Abstract
Herpesviruses cross nuclear membranes (NMs) in two steps, as follows: (i) capsids assemble and bud through the inner NM into the perinuclear space, producing enveloped virus particles, and (ii) the envelopes of these virus particles fuse with the outer NM. Two herpes simplex virus (HSV) glycoproteins, gB and gH (the latter, likely complexed as a heterodimer with gL), are necessary for the second step of this process. Mutants lacking both gB and gH accumulate in the perinuclear space or in herniations (membrane vesicles derived from the inner NM). Both gB and gH/gL are also known to act directly in fusing the virion envelope with host cell membranes during HSV entry into cells, i.e., both glycoproteins appear to function directly in different aspects of the membrane fusion process. We hypothesized that HSV gB and gH/gL also act directly in the membrane fusion that occurs during virus egress from the nucleus. Previous studies of the role of gB and gH/gL in nuclear egress involved HSV gB and gH null mutants that could potentially also possess gross defects in the virion envelope. Here, we produced recombinant HSV-expressing mutant forms of gB with single amino acid substitutions in the hydrophobic “fusion loops.” These fusion loops are thought to play a direct role in membrane fusion by insertion into cellular membranes. HSV recombinants expressing gB with any one of four fusion loop mutations (W174R, W174Y, Y179K, and A261D) were unable to enter cells. Moreover, two of the mutants, W174Y and Y179K, displayed reduced abilities to mediate HSV cell-to-cell spread, and W174R and A261D exhibited no spread. All mutant viruses exhibited defects in nuclear egress, enveloped virions accumulated in herniations and in the perinuclear space, and fewer enveloped virions were detected on cell surfaces. These results support the hypothesis that gB functions directly to mediate the fusion between perinuclear virus particles and the outer NM.
Herpesvirus glycoproteins gB and gH/gL participate in two separate membrane fusion events that occur during different stages of virus replication. First, during virus entry into cells, gB and gH/gL promote fusion between the virion envelope and either the plasma membrane or endosomes (reviewed in references 6, 21, 27, and 39). Second, herpes simplex virus (HSV) gB and gH (likely complexed to form a heterodimer with gL), and likely homologues in other herpesviruses, promote nuclear egress (12). Herpesvirus capsids are produced in the nucleus and cross the nuclear envelope (NE) by envelopment at the inner nuclear membrane (NM), producing perinuclear virions that then fuse with the outer NM (reviewed in references 35 and 36). There is evidence that HSV gB and gH/gL function in a redundant fashion in fusion between enveloped, perinuclear virus particles and the outer NM (12), whereas both gB and gH/gL are essential for entry fusion (8, 13, 38). Much more is known about the mechanisms involved in entry fusion than those involved in egress fusion, and many important questions remain in terms of how these two membrane fusion processes relate to each other.
Entry of HSV into cells involves interactions between the viral receptor-binding protein gD and the gD receptors (16, 28, 30, 37). When gD binds to its receptors, there are conformational changes in gD which apparently activate gB and gH/gL, so that these glycoproteins promote fusion involving the virion envelope and cellular membranes (21, 32). By using split green fluorescent protein fusion proteins, also denoted bimolecular complementation, two groups showed that gD binding to gD ligands triggers interactions between gB and gH/gL and that this is accompanied by cell-cell fusion (1, 2). There is also evidence that gB and gH/gL contribute to different stages of membrane fusion. When gH/gL is expressed with gD, there is hemifusion (mixing of the outer leaflets of membranes) of adjacent cells, and this partial fusion is apparently mediated by gH/gL (41). However, full fusion (mixing of both inner and outer leaflets) occurs only when gB is coexpressed with gD and gH/gL (41). Also supporting a role for gH in membrane fusion, peptides based on heptad repeats in gH can disrupt model membranes (14, 15, 17). HSV gB is a class III fusion protein, structurally similar to vesicular stomatitis virus G protein, with a three-stranded coil-coil barrel in the central region of the molecule reminiscent of class I fusion proteins, e.g., influenza virus hemagglutinin (22). Therefore, herpesvirus gB and gH/gL differ substantially from the fusion proteins expressed by all other well-studied viruses because both gB and gH/gL participate directly in membrane fusion, apparently functioning in different aspects of entry fusion.
HSV gB and other viral class III fusion proteins differ from class I fusion proteins that have N-terminal, hydrophobic fusion peptides because class III fusion proteins possess internal bipartite “fusion loops” composed of both hydrophobic and hydrophilic residues (3, 22). In the solved structure of the HSV gB ectodomain, which might represent a postfusion form of the protein, the fusion loops are located near the base of the molecule, adjacent to the virion envelope (22). Mutant forms of gB with single amino acid substitutions in these fusion loops displayed diminished cell-cell fusion activity when transfected into cells with gD and gH/gL (20). Cell-cell fusion approximates the fusion that occurs during entry, defining the minimal fusion machinery, although there are differences between entry and cell-cell fusion (10). Moreover, full-length gB molecules with fusion loop mutations failed to complement gB null HSV (19). Recently, it was demonstrated that the HSV gB extracellular domain can interact with liposomes in vitro and that this binding depends upon gB's fusion loops (19).
Herpesvirus capsids are assembled in the nucleus and acquire an envelope by budding through the inner NM. For a short time, enveloped virus particles are found in the space between the inner and outer NMs (perinuclear space), but then the envelopes of these particles fuse with the outer NM, releasing capsids into the cytoplasm (reviewed in references 35 and 36). Cytoplasmic capsids acquire a second envelope by budding into the trans-Golgi network, and this secondary envelopment involves redundant or additive functions of gE/gI and gD, i.e., either of these glycoproteins will suffice (11). The second step of the nuclear egress pathway involving membrane fusion between the envelope of perinuclear particles and the outer NM requires HSV glycoproteins gB and gH/gL (12). HSV double mutants lacking both gB and gH accumulate enveloped virus particles in the perinuclear space and in herniations, i.e., membrane vesicles that bulge into the nucleoplasm and derive from the inner NM (12). These observations, coupled with the evidence that gB and gH/gL are fusion proteins, suggested that gB and gH/gL promote the fusion between virus particles and the outer NM. However, there is one important difference between nuclear egress fusion and entry fusion. Virus mutants lacking either gB or gH are unable to enter cells, but such mutants have fewer defects in nuclear egress than double mutants lacking both gB and gH (12). Thus, as with secondary envelopment that involves gD and gE/gI, glycoproteins gB and gH/gL act in a redundant or additive fashion to mediate the fusion between the envelope of perinuclear virus particles and the outer NM. It is also important to note that there appear to be other mechanisms by which HSV particles can exit the perinuclear space. For example, although a substantial number of gB gH null double mutants accumulated in herniations (increased by ∼10-fold), some virions were seen on cell surfaces, although their numbers were reduced by ∼2.5- to 5-fold compared with those of wild-type HSV (12, 46).
