Abstract
Fusion of enveloped viruses with host cells is triggered by either receptor binding or low pH but rarely requires both except for avian sarcoma leukosis virus (ASLV). We recently reported that membrane fusion mediated by an oncogenic Jaagsiekte sheep retrovirus (JSRV) envelope (Env) requires an acidic pH, yet receptor overexpression is required for this process to occur. Here we show that a soluble form of the JSRV receptor, sHyal2, promoted JSRV Env-mediated fusion at a low pH in normally fusion-negative cells and that this effect was blocked by a synthetic peptide analogous to the C-terminal heptad repeat of JSRV Env. In contrast to the receptor of ASLV, sHyal2 induced pronounced shedding of the JSRV surface subunit, as well as unstable conformational rearrangement of its transmembrane (TM) subunit, yet full activation of JSRV Env fusogenicity, associated with strong TM oligomerization, required both sHyal2 and low pH. Consistently, sHyal2 enabled transduction of nonpermissive cells by JSRV Env pseudovirions, with low efficiency, but substantially blocked viral entry into permissive cells at both binding and postbinding steps, indicating that sHyal2 prematurely activates JSRV Env-mediated fusion. Altogether, our study supports a model that receptor priming promotes fusion activation of JSRV Env at a low pH, and that the underlying mechanism is likely to be different from that of ASLV. Thus, JSRV may provide a useful alternate model for the better understanding of virus fusion and cell entry.
Fusion is a fundamental event in the life cycle of enveloped viruses and is essential for viral replication. While viral fusion proteins are highly divergent in primary sequence, their structures and modes of activation share striking similarities, permitting their classification into two major groups (41). Class I fusion proteins, as exemplified by the retrovirus envelope (Env) and influenza virus hemagglutinin (HA), are composed mainly of alpha-helices, and they are present as metastable trimers on the viral surface (11). Class II fusion proteins, represented by alphavirus E1 and flavivirus E, contain predominantly beta-sheets and exist as dimers in the prefusion state (16). Of note, the vesicular stomatitis virus G (VSV-G) and herpesvirus gB proteins were recently assigned to a newly established class III, for fusion proteins combining properties of both class I and class II (13, 30). Despite these differences, one common and intriguing characteristic of all viral fusion proteins is their ability to undergo dramatic conformational rearrangements upon activation, i.e., the formation of trimers of hairpins, which drive fusion between viral and cellular membranes (11, 17).
Retrovirus Env is a typical type I transmembrane protein composed of surface (SU) and transmembrane (TM) subunits and belongs to the class I fusion proteins. SU is responsible for binding to cognate cellular receptors or cofactors, while TM directly mediates membrane fusion (6). Most retroviruses use a pH-independent pathway for entry, during which receptor binding relieves the ability of SU to restrain TM, resulting in conformational changes in TM and subsequent fusion with the cell membrane (11). Interestingly, increasing numbers of retroviruses have recently been shown to require a low pH (3, 15, 24, 28, 31) or pH-dependent protease activities to trigger fusion (18); the latter property has also been demonstrated for some other enveloped viruses (2, 14, 18, 26, 27, 33, 34). Among these, avian sarcoma leukosis virus (ASLV) is unique in that it uses a two-step mechanism for fusion, in which receptor binding primes the second trigger of low pH (24).
Jaagsiekte sheep retrovirus (JSRV) is a simple betaretrovirus etiologically responsible for contagious lung tumors in sheep (12). The native Env protein of JSRV functions as a potent oncogene that induces cell transformation in vitro and in animals (4, 9, 21, 29, 42). The cell entry receptor for JSRV has been identified as hyaluronidase 2 (Hyal2), a glycosylphosphatidylinositol (GPI)-anchored protein belonging to the hyaluronidase family (29); Hyal2 itself has low hyaluronidase activity, and this activity is not associated with JSRV entry and infection (38). Intrigued by the oncogenic nature of JSRV Env, we recently examined the mechanism of JSRV entry and found that JSRV Env-mediated fusion and cell entry require a low pH (1, 8). These observations led us to hypothesize that the pH-dependent fusion activation of JSRV Env may be advantageous for its oncogenesis, given that extreme cell-cell fusion of the plasma membrane at a neutral pH would result in syncytium formation and often cell death. Curiously, we noticed that overexpression of Hyal2 is necessary for JSRV Env to induce membrane fusion at a low pH in vitro, suggesting that Hyal2 may play an active role in the pH-dependent fusion process. Here we provide direct evidence that Hyal2 functions in cooperation with a low pH to trigger the JSRV Env-mediated fusion activation yet exhibits some striking differences from the mechanism of ASLV fusion. The multistep pathway for JSRV Env-mediated fusion activation might be important for its replication fitness and oncogenesis.
MATERIALS AND METHODS
Cell lines, viruses, and reagents.
All mammalian cells used in this study were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum using standard procedures. Drosophila Schneider 2 (S2) cells were grown at 27°C in Express Five SFM medium (Invitrogen, Carlsbad, CA) supplemented with 2 mM l-glutamine (Invitrogen). Moloney murine leukemia virus (MoMLV) pseudotypes bearing JSRV Env (with a FLAG tag at both the N and C termini [termed F-Jenv-F] [8] or at the N terminus only [called F-Jenv] [20]), human immunodeficiency virus type 1 (HIV-1) Env, or VSV-G were produced as previously described (8). Note that the cytoplasmic tail of the HIV-1 Env (strain ADA) used here is deleted (a kind gift of Eric Cohen, Institut de Recherches Cliniques de Montréal, Montreal, Canada) so that it can pseudotype the MoMLV retroviral vector. All primary and secondary antibodies were purchased from Sigma (St. Louis, MO), except for the mixture of B3 and C9 monoclonal antibodies against JSRV SU (43), which was kindly provided by Dusty Miller. The peptides corresponding to the JSRV N-terminal heptad repeat (N-HR1) (DKKIEDRLSALYDVVRVLGE) and C-terminal heptad repeat (C-HR2) (FNTNLSLDLLQLHNEILDIENS) were synthesized by Alpha Diagnostic International (San Antonio, TX), and were solubilized in water and reconstituted in 14% dimethyl sulfoxide (C-HR2 is insoluble in water), respectively.
sHyal2 production and purification.
