /2nd instar larvae were collected on molasses plates supplemented with yeast paste. Genotypes: wild-type- y w (Bloomington Stock Center), lacZ lineage labeling- hsFLP ; X15-29 / X15-33(Harrison and Perrimon, 1993), MARCM lineage labeling- hsFLP ; FRT42D Gal80 / FRT42D (Lee and Luo, 2001); Tub-Gal4/ UAS-GFP, FLP-out system: hsFLP; TubFRT>CD2>FRTGal4, UAS-GFP(Struhl and Basler, 1993). The following stocks were generous gifts: brachyenteron (byn)-Gal4 (Shigeo Takashima, UCLA); UAS-hid, UAS-reaper (rpr) (Zhou et al., 1997, Toshie Kai, Temasek Laboratory); UAS-Ricin (Hidalgo et al., 1995) and UAS-DTI (Han et al., 2000, both from Hugo Bellen, Baylor University).
Lineage analysis and transgene expression
Animals at various stages from embryo to adult bearing either a LacZ or MARCM marking system were heat-shocked once for either 30 min (LacZ, adult) or 60 min (all other experiments) in a 37°C water bath. After heat-shock flies were maintained at 22°C for the indicated number of days. 3rd instar larvae were heat shocked during the wandering stage. Pupae were collected as white prepupae, and the time collected was noted as hour zero. All adult flies were aged 4-7 days prior to heat-shock. This was done to avoid confusing adult homeostasis with the final divisions of hindgut development, which we observed were not always completed until 1-2 days of adulthood. For FLP-out experiments, 4-7 day adult flies were heat-shocked 3-4 times for 40 minutes within a 48-hour period. GAL80ts byn Gal4-driven UAS-hid UAS-rpr, UAS-DTI, or UAS-Ricin expression was induced by placing the flies in a 30°C incubator after adults had been aged for 8-14 days at 18°C. Counts of clone number or size are expressed as mean +/− the standard deviation.
Immunostaining/microscopy
Larval and adult hindguts were dissected in buffer as described by Ohlstein and Spradling (2006), then fixed in 3.7% formaldehyde + 0.3% Triton-X for 15 minutes. Antibody staining was performed in 0.3% Triton-X and 10% normal goat serum. Antibodies used: anti-Wg (mouse, Developmental Studies Hybridoma Bank (DSHB) concentrated supernatant, 1:500), anti-GFP (mouse, Invitrogen, 1:2000), anti-BrdU (Rat, Serotec, 1:100), anti-Beta Galactosidase (Chicken, Abcam, 1:2000), anti-Phospho-H3 Ser10 (Mouse or Rabbit, Cell Signaling, 1:2000), anti-Prospero (DSHB, 1:50). DAPI staining was at 5μg/ml. For BrdU staining, samples were denatured for 1 hour in 200U/ml DNAseI. Alternatively, for lacZ analysis animals were stained with X-Gal (Margolis and Spradling, 1995) as this procedure improved the sensitivity within the adult ileum. All immunostained images were captured on a Zeiss Axioimager ZI equipped with an Apotome system, using 20X or 40X objectives. DNA content was measured using the “Measure” feature in ImageJ software and comparing images captured with constant gain settings.
Calculation of the mean position of BrdU-labeled cells (Fig. 3F, 4F)
The distribution of each BrdU positive cell, either before or after BrdU pulse, was measured in units of cell diameter from the anterior- most position in the pylorus or ileum, defined as the junction with the midgut for the pylorus and as the first polyploid cell adjacent to the pylorus for the ileum. The pylorus is approximately 30 cell diameters long and the ileum approximately 60 cell diameters. The average position of the labeled cells was calculated for each time point using these units.
Calculation of the mean lifetime of larval imaginal ring cells
The elapsed time between L1 clone induction and L3 clone analysis was 3.5 days or 84 hours, and the mean clone size was 20. During this time there were log220 = 4.3 divisions plus 1 additional division since only a single labeled cell usually emerges from the first mitosis in the lacZ system. Therefore, the mean division time = 84 hours/5.3 divisions = 16 hours.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ben Ohlstein for discussions, and Shigeo Takashima, Toshie Kai, Hugo Bellen, and Jessica Treisman for stocks. Joe Gall, Lucy Morris, and Todd Nystul provided useful comments on the manuscript. Don Fox is a fellow of the Jane Coffin Childs Memorial Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
The Supplemental DATA comprise 3 Figures and 1 Table. These materials can be found with this article online at http://www.cell.com/cell-stem-cell/supplemental/S ????
REFERENCES
- Ahmad K, Golic K. Somatic reversion of chromosomal position effects in Drosophila melanogaster. Genetics. 1996;144:657–660. doi: 10.1093/genetics/144.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell host & microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Casali A, Batlle E. Intestinal Stem Cells in Mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mechanisms of development. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Fox DT, Morris LX, Nystul T, Spradling AC. Stembook. The Stem Cell Research Community; 2009. Lineage Analysis of Stem Cells. [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Han DD, Stein D, Stevens LM. Investigating the function of follicular subpopulations during Drosophila oogenesis through hormone-dependent enhancer-targeted cell ablation. Development. 2000;127:573–583. doi: 10.1242/dev.127.3.573. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Urban J, Brand AH. Targeted ablation of glia disrupts axon tract formation in the Drosophila CNS. Development. 1995;121:3703–3712. doi: 10.1242/dev.121.11.3703. [DOI] [PubMed] [Google Scholar]
- Hong A, Narbonne-Reveau K, Riesgo-Escovar J, Fu H, Aladjem M, Lilly M. The cyclin-dependent kinase inhibitor Decapo promotes replication licensing during Drosophila endocycles. EMBO J. 2007;26:2071–82. doi: 10.1038/sj.emboj.7601648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends in neurosciences. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lengyel JA, Iwaki DD. It takes guts: the Drosophila hindgut as a model system for organogenesis. Developmental biology. 2002;243:1–19. doi: 10.1006/dbio.2002.0577. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Finegold M, Grompe M. The repopulation potential of hepatocyte populations differing in size and prior mitotic expansion. The American journal of pathology. 1999;155:2135–2143. doi: 10.1016/S0002-9440(10)65531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsouli C, Perrimon N. Developmental biology: Our fly cousins’ gut. Nature. 2008;454:592–593. doi: 10.1038/454592a. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Robertson C. Metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J Morphol. 1936;59:351–399. [Google Scholar]
- Snodgrass R. In Principles of Insect Morphology. Mc-Graw Hill; New York: 1935. pp. 347–388. [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Lindeman GJ, Visvader JE. The emerging picture of the mouse mammary stem cell. Stem cell reviews. 2007;3:114–123. doi: 10.1007/s12015-007-0018-2. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. PNAS. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.