HSV entry fusion is triggered by gD binding to one of its ligands. However, it is not clear what triggers fusion of the envelope of perinuclear particles with the outer NM. gD, gB, gH, gM, gK, and other viral membrane proteins are all present in NMs and in perinuclear virus particles (4, 12, 25, 40, 42, 44). It seems unlikely that there are substantial quantities of known gD receptors in NMs, although this has not been carefully examined and there may well be unidentified gD receptors present in NMs. However, if fusion at NMs is not activated by gD binding to gD receptors, there must be other mechanisms to trigger this fusion. There is evidence that HSV gK negatively regulates fusion at the NE because (i) overexpression of gK causes enveloped virus particles to accumulate in the perinuclear space (25) and (ii) gK is primarily localized to the endoplasmic reticulum and NM and is not substantially found in extracellular virions (26, 34). Another potential regulatory mechanism for fusion at the outer NM involves phosphorylation of the cytoplasmic domain of gB by the HSV kinase US3 (46). An HSV recombinant lacking gH and expressing a mutant gB with a substitution, T887A, affecting an amino acid in the gB cytoplasmic domain displayed reduced US3-dependent phosphorylation and accumulated enveloped virus particles in herniations (46). This mutation in gB did not alter HSV entry into cells (31, 46). Together, these results suggest that HSV fusion with the outer NM differs from entry fusion in some, but likely not all, important mechanistic details.
Given that both gB and gH/gL are well established as fusion proteins for virus entry, we hypothesized that these glycoproteins directly mediate the membrane fusion that occurs between the envelope of perinuclear virus particles and the outer NM (12, 46). However, there are other possibilities. For example, it is conceivable that loss of both gB and gH alters the structure of the envelope of perinuclear HSV virions so that other HSV glycoproteins (that directly promote fusion) are affected. To address this issue and extend our understanding of how gB functions in nuclear egress fusion, we constructed HSV recombinants that express mutant forms of gB with substitutions in the fusion loops. These viruses also lacked gH, making nuclear egress totally dependent on a functional form of gB. By propagating these recombinants using gH-expressing cells, we could produce virus particles including gH and the mutant gB molecules. These HSV recombinants expressing gH as well as gB fusion loops, W174R, W174Y, Y179K, and A261D, were all unable to enter cells. However, two recombinants, expressing W174Y and Y179K, exhibited some cell-to-cell spread while the other two, expressing W174R and A261D, did not spread beyond single infected cells. All four recombinants infected into cells lacking gH exhibited defects in nuclear egress. These results provide strong support for the hypothesis that gB acts directly to mediate the fusion of the virion envelope with the outer NM during HSV egress.
MATERIALS AND METHODS
Cells and viruses.
HaCaT cells represent a human keratinocyte cell line (7) and were maintained in Dulbecco's minimal essential medium (DMEM) (Invitrogen) with 8% fetal bovine serum (FBS) (ISC BioExpress, Kaysville, UT). F6 cells (a gift from Tony Minson, Cambridge University) were derived from Vero cells, express HSV type 1 (HSV-1) gH (13), and were maintained in DMEM with 8% FBS and 150 μg/ml G418 (Mediatech, Herndon, VA). F6/gB12 cells were derived from F6 cells, express both gB and gH (12), and were maintained in Dulbecco's modified Eagle's medium lacking histidine and supplemented with 8% FBS, 150 μg/ml G418, and 0.4 mM histidinol (Sigma). F-BACgH-, which denotes a virus derived from HSV strain F that was generated by transfecting cells with the bacterial artificial chromosome (BAC) plasmid and that cannot express gH (12), was propagated on F6 cells (13). F-BAC-gBgalK/gH-, lacking both gB and gH genes (46), was propagated, and the titers of the virus on F6/gB12 cells were determined.
Construction of recombinant HSV.
The gB fusion loop point mutations were previously engineered into plasmid copies of the gB gene, and here, these gB sequences were transferred into a BAC copy of the HSV-1 (strain F) genome (23). gB sequences in plasmids (20) were excised using XhoI and BglII, DNA fragments were gel purified using the QIAquick gel extraction kit (Qiagen, MD), and then DNA was introduced into SW102 bacteria that contain BAC-gBgalK/gH-, as described previously (46). BAC-gBgalK/gH- contains galK sequences replacing gB sequences and a kanamycin resistance gene cassette replacing gH sequences (46). Recombination between the DNA fragments containing gB fusion loop mutations and gB sequence-flanking galK involved temperature-sensitive lambda prophage recombination proteins coupled with selection against galK using 2-deoxy-galactose, as described previously (43). The resulting BAC recombinants were screened by PCR amplification of the gB sequences, including the mutations, followed by DNA sequencing. To produce HSV from BACs, BAC DNA was prepared using the plasmid maxikit (Qiagen, MD) and then was transfected into F6/gB12 cells using TransFectin lipid reagent (Bio-Rad, Hercules, CA). The HSV recombinants expressing fusion loop mutations (and lacking gH) were propagated, and the titers of the recombinants on F6/gB12 cells were determined.
Preparation of supernatant virions from F6 cells.
For entry assays and to characterize incorporation of mutant gB molecules into virions, extracellular virus particles were obtained from F6 cells that express gH but not gB. F6 cells were infected using 5 PFU/cell of each recombinant HSV (derived from F6/gB12 cells) in DMEM with 1% FBS. Cell culture supernatants were collected after 18 h of infection and centrifuged at 850 × g to remove any cells, and then virus particles were centrifuged at 71,000 × g through a 20% dextran-10 cushion in Beckman SW32 centrifuge tubes at 4°C. The virus pellet was resuspended in 1 ml of phosphate-buffered saline (PBS) containing 1% FBS centrifuged at 76,000 × g in a Beckman TLA for 30 min at 4°C. The virus pellet was resuspended in 50 to 100 μl and sonicated briefly on ice.
Western blotting.