Drosophila S2 cells stably expressing soluble Hyal2 (sHyal2) (39) (a kind gift of Vladimir Vigdorovich and Dusty Miller) were induced by 1 mM CuSO4 for 5 to 7 days at 27°C; the culture medium was harvested; and sHyal2 protein was purified using nickel-nitrilotriacetic acid columns (Qiagen, Valencia, CA). The purity of sHyal2 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and it was quantified by Bradford assays.
Cell surface staining.
Cell surface staining was performed by flow cytometry as previously described (19), except that an anti-FLAG antibody, or 0.5 μg of sHyal2 followed by an anti-His antibody, was incubated with 293 cells expressing FLAG-tagged JSRV Env.
Syncytium induction assay.
Syncytium induction assays were performed as described previously (8), with minor modifications. Briefly, 293 cells were cotransfected with plasmids encoding JSRV Env and pCMV-GFP; 24 h posttransfection, cells were incubated with different amounts of sHyal2 for 1 h at 4°C, followed by incubation at 37°C for 30 min. Cells were then treated with prewarmed pH 7.4 or pH 5.0 buffer for 5 min and were incubated for 1 h at 37°C before being examined for fusion and photographed. For syncytium induction involving heptad repeat peptides, transfected cells were incubated with a peptide or sHyal2 for 1 h at 4°C, followed by incubation at 37°C for 30 min; cells were then incubated with fresh medium containing either sHyal2 or the peptide at 4°C for 1 h, followed by recovery at 37°C for 30 min. The sHyal2- or peptide-treated cells were incubated with a pH 5.0 buffer for 5 min and were then analyzed for fusion using fluorescence microscopy.
Metabolic labeling.
Metabolic labeling was performed as described previously (8), except that different amounts of sHyal2 were incubated with 293 cells during the last 3 h of a 6-h chase period. If needed, a pH 5.0 pulse was subsequently applied for 5 min. Cell lysates and culture media containing the [35S]Met-Cys-labeled JSRV Env were harvested, neutralized with 0.1 M Tris (pH 7.5) if necessary (pH 5.0 treated), and immunoprecipitated using anti-FLAG beads. Samples were boiled in a buffer containing 1% SDS and 1% β-mercaptoethanol for 10 min, resolved by 10% SDS-PAGE, and analyzed by autoradiography.
Oligomerization assay.
JSRV Env pseudovirions were pelleted by centrifugation for 3 h at 185,000 × g on a 1.5-ml 20% sucrose cushion and were resuspended in phosphate-buffered saline (PBS). Purified pseudovirions were incubated with appropriate concentrations of sHyal2 or equal volumes of PBS on ice for 30 min, followed by incubation at 37°C for 30 min. The complex was then treated with a pH 7.4 or pH 5.0 buffer (acidified with a predetermined volume of 0.1 N HCl) for 5 min at 37°C and was neutralized with 0.1 N NaOH if necessary. Unless otherwise stated, the complex was cross-linked by 25 μM dithiobis[succinimidylpropionate] (DSP) (Pierce, Rockford, IL) for 30 min at room temperature, and the remaining reactive DSP was quenched by ∼0.2 M Tris (pH 8.0). For oligomerization assays involving different temperatures, the virion-sHyal2 complex was incubated for 5 min at different temperatures before being cross-linked with 25 μM DSP. To assess the stability of the oligomers, the virion-sHyal2 complex, treated with either a pH 7.4 or a pH 5.0 buffer, was incubated with different concentrations of SDS or urea for 5 min at 37°C. All samples, unless otherwise specified, were incubated in a nonreducing sample buffer containing 0.1% SDS for 5 min at 37°C before being resolved by 7.5% SDS-PAGE, followed by Western blotting using an anti-FLAG or anti-SU antibody, as previously described (20).
Infection.
For infection of nonpermissive cells, green fluorescent protein (GFP)-encoding MoMLV pseudoparticles bearing JSRV Env, HIV-1 Env, or VSV-G were bound to HeLa cells in Dulbecco's modified Eagle medium containing 5 μg/ml Polybrene by spinoculation at 1,680 × g for 2 h at 4°C. Appropriate amounts of sHyal2 were incubated with the cells for 48 h before the cells were analyzed by flow cytometry. To assess the effect of sHyal2 on permissive cells, HTX cells were prebound by virions in a medium containing Polybrene for 1 h at 4°C; cells were washed to remove unbound virions and were then incubated with sHyal2 for 1 h at 4°C. Alternatively, pseudovirions were incubated with appropriate amounts of sHyal2 on ice for 30 min, and the virus-sHyal2 complex was added to HTX cells for binding at 4°C for 2 h, followed by washes to remove unbound virus. In both cases, cells were incubated for 48 h before analysis using flow cytometry to measure the percentages of GFP-positive cells. The relative rate of infection was calculated by using cells that had not been treated with sHyal2 as a reference.
RESULTS
sHyal2 mediates syncytium induction by JSRV Env at a low pH.
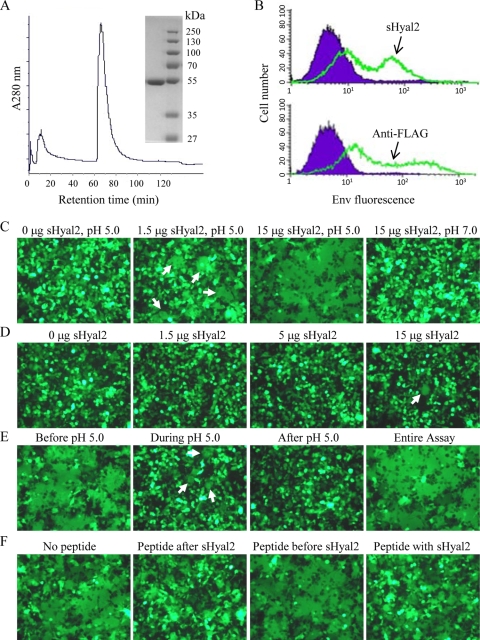
To address the possible role of Hyal2 in JSRV Env-mediated fusion, we produced a soluble form of Hyal2 (sHyal2) using a Drosophila S2 cell line that stably expresses sHyal2 (kindly provided by Vladimir Vigdorovich and Dusty Miller). In this system, the GPI anchor of Hyal2 was replaced by a histidine tag, and the expressed proteins were secreted into the cell culture medium. We used nickel-nitrilotriacetic acid columns to isolate sHyal2 to a high purity (>95%) (Fig. 1A), and its ability to interact with JSRV Env was determined by flow cytometry, which demonstrated specific binding of sHyal2 to 293 cells expressing FLAG-tagged JSRV Env (F-Jenv-F) (Fig. 1B).