To characterize extracellular virus preparations, equal volumes of partially purified viruses were separated by electrophoresis using a 6% or 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel. Proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore), and the membranes were blocked by incubation with 2% nonfat milk, 1% fish gelatin, 0.5% goat serum, and 0.25% polyvinyl pyrrolidone. Membranes were then probed with antibodies in blocking buffer diluted equally in PBS containing 0.1% Tween 20. Membranes were incubated with monoclonal antibody (MAb) DL6 specific to gD (9), ascites fluid diluted to 1:5,000, VP5-specific MAb 3B6 (Virusys, Sykesville, MD) diluted 1:10,000, anti-gB rabbit polyclonal serum R68 (5) diluted 1:7,500, or anti-gH rabbit serum R137 (18). Blots were washed with PBS containing 0.1% Tween 20 and incubated with mouse anti-rabbit horseradish peroxidase-conjugated antibody (Sigma) or rabbit anti-mouse horseradish peroxidase-conjugated secondary antibody (Sigma), and then secondary antibodies were detected using a chemiluminescence kit (Pierce Chemicals). A Lumi-Imager (Boehringer Mannheim) was used, and band densities were measured by quantitative imaging.
Entry assays involving immunoprecipitation of HSV proteins.
HaCaT cells were infected with extracellular HSV virions prepared from F6 cells using 10 PFU/cell for F-BACgH- (the titers of the virus on F6 cells were determined) and the same quantities of virions for other recombinants, as determined by quantifying VP5 in virus preparations by Western blotting. After 5 h, cells were washed with PBS containing 1 mM MgCl2 and 1 mM CaCl2 (PBS++) and then labeled with [35S]methionine/cysteine (PerkinElmer, Boston, MA) for 2 h. Radiolabeled cells were lysed in 1% NP-40-0.5% deoxycholate, 50 mM Tris-HCl (pH 7.2), 100 mM NaCl containing 2 mg/ml bovine serum albumin, and 1.0 mM phenylmethylsulfonyl fluoride and frozen at −70°C, and later the extracts were clarified by centrifugation at 100,000 × g for 60 min. HSV proteins were immunoprecipitated using anti-HSV-1 polyclonal sera (Dako, Carpinteria, CA) or anti-gD MAb DL6 as described previously (45), and then proteins were separated by gel electrophoresis and imaged using a phosphorimager.
Cell-to-cell spread analyses.
F6 cell monolayers were infected with approximately 100 to 200 PFU of each recombinant HSV derived from F6/gB12 cells and then incubated with DMEM containing 1% FBS and 0.2% human gamma globulin (to restrict virus spread through the media) for 2 days. Cells were washed with PBS++ and fixed with PBS++ containing 2% formaldehyde for 10 min at room temperature. The fixed cells were permeabilized with 0.5% Triton X-100, 0.5% sodium deoxycholate, and 2% goat serum in PBS for 30 min and then incubated with polyclonal rabbit anti-HSV antibodies (Dako) for 1 h, washed, and subsequently incubated with Alexa Fluor 495-conjugated mouse anti-rabbit antibodies (Molecular Probes, Eugene, OR). The monolayers were viewed and photographed using a Nikon fluorescent microscope.
Electron microscopy.
HaCaT cells were infected using 10 to 20 PFU/cell of each recombinant HSV derived from F6/gB12 cells for 18 h, and then the cells were washed three times with PBS++ and fixed in Ito and Karnovsky's fixative (1.6% paraformaldehyde, 2.5% glutaraldehyde, and 0.5% picric acid in 0.1 M sodium cacodylate). The samples were dehydrated and viewed with an electron microscope, as described previously (11).
RESULTS
Construction of HSV recombinants expressing gB fusion loop mutations and lacking gH.
Mutant forms of gB containing point mutations affecting the fusion loops were previously characterized by transfection into cells for their ability to cooperate with gD and gH/gL to promote fusion of cells (21) and for complementation of a gB null mutant (19, 20). We wished to recombine certain of these mutations into HSV in order to characterize how these proteins function in virus entry, cell-to-cell spread, and especially, in egress of HSV from cells (fusion at the outer NM). For studies of nuclear egress, it was necessary to construct viruses expressing mutant gB molecules, but also lacking gH, given that gB and gH can act in a redundant fashion in this process (12). Four HSV recombinants expressing substitutions in either of the two gB fusion loops, W174Y, W174R, Y179K, and A261D, were constructed using an HSV-1 genome subcloned into a BAC, as described previously (46). Briefly, the mutant gB genes were removed from plasmids (20), and these DNA sequences were electroporated into bacteria containing BAC-gBgalK/gH- sequences. The gB gene in BAC-gBgalK/gH- had been previously replaced by the galK gene, and this BAC lacks gH coding sequences (46). Recombination between gB sequences (containing fusion loop mutations) in plasmids and gB sequences upstream and downstream of galK in the BACs produced BACs with the galK gene replaced by gB sequences, including fusion loop mutations. Bacteria containing galK BAC can be selected using 2-deoxy-galactose, which is toxic to bacteria expressing galK (43). Using PCR, we confirmed that BACs contained mutant gB sequences (not shown). The recombinant BACs containing gB mutations were transfected into complementing F6/gB12 cells that express gB and gH (12), and viruses were produced and denoted as follows: F-BAC-gB(W174Y)/gH-, F-BAC-gB(W174R)/gH-, F-BAC-gB(Y179K)/gH-, and F-BAC-gB(A261D)/gH-. Previously, we described an HSV recombinant lacking gH sequences and expressing wild-type gB, denoted F-BACgH- (12), and a recombinant lacking both gB and gH sequences, denoted F-BAC-gBgalK/gH- (46).
During preliminary characterization of these HSV gB fusion loop recombinants, we observed defects in virus replication in double complementing F6/gB12 cells. For example, F-BAC-gB(W174R)/gH- and F-BAC-gB(A261D)/gH- produced virus titers of 1 × 107 to 3 × 107 PFU/ml using F6/gB12 cells. Using the same F6/gB12 cells, stocks of the gB null and gH null F-BAC-gBgalK/gH- were routinely 10- to 20-fold higher (1 × 108 to 5 × 108 PFU). F-BAC-gB(W174Y)/gH-, which expresses a gB molecule that possesses some (50%) cell-cell fusion (20), produced higher titers of virus (0.5 × 108 to 2 × 108 PFU). The complementing F6/gB12 cells produce wild-type gB, and this is inserted into the virion envelope along with mutant gB molecules. gB forms trimers in the virion envelope (22). Thus, it appeared that mutant gB molecules that fail to function in some capacity (membrane fusion) were incorporated into oligomers with wild-type gB, and these mutant gB molecules acted in a dominant-negative fashion.
Characterization of the proteins present in extracellular HSV virions.
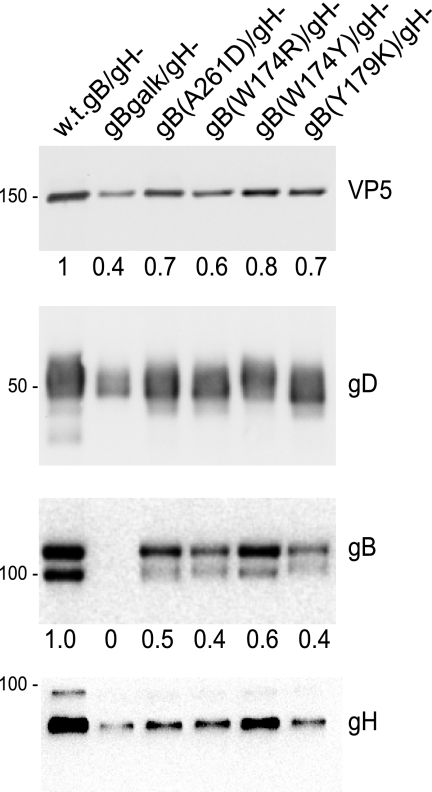
As a measure of whether mutant gB molecules could be introduced into virus particles, we infected F6 cells that express gH but not gB with HSV recombinants and obtained cell culture supernatants. These supernatants were harvested well before the cells began to lyse. Extracellular virions were partially purified by high-speed centrifugation through dextran-10 cushions. The virions were disrupted with 2% SDS and subjected to SDS-polyacrylamide gel electrophoresis and characterized by Western blotting. F-BACgH- (wild-type gB and lacking gH) was used as the comparison and showed substantial amounts of VP5 (major capsid protein), gD, and gB. Note that gH is present because these viruses were prepared using F6 cells that express gH. There were lower levels of VP5, gD, and gH in preparations of extracellular virions from cells infected with gB fusion loop recombinants, ranging from 40 to 80% of that observed with F-BACgH- (Fig. 1). For each of the gB mutants, as well as for the double mutant F-BAC-gBgalK/gH-, there was a moderate decrease in extracellular virus particles compared with extracellular virus produced by F-BACgH-. The extracellular virions produced by all four fusion loop mutants contained gB, although we noted small reductions in the amounts of these mutant gB molecules compared with the amounts of VP5 and gD present in the same virus particles (Fig. 1). We concluded that mutant gB molecules were present in extracellular virions. For subsequent studies involving entry of these particles, we used VP5 levels to equalize the amounts of input virus added to cells.
FIG. 1.
Characterization of HSV proteins present in extracellular virions. F6 cells that express gH were infected with the following HSV recombinants for 18 h, and then virions were purified from cell culture supernatants: F-BACgH- that expresses wild-type (w.t.) gB but no gH (w.t.gB/gH-), F-BAC-gBgalK/gH- that expresses no gB and no gH, F-BAC-gB(A261D)/gH-, F-BAC-gB(W174R)/gH-, F-BAC-gB(W174Y)/gH-, or F-BAC-gB(Y179K)/gH-. The virions were subjected to Western blotting for VP5, gD, gB, and gH. VP5 and gB were quantified as indicated in Materials and Methods. Molecular weight markers are shown on the left side of the panels.
Entry of virus particles expressing gB fusion loop mutations and wild-type gH.
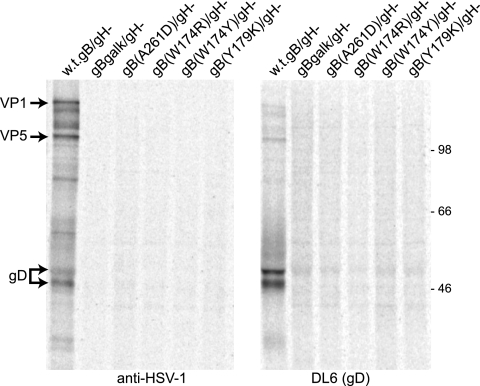
Previous transfection experiments involving gB fusion loop mutants entering into cells in conjunction with gD and gH/gL produced various levels of cell-cell fusion, as follows: gB(W174R) and gB(A2161D) exhibited ∼9% cell-cell fusion activity, gB(W174Y) exhibited ∼50% fusion, and gB(Y179K) showed no cell-cell fusion (20). To measure the entry of recombinant HSV expressing these mutant gB molecules, we prepared virions derived from F6 cells in order to supply gH, as described above. The titers of F-BACgH- virions were determined using plaque assays with F6 cells, and then these F-BACgH- virions were applied to HaCaT cells using 10 PFU/cell. HaCaT cells are a human keratinocyte cell line that might be considered to represent HSV infection in vivo well. HSV replicates well in these cells, producing higher titers and larger plaques than those produced by monkey Vero cells (45). Similar quantities of the other HSV recombinants, F- BAC-gB(W174R)/gH-, F-BAC-gB(W174Y)/gH-, F-BAC-gB(Y179K)/gH-, and F-BAC-gB(A261D)/gH-, were applied to HaCaT cells by basing the quantity of input viruses on the quantities of VP5 measured by using Western blotting and quantitative imaging of VP5 bands. This estimate of the quantities of virus particles containing capsids should accurately measure particles that can initiate infection, as we would not anticipate defects in tegument proteins, and as described above, glycoproteins gB and gD are present at normal levels. Cells were infected for 5 h and then radiolabeled for 2 h with [35S]methionine/cysteine. Detergent extracts of the cells were then immunoprecipitated using anti-HSV polyclonal antibodies or a MAb specific to the early protein gD. Viral proteins such as gD, VP1, and VP5 were observed in HaCaT cells infected with F-BACgH- (Fig. 2). However, few or no viral proteins were detected in cells infected with any of the recombinants expressing gB fusion loop mutations. This was consistent with the conclusion that all four of these mutant forms of gB were largely unable to mediate entry into cells.
FIG. 2.