FIG. 1.
sHyal2 mediates syncytium formation by JSRV Env in 293 cells at low pH, the effect of which is inhibited by a synthetic C-HR2 peptide. (A) Size exclusion chromatography and SDS-PAGE of sHyal2. sHyal2 (∼50 kDa) was purified by fast protein liquid chromatography, and the purified sHyal2 was analyzed by SDS-PAGE and Coomassie blue staining. (B) sHyal2 specifically binds to 293 cells expressing a FLAG-tagged JSRV Env (top); expression of the latter on the cell surface was confirmed by an anti-FLAG antibody (bottom). (C) Syncytium formation by JSRV Env in 293 cells (expressing GFP) is induced only by sHyal2 plus pH 5.0 (central panels), but not by either one alone (left and right panels). (D) A JSRV Env chimera, containing the ENTV SU and with reduced binding to Hyal2, requires an increased concentration of sHyal2 for fusion (see the text for details). (E) sHyal2 acts before but not after the pH 5.0 pulse for fusion induction. These experiments employed 5 μg sHyal2. (F) The JSRV C-HR2 peptide acts after sHyal2 incubation and before a low-pH pulse to inhibit fusion. The assays employed 5 μg sHyal2 and 30 μg C-HR2 peptide, respectively. Arrows are placed to indicate the fused cells only when fusion is not obvious.
We first examined whether sHyal2 could induce membrane fusion of JSRV Env in 293 cells at a low pH. In the absence of sHyal2, no syncytia were observed (left panel, Fig. 1C), as we previously reported (8). However, preincubation of the Env-expressing 293 cells with 0.5 to 15 μg/ml of sHyal2 for 1 h, followed by a pH 5.0 pulse for 5 min, led to syncytium formation, albeit to different extents (central panels, Fig. 1C; also data not shown). The concentration of sHyal2 required to induce syncytia under these conditions (pH 5.0 for 5 min) was ∼1 μg/ml, and no further increase in fusion was observed when more than 5 μg/ml sHyal2 was applied. In contrast, cells preincubated with 15 μg/ml of sHyal2, followed by a neutral-pH pulse, showed no apparent fusion (right panel, Fig. 1C). These results indicate that both sHyal2 and a low pH are required for syncytium induction by JSRV Env.
To show that the effect of sHyal2 on fusion takes place through JSRV SU, we performed a similar syncytium formation assay using a JSRV Env chimera that contains the SU from a closely related enzootic nasal tumor virus (ENTV) (referred to as EJ in reference 7) and shows reduced binding to Hyal2 (7). Indeed, this chimeric Env required a much increased concentration of sHyal2 (>15 μg/ml) for syncytium induction, and the percentage and size of syncytia were also much lower than those of JSRV Env (Fig. 1D). The reduced fusogenicity of this chimera was not a result of lower Env expression; it exhibited slightly greater Env expression than did wild-type Env (7). Together, these results demonstrate that Hyal2 is required to induce fusion of JSRV Env at a low pH, most likely by acting through its interaction with the JSRV SU.
Membrane fusion mediated by sHyal2 at a low pH is blocked by a C-heptad repeat peptide of JSRV Env.
We next sought to determine the step(s) of the fusion process at which Hyal2 is required for syncytium formation by JSRV Env in 293 cells. To this end, we incubated Env-expressing 293 cells with sHyal2 before, during, or after pH 5.0 treatment and examined the effects of sHyal2 and low pH on Env-mediated fusion induction. Incubation of sHyal2 after or during low-pH treatment induced no fusion or mild fusion (Fig. 1E, central panels), whereas incubation of sHyal2 prior to low-pH treatment, or inclusion of sHyal2 in the entire fusion assay, led to robust syncytium formation with an increased number and size of syncytia (Fig. 1E, left and right panels). Importantly, a synthetic peptide analogous to JSRV Env C-HR2, but not to N-HR1 (data not shown), substantially blocked syncytium formation (Fig. 1F). Of note, the effect of JSRV C-HR2 on Env-mediated fusion was most pronounced when the peptide was added between incubation with sHyal2 and the low-pH pulse, not before sHyal2 incubation (Fig. 1F), suggesting that the C-HR2 peptide acts after receptor binding but before low-pH triggering.
To test if a low pH could modulate the requirement of sHyal2 for fusion, we preincubated 293 cells with different amounts of sHyal2, followed by treatment with different low-pH conditions for 5 min. We observed that, although 1 μg/ml of sHyal2 induced visible syncytia at pH 4.0, 5 μg of sHyal2 was necessary to trigger discernible fusion at pH 5.0, and even 10 μg or more of sHyal2 barely triggered fusion at pH 6.0 (data not shown). These results, together with the effect of C-HR2 on syncytium formation shown above (Fig. 1F), suggest that sHyal2 functions in cooperation with a low pH to trigger JSRV Env-mediated fusion, likely by inducing one or more fusion intermediates that are sensitive to binding by the JSRV C-HR2 peptide.
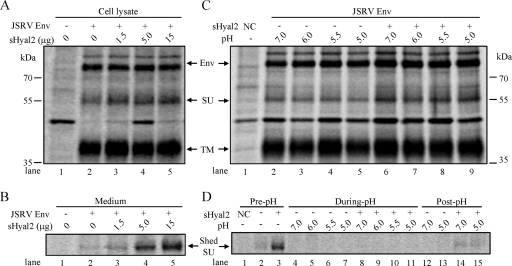
sHyal2 promotes shedding of JSRV SU in cells expressing JSRV Env.