Entry of HSV recombinants expressing gB fusion loop mutations. Preparations of virions derived from F6 cells (that express gH) were harvested from culture supernatants, and VP5 levels were quantified by using Western blot experiments (see Fig. 1). F-BACgH-, which expresses wild-type gB and no gH, was also derived from F6 cells, and the titers of the virus on F6 cells were determined. HaCaT cells were infected with a dose of F-BACgH- corresponding to 10 PFU/cell. Other HaCaT cells were infected with HSV recombinants expressing gB fusion loops using equal quantities of particles compared with that of F-BACgH- (particles quantified by VP5 Western blotting). After 5 h of infection, the HaCaT cells were labeled for 2 h with [35S]methionine/cysteine, and then detergent extracts of the cells were subjected to immunoprecipitation using anti-HSV polyclonal antibodies (left) or anti-gD MAb DL6 (right).
Cell-to-cell spread mediated by gB fusion loop mutants.
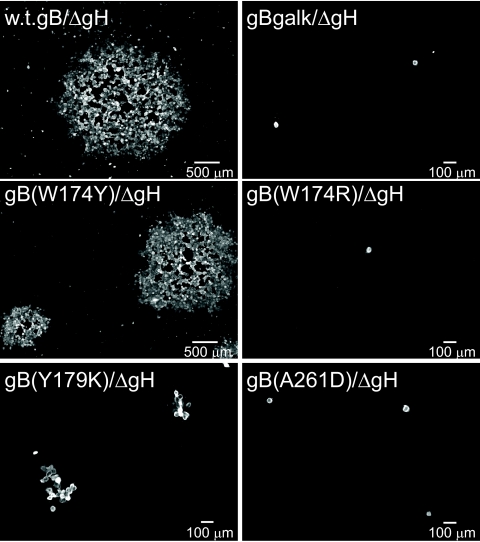
It was also of interest to determine whether any of the gB fusion loop mutants could function to promote spread of HSV from cell to cell. The mutant viruses were grown on F6/gB12 cells that supply both wild-type gB and gH, and the titers of the viruses derived from these cells on F6/gB12 cells were determined. Since wild-type gB was supplied, these virus particles could enter F6 cells that express gH and not gB. However, virus spread among F6 cells was mediated not by wild-type gB, but by mutant gB molecules. Monolayers of F6 cells were infected with 100 to 200 PFU of each of the HSV recombinants for 2 h, and then cells were incubated for 2 days with anti-HSV neutralizing antibodies (to contain spread through media). The monolayers were fixed, permeabilized, and then stained with polyclonal anti-HSV antibodies to detect HSV-infected cells. As expected, F-BACgH-, which expresses wild-type gB, spread to form plaques involving over 100 cells (Fig. 3). The gB null mutant F-BAC-gBgalK/gH- (derived from F6/gB12 cells) entered cells but did not spread beyond a single infected cell. Again, this was expected, as gB is known to be essential for virus spread. Recombinants F-BAC-gB(A261D)/gH- and F-BAC-gB(W174R)/gH- also did not spread beyond a single infected cell (Fig. 3), consistent with the conclusion that these mutant forms of gB did not function in spread. F-BAC-gB(Y179K)/gH- also produced some single infected cells, but the majority of plaques involved 3 to 15 infected cells. This observation was surprising given that gB(Y179K) has the most-profound effects in cell-cell fusion assays, exhibiting no fusion (0%), while others such as mutant gB(A261D) and gB(W174R) displayed modest fusion (9%) (20). Recombinant F-BAC-gB(W174Y)/gH- could also spread cell to cell, forming some plaques that were similar in size to those produced by F-BACgH-, although other plaques were moderately smaller (Fig. 3). gB(W174Y) retained ∼50% of the cell-cell fusion observed with wild-type gB. Thus, we concluded that lower levels of membrane fusion activity associated with mutant W174Y can largely suffice for the cell-to-cell spread of virus. F-BAC-gB(Y179K)/gH-, which expresses a mutant gB that mediated no cell-cell fusion (20) and that did not enter cells (Fig. 3), exhibited some cell-to-cell spread, suggesting that there was a low level of membrane fusion activity associated with gB(Y179K).
FIG. 3.
Cell-to-cell spread of HSV recombinants expressing gB fusion loop mutations. Preparations of virus particles were derived from F6/gB12 cells that express both gB and gH, and then the titers of these viruses on F6/gB12 cells were determined. Approximately 100 to 200 PFU each of F-BACgH- (wild-type [w.t.] gB), F-BAC-gBgalK/gH- (no gB), F-BAC-gB(W174Y)/gH-, F-BAC-gB(W174R)/gH-, F-BAC-gB(Y179K)/gH-, and F-BAC-gB(A261D)/gH- were incubated with F6 cells for 2 h, and then the cells were washed and incubated with human gamma globulin (a source of anti-HSV antibodies) for 2 days. The cells were fixed, permeabilized, and stained with rabbit anti-HSV polyclonal antibodies and then with a fluorescent anti-rabbit immunoglobulin G antibody, and images of plaques were obtained using a fluorescent microscope. ΔgH, gH-.
Role of gB fusion loops in nuclear egress.