SU shedding is regarded as an important, though not necessary, feature of retrovirus fusion and infection (11). To address the possible effects of sHyal2 on JSRV SU shedding, Env-expressing 293 cells were pulse-chase labeled with [35S]Met-Cys in the presence of different amounts of sHyal2. While sHyal2 had no effect on JSRV Env levels over the 3-h chase period (Fig. 2A), as would be expected, the shedding of JSRV SU into the culture medium was clearly detected, in an sHyal2 dose-dependent manner (Fig. 2B). We also treated the labeled 293 cells with different pH conditions, i.e., pH 7.0, 6.0, 5.5, or 5.0, but observed no SU shedding in any of the pH-treated cells, either during the 5-min treatment interval (Fig. 2D, lanes 4 to 7), or in the 1-h recovery period (Fig. 2D, lanes 12 and 13). In the presence of sHyal2, while SU shedding was detected at pH 5.0 during the 1-h recovery period, its level was similar to that at pH 7.0 (Fig. 2D, compare lane 15 with lane 14). Again, as expected, different pH treatments had no effect on JSRV Env expression and processing (Fig. 2C). Collectively, these data demonstrate that sHyal2, but not low pH, promotes JSRV SU shedding in Env-expressing 293 cells, suggesting that sHyal2 may cause conformational changes in SU on the cell surface, resulting in a weakened association between SU and TM (see Discussion).
FIG. 2.
sHyal2, but not low pH, promotes shedding of JSRV SU. (A and B) 293 cells expressing JSRV Env were metabolically labeled for 1 h and then chased for 3 h before the addition of the indicated amounts of sHyal2. Cells were chased for an additional 3 h before being lysed. Cell lysates and culture media were harvested and immunoprecipitated using anti-FLAG beads. Samples were resolved by SDS-PAGE and analyzed by autoradiography. (A) Env expression and processing in cell lysates. (B) JSRV SU shedding into the culture medium. (C and D) Metabolic labeling was performed as described for panels A and B, except that cells were incubated in the absence or presence of 5 μg sHyal2. The culture medium was harvested (pre-pH), and the cells were treated with the indicated pH buffers for 5 min at 37°C. The pH buffers were harvested and neutralized (during pH), and the cells were cultured for an additional 1 h. The culture medium was harvested (post-pH), and the cells were lysed. The cell lysates (C) and harvested supernatants or neutralized pH buffers (D) were immunoprecipitated using anti-FLAG beads and were analyzed by autoradiography. Env, JSRV Env precursor; NC, parental 293 cells not expressing JSRV Env. The strong band appearing in the parental 293 cells (panels A and C, lanes 1), and sometimes in the Env-expressing 293 cells, is likely a cellular protein that was nonspecifically pulled down by anti-FLAG beads.
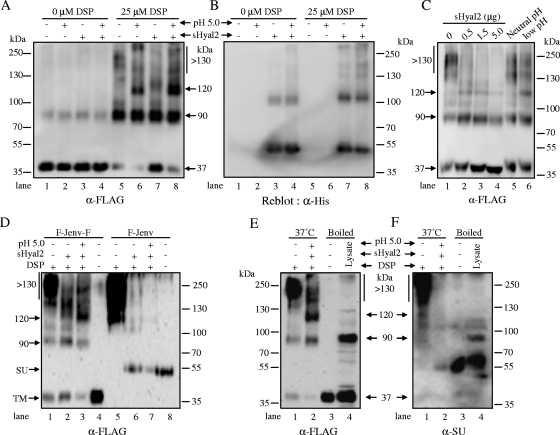
sHyal2 and a low pH induce conformational refolding of TM in JSRV Env-pseudotyped retroviral particles.
To acquire biochemical evidence for the role of Hyal2 in JSRV Env-mediated fusion activation, we developed an oligomerization assay and assessed possible conformational changes of JSRV TM induced by sHyal2, a low pH, or both. In the absence of the cross-linker, DSP, JSRV TM exists predominantly as a monomer of ∼37 kDa, although a minor species of ∼90 kDa, possibly a TM dimer, was also detected in all samples (Fig. 3A, lanes 1 to 4). In the presence of DSP, in addition to the enhanced 90-kDa form, a ∼120-kDa species was apparently induced by a low pH (Fig. 3A, lane 6), and its intensity was substantially enhanced by preincubation with sHyal2 (Fig. 3A, lane 8). Interestingly, sHyal2 alone also appeared to induce a slight formation of the ∼120-kDa species (Fig. 3A, lane 7; note the very faint band underneath the smeared bands), as well as to enhance the 37-kDa monomers as opposed to the larger, high-molecular-weight (HMW) species (>130 kDa) (Fig. 3A, compare lane 7 with lane 5). The latter observations were further confirmed by an independent experiment showing that sHyal2 induces the formation of 120-kDa species, increases the proportions of TM monomers, and reduces those of HMW species (Fig. 3C, lanes 1 to 4).
FIG. 3.
JSRV TM oligomerization induced by sHyal2 and low pH. (A) Purified JSRV Env pseudovirions were incubated with or without 1.5 μg of sHyal2, followed by treatment with a pH 7.4 or pH 5.0 buffer. Samples were either cross-linked by 25 μM DSP or left untreated, and TM oligomerization was analyzed by Western blotting using an anti-FLAG (α-FLAG) antibody. (B) The membrane was then stripped and reblotted using an anti-His (α-His) tag antibody to detect sHyal2. (C) Effect of sHyal2 on TM oligomerization. Purified virions were incubated with the indicated amounts of sHyal2 or were treated with a neutral or a low pH, which served as negative and positive controls, respectively. Samples were cross-linked and analyzed by Western blotting. (D) Purified JSRV pseudovirions bearing F-Jenv-F or F-Jenv were either treated with 1.5 μg sHyal2, alone or in combination with pH 5.0, or left untreated. Samples were either cross-linked by DSP (lanes 1 to 3 and 5 to 7) or boiled without cross-linking (lanes 4 and 8). Note that fivefold more virus was used for F-Jenv than for F-Jenv-F, in order to enhance the detection of SU. (E and F) Purified pseudovirions bearing F-Jenv-F were either left untreated or treated with 1.5 μg sHyal2 plus pH 5.0; then they were cross-linked with DSP. The cross-linked virus samples (indicated as “37°C”) and boiled virions and cell lysates (“Boiled”) were resolved by SDS-PAGE and analyzed by Western blotting using an anti-FLAG antibody (E) or an anti-JSRV SU (α-SU) antibody (F).
Given that the JSRV pseudovirions used for the oligomerization assay harbor an Env that is FLAG tagged on both SU and TM (called F-Jenv-F), it is possible that the 120-kDa form induced by sHyal2 and a low pH also contains JSRV SU and/or sHyal2, in addition to TM. This is despite the fact that, for unknown reasons, the anti-FLAG antibody does not normally detect the FLAG-tagged JSRV SU by Western blotting (7, 20) yet functions efficiently for flow cytometry (Fig. 1B) and immunoprecipitation (Fig. 2). Reblotting of the polyvinylidene difluoride membrane that was used for Fig. 3A with an anti-His antibody (sHyal2 is tagged with His) revealed two major bands, i.e., ∼50- and ∼100-kDa species (likely corresponding to the monomer and dimer of sHyal2, respectively) (Fig. 3B), but no band corresponding to ∼120 kDa was observed, suggesting that the 120-kDa species observed in Fig. 3A does not contain sHyal2. To exclude the possibility that the 120-kDa species may contain JSRV SU, we performed an oligomerization assay using highly concentrated JSRV pseudovirions bearing an Env that is FLAG tagged only on the SU (F-Jenv) and compared its oligomerization patterns with those of F-Jenv-F. Again, no 120-kDa species was detected for the F-Jenv construct (Fig. 3D, lane 7), despite the loading of ∼5-fold more of this construct than of F-Jenv-F (Fig. 3D). Notably, a 55-kDa band was found in the samples treated with sHyal2 and in those treated with sHyal2 plus a low pH (Fig. 3D, lanes 6 and 7, respectively); this size is similar to that of boiled viral particles (Fig. 4D, lane 8), confirming that sHyal2 does promote JSRV SU shedding from the pseudovirions, as revealed in Fig. 3. The effect of sHyal2 on SU shedding, as well as the lack of SU in the 120-kDa species, was further confirmed by using an anti-SU antibody on the F-Jenv-F construct (Fig. 3F), although the signal for SU was very weak, even with prolonged exposure (Fig. 3F, lane 2). Again, it is evident that the anti-FLAG antibody was unable to detect JSRV SU by Western blotting, even from samples of boiled viral particles and cell lysates (compare Fig. 3E, lanes 3 and 4, with Fig. 3F, lanes 3 and 4). Taken together, these results strongly support the notion that the 120-kDa species is predominantly derived from JSRV TM and does not appear to contain JSRV SU or sHyal2. However, we cannot completely rule out the possibility that the anti-FLAG and/or anti-SU antibody used here may not recognize the JSRV SU possibly present in the 120-kDa form.
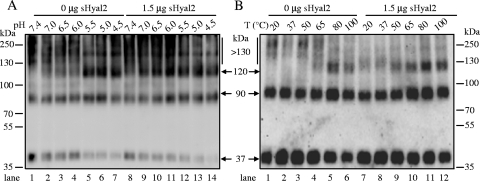
FIG. 4.
sHyal2 modulates the thresholds of low pH and temperature required for JSRV TM oligomerization. JSRV pseudovirions, preincubated with 1.5 μg sHyal2 or an equal volume of PBS, were treated with the indicated pH conditions (A) or temperatures (B) for 5 min, followed by cross-linking with DSP, and were analyzed by Western blotting using an anti-FLAG antibody.
sHyal2 modulates the threshold of low pH or heat required for TM oligomerization.
We next tested if sHyal2 could modulate the thresholds of low pH and/or high temperature required for TM oligomerization. In the absence of sHyal2, the pH threshold for inducing the ∼120-kDa species was ∼pH 5.5 (Fig. 4A, lane 5), but it shifted to ∼pH 6.5 or 7.0 in the presence of 1.5 μg of sHyal2 (Fig. 4A, lanes 9 and 10). These pH thresholds appeared to be relatively higher than those observed in the fusion assays (7), possibly because the biochemical oligomerization assay is more sensitive than the phenotype-based fusion assay. Similarly, the temperature threshold for detecting the ∼120-kDa species was reduced from 65°C to 50°C in the presence of sHyal2 (Fig. 4B, compare lane 9 with lane 4). Taken together, these data demonstrate that sHyal2 effectively reduces the energy barriers that must be overcome by low pH or heat in order to form the ∼120-kDa species. Our results also demonstrate that the JSRV Env protein in the mature MoMLV pseudoparticles is metastable, like those of influenza virus HA and ASLV Env (5, 22, 32, 37).
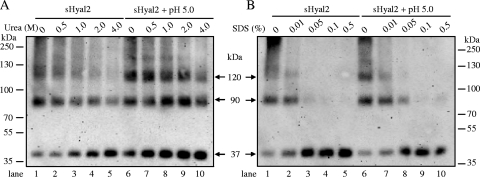
Stability of JSRV TM oligomers induced by sHyal2 and low pH.
The stability of JSRV TM oligomers, in particular the ∼120-kDa species, which was specifically induced by a low pH and/or sHyal2, was also assessed. TM oligomers induced by a combination of low pH and sHyal2 were stable in urea at concentrations up to 2 M (Fig. 5A, lanes 6 to 9), whereas sHyal2-induced oligomers were relatively unstable and disassociated even with 0.5 M urea (Fig. 5A, lanes 2 to 5). Interestingly, the ∼120-kDa species, induced either by a low pH plus sHyal2 or by sHyal2 alone (Fig. 5B, lanes 1 to 5), were sensitive to SDS treatment, even at very low concentrations (0.01% or higher). These results are in sharp contrast to those reported for ASLV, whose six-helix bundles (6-HBs) and other HMW species are virtually resistant to 1 to 2% SDS, even without cross-linking (22, 24). However, our data are similar to those for MoMLV, whose TM oligomers are also less stable in SDS (35).