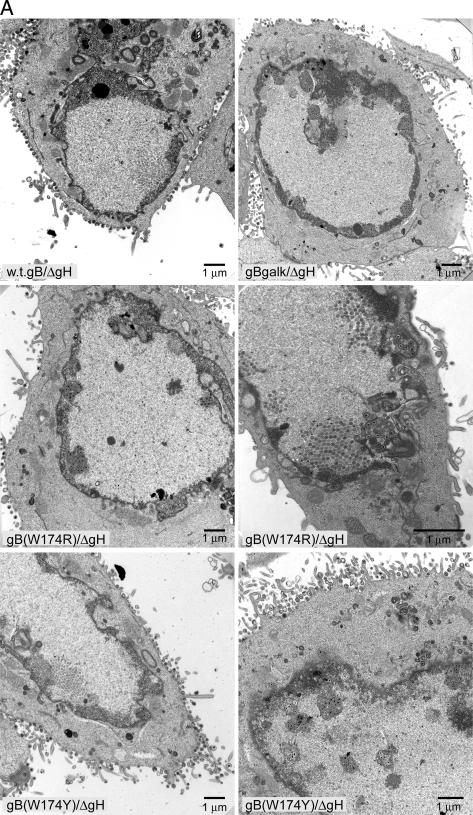
We previously concluded that either gB or gH (likely complexed with gL) can function in the second stage of nuclear egress (12). This conclusion was based primarily on the phenotypes of null mutants lacking both gB and gH. It is conceivable that gB and gH function in nuclear egress, not by promoting membrane fusion but in some other capacity. To test this further, we characterized HSV recombinants expressing gB fusion loop mutations and lacking gB in terms of accumulation of enveloped particles in herniations and in the perinuclear space. F-BACgH- (with wild-type gB) largely produced enveloped particles on the surfaces of HaCaT cells (Fig. 4A), as described previously (12). In contrast, F-BAC-gBgalK/gH- (lacking both gB and gH) accumulated enveloped particles in herniations and in the perinuclear space and fewer cell surface virions (Fig. 4A). As seen previously (12), relatively few enveloped virions were present in the cytoplasm, and we observed no differences in the numbers of unenveloped capsids in the nucleoplasm with those of any of the mutants (not shown). In order to quantify defects in assembly, we counted enveloped particles present in perinuclear spaces and herniations, as well as those on cell surfaces, and then calculated a ratio of these two populations of particles (perinuclear enveloped particles/cell surface enveloped particles) (Table 1). The nuclear/cell surface particle ratio for F-BACgH- (with wild-type gB) was 0.06, i.e., only about 5.6% of the total number of enveloped particles counted were present in herniations or as perinuclear enveloped particles (Fig. 4A; Table 1). In contrast, for F-BAC-gBgalK/gH- (no gB) this ratio was 1.9, i.e., about 65% of all enveloped particles counted were present in the perinuclear space or in herniations. Thus, the difference between wild-type gB and a gB null mutant was 31-fold by this measure.
FIG. 4.
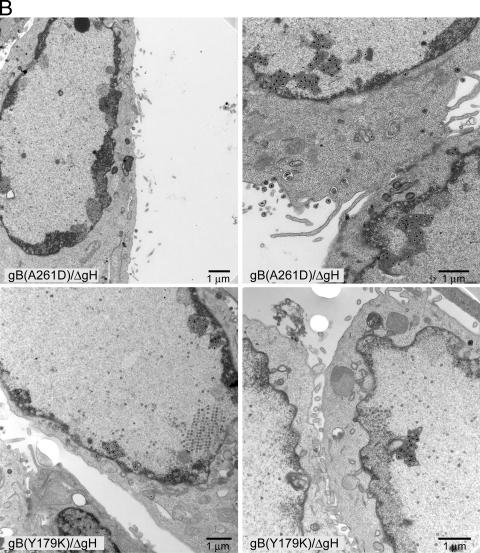
Analyses of defects in nuclear egress associated with gB fusion loop mutants. (A) HSV recombinants F-BACgH- (wild-type [w.t.] gB), F-BAC-gBgalK/gH- (no gB), F-BAC-gB(W174R)/gH-, and F-BAC-gB(W174Y)/gH- were derived from F6/gB12 cells (which express gB and gH) and used to infect HaCaT cells for 18 to 20 h, and then the cells were fixed, stained, and viewed by electron microscopy. (B) HaCaT cells were infected with F-BAC-gB(A261D)/gH- or F-BAC-gB(Y179K)/gH- as described in the legend to panel A and viewed by electron microscopy. ΔgH, gH-.
TABLE 1.
Nuclear egress of HSV recombinants expressing gB fusion loops and lacking the gH gene
| Virusa | No. of: |
Ratiod | |
|---|---|---|---|
| Enveloped perinuclear virionsb | Cell surface virionsc | ||
| F-BACgH- (expressing wild-type gB) | 125 | 2,087 | 0.06 |
| F-BAC-gB(W174Y)/gH- | 891 | 1,767 | 0.50 |
| F-BAC-gB(W174R)/gH- | 1,251 | 736 | 1.7 |
| F-BAC-gB(A261D)/gH- | 2,006 | 1,180 | 1.7 |
| F-BAC-gB(Y179K)/gH- | 1,892 | 901 | 2.1 |
| F-BAC-gBgalK/gH- (expressing no gB) | 1,182 | 622 | 1.9 |
HaCaT cells were infected with HSV recombinants and then viewed by an electron microscope, and enveloped virus particles were counted.
Enveloped virus particles present in herniations or found between the inner and outer NEs.
Enveloped virus particles present on the surface of cells.
The number of enveloped perinuclear virions/number of cell surface virions.
Recombinants F-BAC-gB(W174R)/gH- and F-BAC-gB(A261D)/gH-, both of which failed to enter cells and spread between cells, accumulated herniations and enveloped virions within NMs (Fig. 4A and B), and exhibited a ratio of nuclear/cell surface virions of 1.7 (62% of all enveloped particles were perinuclear) (Table 1). Similarly, F-BAC-gB(Y179K)/gH-, which failed to enter cells and spread poorly, formed herniations and accumulated virions in the perinuclear space (Fig. 4B), and the ratio was 2.1 (67% of all enveloped particles were perinuclear) (Fig. 4B; Table 1). We consider these three mutants, W174R, A261D, and Y179K (ratios of 1.7 to 2.1), not to be different from the gB and gH null mutant (1.9) in nuclear egress. Recombinant F-BAC-gB(W174Y)/gH- exhibited an intermediate phenotype, i.e., some cells showed herniations, but there were also numerous cell surface virions (Fig. 4A), and the ratio of nuclear/cell surface virions was 0.5 (33% of all enveloped particles were perinuclear) (Table 1). This mutant could also spread well with cell-to-cell spread (Fig. 3) and exhibited 50% cell-cell fusion (20). It should be noted that individual HSV-infected cells differ substantially in the numbers of capsids and enveloped virions per cell. Thus, the application of statistics, comparing one cell to another, produces large standard deviations, and it is not useful to compare one cell to another. Instead, we routinely count particles in 10 to 20 representative cells and add thousands of particles together, producing an accurate picture of the defects in HSV egress (11, 12). Therefore, the phenotypes of gB fusion loop mutants in nuclear egress mirrored the defects in cell-cell fusion, entry, and spread. These data support the hypothesis that gB functions in nuclear egress by mediating membrane fusion involving the enveloped virion and the outer NM.