FIG. 5.
Stability of TM oligomers induced by sHyal2 and low pH. JSRV Env pseudovirions, preincubated either with 1.5 μg sHyal2 alone or with 1.5 μg sHyal2 plus pH 5.0, were subjected to the indicated concentrations of urea (A) or SDS (B) for 5 min at 37°C before being cross-linked by DSP and analyzed by Western blotting.
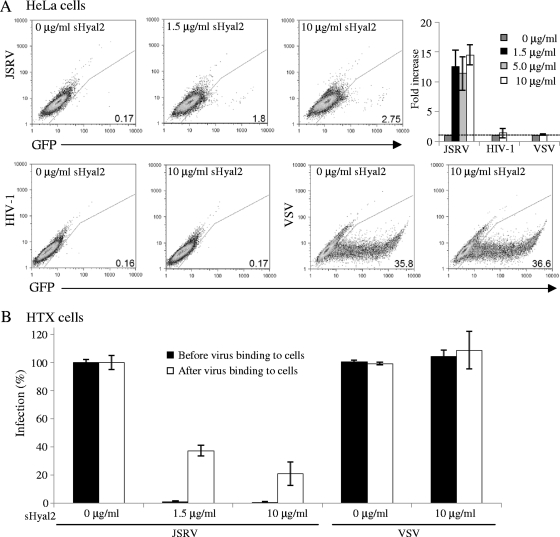
sHyal2 enables JSRV Env pseudovirions to transduce nonpermissive cells but blocks their entry into permissive cells.
The apparent role of sHyal2 in JSRV Env-mediated fusion prompted us to further test whether sHyal2 can induce JSRV vector transduction of nonpermissive cells. To this end, JSRV Env pseudovirions were allowed to bind to nonpermissive HeLa cells at 4°C for 2 h, and the virion-cell complexes were incubated with sHyal2 for 48 h before the cells were analyzed for infectivity by flow cytometry. As shown in Fig. 6A, sHyal2 enabled the transduction of HeLa cells by JSRV pseudovirions (albeit with low efficiency, ∼2 to 3%) but had no effect on that by HIV-1 Env (nonpermissive in HeLa cells; efficiency, ∼0.16 to 0.3%) or VSV-G (permissive in HeLa cells; efficiency, ∼35%). In contrast, sHyal2 markedly reduced the transduction efficiency of JSRV-permissive HTX cells, which were prebound with JSRV pseudovirion particles, in a dose-dependent manner (Fig. 6B). As would be expected, preincubation of JSRV pseudovirions with sHyal2 (1.5 μg/ml) before binding to HTX cells virtually blocked vector transduction (Fig. 6B). These results strongly argue that, in addition to blocking virus binding to target cells, sHyal2 also inactivates JSRV Env pseudovirion infectivity, possibly by triggering premature conformational changes of TM, as shown in Fig. 3. The potent effect of sHyal2 on the infectivity of JSRV Env pseudovirions was in sharp contrast to that of low pH, which has no significant effect (8). These results are also consistent with the observation that syncytium induction mediated by sHyal2 was not strictly dose dependent (Fig. 1C), and they may explain, at least in part, why the sHyal2-mediated transduction of a JSRV vector into nonpermissive HeLa cells was inefficient and did not occur in a linearly dose dependent manner (Fig. 6A). It is therefore possible that the sHyal2-mediated activation of JSRV Env fusion is cooperative, where multiple interactions between and within the Env trimers of JSRV may occur upon a single binding by sHyal2. A similar mechanism has been reported previously for ASLV (10).
FIG. 6.
sHyal2 enables transduction of nonpermissive cells by JSRV Env pseudovirions (A) but blocks their entry into permissive cells (B). (A) HeLa cells were bound by JSRV Env, HIV-1 Env, or VSV-G pseudovirions encoding GFP by spinoculation, followed by incubation with the indicated amounts of sHyal2. The transduction efficiency was analyzed by flow cytometry 48 h postinfection. The titers of JSRV pseudovirions with the presence of 0, 1.5, and 10 μg of sHyal2 in HeLa cells are 34, 360, and 550 GFP+ cells/ml, respectively. The titers of HIV-1 Env and VSV-G pseudotypes in HeLa cells are ∼35 and 3.6 × 106 GFP+ cells/ml, respectively. Note that the low titer of HIV-1 Env pseudovirions in HeLa cells was not due to the viral stock preparation or infection, because the titer in HeLa-TZM-bl cells expressing CD4 and CCR5 was 5 × 104 GFP+ cells/ml. Representative dot plots are shown, and the percentages of GFP+ cells are given at the lower right. The changes (n-fold) in transduction efficiency in four independent experiments were averaged and plotted. (B) HTX cells either were infected with JSRV Env or VSV-G pseudovirions that had been preincubated (before binding) with the indicated amounts of sHyal2 or were first bound with pseudovirions and then incubated with the indicated amounts of sHyal2 (after binding); see details in Materials and Methods. In both cases, unbound sHyal2 and virions were removed from cells by washing, and viral infectivity was assessed by flow cytometry 48 h postinfection.
DISCUSSION
JSRV is an interesting retrovirus in that its native Env functions as an active oncogene in addition to mediating entry into host cells. However, unlike most retroviruses, which are pH independent, JSRV entry requires dynamin-dependent endocytosis and a low pH (1, 8). Moreover, we observed that membrane fusion mediated by JSRV Env in vitro could be induced only by a low pH in cells that overexpress functional Hyal2 (8), suggesting that Hyal2 may play an active role in the fusion activation process. Using a soluble form of the JSRV receptor, sHyal2, we demonstrate here that a low pH is indeed not sufficient to induce fusion by JSRV Env but that receptor binding is also required for this process to occur (Fig. 1). We conclude that, like ASLV, oncogenic JSRV has borrowed features of both pH-independent and pH-dependent viruses for its fusion activation and entry, which involve stepwise and cooperative triggers from both the receptor and low pH. This multistep fusion pathway used by JSRV and ASLV is also analogous to HIV entry, where CD4 binding to HIV Env triggers initial conformational changes of gp120 that allow subsequent coreceptor binding and fusion, leading to productive infection (11).