DISCUSSION
The two fusion loops present near the base of the solved structure of the HSV gB ectodomain were hypothesized to represent sequences that interact with cellular membranes, participating in a bridge that gB forms between viral and cellular membranes and promoting fusion (22). Consistent with this hypothesis, mutations in these fusion loops in the context of full-length gB substantially reduced cell-cell fusion, complementation of a gB null mutant, and the interactions between gB and liposomes (19, 20). Here, we recombined four of these gB fusion loop mutations into HSV with the major intention of testing whether gB fusion loop activity is required for nuclear egress. However, having HSV recombinants expressing these mutant gB molecules, we also tested virus entry into cells and cell-to-cell spread. Viruses with any of the four mutations, W174Y, W174R, Y179K, and A261D, showed little or no entry of HSV into cells, as indicated by highly diminished expression of newly synthesized viral proteins in cells (summarized in Table 2). It is formally possible that these mutations might have affected some postentry stage of HSV replication, although this is unlikely given that gB is essential for entry and not known to play a role in postentry steps that lead to early viral protein synthesis. Of special interest was the mutation W174Y, which retained almost 50% of its ability to cause cell-cell fusion when transfected into cells, yet F-BAC-gB(W174Y)/gH- did not obviously mediate entry into cells. The inability of F-BAC-gB(W174Y)/gH- to enter cells was consistent with very low (5%) complementation of a gB null mutant by the gB(W174Y) molecule (19). Even though F-BAC-gB(W174Y)/gH- did not enter cells well, this virus could spread well from cell to cell, forming some plaques that were similar in size to those of wild-type HSV, although other plaques were smaller. Therefore, the reduced fusion activity of gB(W174Y) was insufficient for entry and complementation but was sufficient for cell-to-cell spread and cell-cell fusion. Two other mutants, gB(W174R) and gB(A261D), which retained about 9% of their activity in cell-cell fusion assays and had no complementation activity (19), were unable to mediate entry and did not mediate cell-to-cell spread (see Table 2). A comparison of these three mutant gB molecules suggests that gB must attain a threshold level of fusion activity that is substantially higher for entry of extracellular virions than that required for cell-cell fusion and cell-to-cell spread. Cell-to-cell spread can occur at relatively normal rates with less fusion activity, e.g., with gB(W174Y), which exhibited 50% cell-cell fusion activity yet did not promote entry and did not complement a gB null mutant. Cell-to-cell spread involves virus particles secreted onto cell surfaces at sites of cell-cell contact, and virus is transferred across these cell-cell junctions to enter adjacent cells (24, 29). Virions moving across cell junctions apparently have major advantages in entering other cells, as they are presented directly to the adjacent cells in a relatively concentrated form and can rapidly move into the neighboring cell. In contrast, extracellular virions in solution are less concentrated and must seek out host cells, and this appears to be less efficient. Thus, lower levels of gB fusion activity may suffice for cell-to-cell spread.
TABLE 2.
Summary of gB fusion loop mutants in fusion, complementation, cell-to-cell spread, and nuclear egress
| Expression of fusion loop mutant | % Cell-cell fusiona | % Complementationb | Entryc | Spreadd | Herniationse |
|---|---|---|---|---|---|
| Wild-type gB | 100 | 100 | + | Normal | − |
| gB(W174Y) | 46 | 5 | − | Near normal | +/− |
| gB(W174R) | 9 | 0 | − | None | + |
| gB(A261D) | 9 | 0 | − | None | + |
| gB(Y179K) | 0 | 0 | − | Reduced | + |
| No gB | 0 | 0 | − | None | + |
The CHO cell-cell fusion assay was used, involving transfection of plasmids encoding mutant gB, wild-type gD, and gH/gL (20).
Vero cells were transfected with plasmids encoding mutant gB and then infected with an HSV gB-null mutant, and complementation was measured (19).
HaCaT cells were infected with HSV recombinants expressing gB mutants (produced using gH-complementing F6 Vero cells), and expression of newly-synthesized HSV proteins was measured (Fig. 2).
Cell-to-cell spread involving F6 (gH-expressing) Vero cells (Fig. 3).
Nuclear egress in HaCaT cells infected with HSV recombinants expressing mutant gB and no gH was measured by counting the numbers of enveloped virus particles in herniations and perinuclear virions rather than the number of virions on cell surfaces (Table 1).
Results with F-BAC-gB(Y179K) were also consistent with the hypothesis that low levels of gB fusion activity are sufficient for cell-to-cell spread but not for entry. The gB(Y179K) molecule exhibited no detectable cell-cell fusion activity and did not complement a gB null HSV (19, 20), and F-BAC-gB(Y179K)/gH- displayed no capacity to enter cells (Table 2). Surprisingly, F-BAC-gB(Y179K)/gH- spread between cells, moving to 3 to 15 other cells in most plaques. Based on this observed cell-to-cell spread, we suspect that gB(Y179K) has some fusion activity, not detected in other assays. Again in this case, HSV particles shed onto the surfaces of infected cells must be able to enter into closely adjacent cells, inefficiently but nonetheless to some extent, by a pathway that requires only very low levels of gB fusion activity. Again, this highlights substantial differences between entry of extracellular virions and spread of virions across cell junctions.
One might argue that our entry assay failed to detect small amounts of virus entering cells that promoted only low levels of HSV protein expression. However, it was clear that all four mutants, including F-BAC-gB(W174Y)/gH-, did not differ substantially from the gB null mutant in terms of de novo viral protein synthesis, yet HSV expressing wild-type gB produced copious amounts of viral proteins. Moreover, none of these mutants showed any significant entry, and none complemented a gB null mutant, except gB(W174Y), which produced only 5% complementation (Table 2). Another issue that must be considered in comparing these various assays of gB fusion activity involves the use of different cell types. Previous studies describing cell-cell fusion with gB fusion loop mutants involved Chinese hamster ovary (CHO) cells (20), while complementation of gB null HSV involved Vero cells (19). Our cell-to-cell spread assays necessitated expression of gH, and thus, F6 cells (derived from Vero cells) were used. Entry assays and nuclear egress assays (see below) involved HaCaT keratinocytes, cells that mimic HSV infection in vivo in a number of ways. HSV cell-to-cell spread in HaCaT cell monolayers is very rapid, and large plaques are produced (45). Moreover, large numbers of infectious particles are produced in these cells following infection with wild-type HSV, and numerous herniations are produced following infection with gB and gH null HSV (12). HaCaT cells express very high levels of nectin-1 (10- to 20-fold higher than those in Vero cells) (45). Importantly, none of the gB fusion loop mutants entered HaCaT cells, a process we might expect to be efficient at the level of receptor binding. Thus, the differences we observed with F-BAC-gB(W174Y)/gH- and F-BAC-gB(Y179K)/gH-, which could not enter HaCaT cells and yet could spread between F6 Vero cells, cannot be explained by lower levels of gD receptors. Other differences between these cells, e.g., lipid compositions or other properties, might explain certain differences. More likely to us is the hypothesis described above, suggesting that entry of extracellular virions added to cells in solution is much less efficient than transfer of virus particles across cell junctions.