However, there are some striking differences between JSRV and ASLV in terms of fusion activation by receptor binding or priming. First, we show that sHyal2 induces pronounced JSRV SU shedding in 293 cells at a neutral pH (Fig. 2), and this is in sharp contrast to findings for ASLV, where soluble Tva does not cause apparent disassociation between SU and TM (36) (see detailed discussions below). Second, we find that the ∼120-kDa species, likely the 6-HB, or a pre-6-HB intermediate, of JSRV Env, is much less stable in SDS (Fig. 5A) than its ASLV Env counterpart, which is resistant to SDS at concentrations up to 2% (22, 24). This could be due to the relatively short lengths of the N- and C-HRs of JSRV Env compared to those of ASLV (25) and/or to their intrinsic differences in specific amino acid interactions, or technical limitations. The relative low stability of JSRV TM oligomers may explain, at least in part, why cross-linking is required for the detection of JSRV TM oligomerization (Fig. 3). Third, sHyal2 potently inactivates the infectivity of JSRV Env pseudovirions in a step(s) after virus binding (Fig. 6B), which is consistent with the observation that sHyal2 induces the TM conformational refolding of JSRV Env at a neutral pH (Fig. 3C). This feature appears also to be different from that of ASLV, in which both soluble Tva and a low pH are required to inactivate viral infectivity (37).
We propose the following model for JSRV Env-mediated fusion activation. Upon receptor binding, JSRV SU undergoes conformational changes that cause it to disassociate from TM. This would relieve its intrinsic restriction on TM, resulting in exposure of the fusion peptide and its subsequent insertion into the plasma membrane. Although our current study did not directly address the conformational changes of SU and the association of TM with the cell membrane, it was evident that sHyal2 promotes JSRV SU shedding in both Env-expressing cells (Fig. 2) and Env pseudovirions (Fig. 3). It is notable that JSRV SU contains a CXXC motif, a conserved amino acid sequence that has previously been shown to be important for fusion activation in other retroviruses by mediating the switch from an intersubunit (SU-TM) to an intra-SU subunit disulfide bond (40). Interestingly, pretreatment of JSRV pseudovirions with an alkylating agent, N-ethylmaleimide, failed to block the ability of sHyal2 and low pH to induce the formation of the 120-kDa species (data not shown). While we cannot rule out the possibility that this might be due to the experimental conditions we have used, or the nature of the pseudovirions that were produced from the Hyal2-positive 293 cells and could have been prematurely activated, it is noteworthy that betaretroviruses, including JSRV and ENTV, were recently shown to contain no disulfide bond between SU and TM (John M. Coffin, personal communication). Whether or not the CXXC motif of JSRV SU, and/or the potential formation of receptor-induced thiolates, mediates JSRV SU shedding and fusion activation remains to be investigated.
Once the fusion peptide inserts into the plasma membrane, the TM of JSRV Env may become elongated and form one or more prehairpin intermediates that are sensitive to JSRV C-HR2 peptide binding. At this point, a low pH is necessary to trigger dramatic TM conformational refolding in order to form a 6-HB and to enable subsequent fusion. We found, however, that although the JSRV C-HR2 peptide effectively inhibited JSRV Env-mediated membrane fusion (Fig. 1F; also data not shown), it is unable to block the formation of the ∼120-kDa species, even at extremely high concentrations (up to 2 mg/ml) (data not shown). It is possible that the ∼120-kDa form is a heterogeneous mixture, containing both pre-6-HB intermediates and stable 6-HBs; alternatively, this form may be a precursor of JSRV 6-HB, with a true 6-HB form remaining to be identified. It is noteworthy that the ASLV C-HR2 peptide, R99, does not always block the formation of 6-HB species yet potently inhibits ASLV Env-mediated fusion and infection (22, 23, 25). We should mention that all the JSRV Env pseudovirions used in this study were produced from 293T or 293 cells (because of their high transfection efficiency), and it is therefore possible that the endogenous Hyal2 expressed in these cells may have prematurely activated JSRV Env in the viral particles and contributed to the formation of the ∼120-kDa species by low pH alone, even without sHyal2 preincubation (Fig. 3). Work is under way to address this issue and to further characterize the JSRV fusion intermediate(s) and the formation of its 6-HBs.
Acknowledgments
We thank Vladimir Vigdorovich and Dusty Miller for the generous gift of the S2 cell line stably expressing sHyal2. We also thank the anonymous reviewers for comments and suggestions that improved the article.
This work was supported by the Canadian Institutes of Health Research (CIHR) (to S.-L.L.). M.C. was supported by scholarships from the Natural Sciences and Engineering Research Council of Canada (NSERC). S.-L. Liu holds a Canada Research Chair in Virology and Gene Therapy.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Bertrand, P., M. Côté, Y.-M. Zheng, L. M. Albritton, and S.-L. Liu. 2008. Jaagsiekte sheep retrovirus utilizes a pH-dependent endocytosis pathway for entry. J. Virol. 82:2555-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brindley, M. A., L. Hughes, A. Ruiz, P. B. McCray, Jr., A. Sanchez, D. A. Sanders, and W. Maury. 2007. Ebola virus glycoprotein 1: identification of residues important for binding and postbinding events. J. Virol. 81:7702-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brindley, M. A., and W. Maury. 2005. Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus. J. Virol. 79:14482-14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caporale, M., C. Cousens, P. Centorame, C. Pinoni, M. De las Heras, and M. Palmarini. 2006. Expression of the Jaagsiekte sheep retrovirus envelope glycoprotein is sufficient to induce lung tumors in sheep. J. Virol. 80:8030-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 94:14306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin, J. M., S. H. Hughes, and H. E. Varmus. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 7.Côté, M., T. J. Kucharski, and S.-L. Liu. 2008. Enzootic nasal tumor virus envelope requires a very acidic pH for fusion activation and infection. J. Virol. 82:9023-9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Côté, M., Y.-M. Zheng, L. M. Albritton, and S.-L. Liu. 2008. Fusogenicity of Jaagsiekte sheep retrovirus envelope protein is dependent on low pH and is enhanced by cytoplasmic tail truncations. J. Virol. 82:2543-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dakessian, R., Y. Inoshima, and H. Fan. 2007. Tumors in mice transgenic for the envelope protein of Jaagsiekte sheep retrovirus. Virus Genes 35:73-80. [DOI] [PubMed] [Google Scholar]
- 10.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 12.Fan, H. (ed.). 2003. Current topics in microbiology and immunology, vol. 275. Jaagsiekte sheep retrovirus and lung cancer. Springer, Berlin, Germany. [DOI] [PubMed]
- 13.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 14.Huang, I. C., B. J. Bosch, F. Li, W. Li, K. H. Lee, S. Ghiran, N. Vasilieva, T. S. Dermody, S. C. Harrison, P. R. Dormitzer, M. Farzan, P. J. Rottier, and H. Choe. 2006. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 281:3198-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin, S., B. Zhang, O. A. Weisz, and R. C. Montelaro. 2005. Receptor-mediated entry by equine infectious anemia virus utilizes a pH-dependent endocytic pathway. J. Virol. 79:14489-14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kielian, M. 2006. Class II virus membrane fusion proteins. Virology 344:38-47. [DOI] [PubMed] [Google Scholar]
- 17.Kielian, M., and F. A. Rey. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, P., D. Nachagari, C. Fields, J. Franks, and L. M. Albritton. 2007. Host cell cathepsins potentiate Moloney murine leukemia virus infection. J. Virol. 81:10506-10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, S.-L., F. M. Duh, M. I. Lerman, and A. D. Miller. 2003. Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus Env proteins. J. Virol. 77:2850-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, S.-L., M. I. Lerman, and A. D. Miller. 2003. Putative phosphatidylinositol 3-kinase (PI3K) binding motifs in ovine betaretrovirus Env proteins are not essential for rodent fibroblast transformation and PI3K/Akt activation. J. Virol. 77:7924-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 98:4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama, S., S. E. Delos, and J. M. White. 2004. Sequential roles of receptor binding and low pH in forming prehairpin and hairpin conformations of a retroviral envelope glycoprotein. J. Virol. 78:8201-8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melikyan, G. B., R. J. Barnard, L. G. Abrahamyan, W. Mothes, and J. A. Young. 2005. Imaging individual retroviral fusion events: from hemifusion to pore formation and growth. Proc. Natl. Acad. Sci. USA 102:8728-8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 25.Netter, R. C., S. M. Amberg, J. W. Balliet, M. J. Biscone, A. Vermeulen, L. J. Earp, J. M. White, and P. Bates. 2004. Heptad repeat 2-based peptides inhibit avian sarcoma and leukosis virus subgroup A infection and identify a fusion intermediate. J. Virol. 78:13430-13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pager, C. T., W. W. Craft, Jr., J. Patch, and R. E. Dutch. 2006. A mature and fusogenic form of the Nipah virus fusion protein requires proteolytic processing by cathepsin L. Virology 346:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pager, C. T., and R. E. Dutch. 2005. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 79:12714-12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard-Maureau, M., G. Jarmy, A. Berg, A. Rethwilm, and D. Lindemann. 2003. Foamy virus envelope glycoprotein-mediated entry involves a pH-dependent fusion process. J. Virol. 77:4722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche, S., S. Bressanelli, F. A. Rey, and Y. Gaudin. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187-191. [DOI] [PubMed] [Google Scholar]
- 31.Ross, S. R., J. J. Schofield, C. J. Farr, and M. Bucan. 2002. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 99:12386-12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruigrok, R. W., S. R. Martin, S. A. Wharton, J. J. Skehel, P. M. Bayley, and D. C. Wiley. 1986. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology 155:484-497. [DOI] [PubMed] [Google Scholar]
- 33.Schornberg, K., S. Matsuyama, K. Kabsch, S. Delos, A. Bouton, and J. White. 2006. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 80:4174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons, G., D. N. Gosalia, A. J. Rennekamp, J. D. Reeves, S. L. Diamond, and P. Bates. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 102:11876-11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjoberg, M., B. Lindqvist, and H. Garoff. 2008. Stabilization of TM trimer interactions during activation of Moloney murine leukemia virus Env. J. Virol. 82:2358-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, J. G., and J. M. Cunningham. 2007. Receptor-induced thiolate couples Env activation to retrovirus fusion and infection. PLoS Pathog. 3:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, J. G., W. Mothes, S. C. Blacklow, and J. M. Cunningham. 2004. The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection. J. Virol. 78:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigdorovich, V., A. D. Miller, and R. K. Strong. 2007. Ability of hyaluronidase 2 to degrade extracellular hyaluronan is not required for its function as a receptor for Jaagsiekte sheep retrovirus. J. Virol. 81:3124-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigdorovich, V., R. K. Strong, and A. D. Miller. 2005. Expression and characterization of a soluble, active form of the jaagsiekte sheep retrovirus receptor, Hyal2. J. Virol. 79:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallin, M., M. Ekstrom, and H. Garoff. 2004. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 23:54-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White, J. M., S. E. Delos, M. Brecher, and K. Schornberg. 2008. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 43:189-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wootton, S. K., C. L. Halbert, and A. D. Miller. 2005. Sheep retrovirus structural protein induces lung tumours. Nature 434:904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wootton, S. K., M. J. Metzger, K. L. Hudkins, C. E. Alpers, D. York, J. C. DeMartini, and A. D. Miller. 2006. Lung cancer induced in mice by the envelope protein of jaagsiekte sheep retrovirus (JSRV) closely resembles lung cancer in sheep infected with JSRV. Retrovirology 3:94. [DOI] [PMC free article] [PubMed] [Google Scholar]