We also observed a relatively strong dominant-negative effect of mutant gB molecules. Virus stocks of mutants F-BAC-gB(W174R)/gH- and F-BAC-gB(A261D)/gH- produced on complementing F6/gB12 cells (that express wild-type gB and gH) exhibited 10- to 20-fold-lower titers than those of F-BAC-gBgalK/gH-, which expresses no gB. Since gB forms dimers or trimers, it appears that mutant forms of gB (unable to cause fusion) can also poison oligomers so that the wild-type molecules are also less effective in mediating entry into cells. The complementing cells likely express lower levels of wild-type gB than mutant gB molecules produced from the HSV genome, so that many wild-type molecules are complexed with mutant molecules.
Our principal objective was to assess whether gB fusion loop mutations affected nuclear egress. This addressed the question of whether gB acts directly in the fusion between the viral envelope and the outer NM. It was conceivable that gB and gH/gL might act indirectly in nuclear egress, e.g., loss of gB and gH might alter the envelope of perinuclear virions so that fusion cannot occur. HSV recombinants expressing gB mutants W174R, Y179K, and A261D (and no gH) displayed herniations and perinuclear virions similar to those produced by the gB and gH null mutant F-BAC-gBgalK/gH-. Moreover, the ratios of nuclear/cell surface virions were similar to those of the gB and gH null mutant (Table 1). Many fewer herniations were observed with F-BACgH-, which expresses wild-type gB, consistent with previous studies that loss of gH has little effect on nuclear egress (12). F-BAC-gB(W174Y)/gH- produced an intermediate phenotype (Table 1), in that some cells displayed herniations and other cells did not exhibit herniations and had large numbers of cell surface virions. Thus, the nuclear egress of F-BAC-gB(W174Y)/gH- was consistent with observations of intermediate cell-cell fusion activity (50%) and relatively normal cell-to cell spread (Table 2).
We concluded that the membrane fusion activity of gB is necessary for HSV egress across the NE (in the absence of gH). This is important support for the hypothesis that the two HSV fusion proteins, gB and gH/gL, mediate the second stage of nuclear egress by promoting fusion between the virion envelope and the outer NM. In this respect, the membrane fusion that occurs at the NE has some fundamental similarities to the membrane fusion that occurs during entry. gB and gH/gL might be triggered by gD interactions with known gD receptors or other, as yet unidentified gD receptors. However, HSV gD null mutants do not display defects in nuclear egress (11, 33). Thus, it is very possible that the regulation of gB-mediated fusion at the NE is different from that at the plasma membrane. For example, we proposed that phosphorylation of gB cytoplasmic residue T887 by the HSV kinase US3 might activate gB for fusion (46). US3 is likely to be brought into close proximity with the gB cytoplasmic domain during primary envelopment. Alternatively, gB might be released from the effects of gK, a membrane protein that negatively regulates fusion, is localized to nuclear membranes, and is not found in extracellular virions (25, 26, 34). There may also be other, as yet uncharacterized mechanisms to regulate gB and gH/gL in fusion at the outer NM.
Acknowledgments
We are especially indebted to Michael Webb of the OHSU electron microscopy core facility for his extensive efforts and skill in performing the electron microscopic studies.
This work was supported by National Institutes of Health grants EY018755 to D.C.J., AI076231 and AI056045 to R.J.E., and AI18289 to G.H.C. B.P.H. was supported by predoctoral training grant T32-AI076234.
Footnotes
Published ahead of print on 16 September 2009.
REFERENCES
- 1.Atanasiu, D., J. C. Whitbeck, T. M. Cairns, B. Reilly, G. H. Cohen, and R. J. Eisenberg. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. USA 104:18718-18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avitabile, E., C. Forghieri, and G. Campadelli-Fiume. 2007. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J. Virol. 81:11532-11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backovic, M., G. P. Leser, R. A. Lamb, R. Longnecker, and T. S. Jardetzky. 2007. Characterization of EBV gB indicates properties of both class I and class II viral fusion proteins. Virology 368:102-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., E. Wills, R. J. Jacob, J. Pennington, and B. Roizman. 2007. Glycoprotein M of herpes simplex virus 1 is incorporated into virions during budding at the inner nuclear membrane. J. Virol. 81:800-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme, K. W., and T. Compton. 2006. Virus entry and activation of innate immunity, p. 111-130. In M. Reddehase (ed.), Cytomegaloviruses: biology and immunology. Caister Academic Press, Norfolk, United Kingdom.
- 7.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis-Poynter, N., S. Bell, T. Minson, and H. Browne. 1994. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J. Virol. 68:7586-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farnsworth, A., K. Goldsmith, and D. C. Johnson. 2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77:8481-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farnsworth, A., T. W. Wisner, M. Webb, R. Roller, G. Cohen, R. Eisenberg, and D. C. Johnson. 2007. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. USA 104:10187-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galdiero, S., A. Falanga, M. Vitiello, H. Browne, C. Pedone, and M. Galdiero. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 280:28632-28643. [DOI] [PubMed] [Google Scholar]
- 15.Galdiero, S., M. Vitiello, M. D'Isanto, A. Falanga, C. Collins, K. Raieta, C. Pedone, H. Browne, and M. Galdiero. 2006. Analysis of synthetic peptides from heptad-repeat domains of herpes simplex virus type 1 glycoproteins H and B. J. Gen. Virol. 87:1085-1097. [DOI] [PubMed] [Google Scholar]
- 16.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 17.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handler, C. G., R. J. Eisenberg, and G. H. Cohen. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J. Virol. 70:6067-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannah, B. P., T. M. Cairns, F. C. Bender, J. C. Whitbeck, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J. Virol. 83:6825-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannah, B. P., E. E. Heldwein, G. H. Cohen, and R. J. Eisenberg. 2007. Mutational evidence of internal fusion loops in HSV glycoprotein B. J. Virol. 81:4858-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldwein, E. E., and C. Krummenacher. 2008. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 65:1653-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 23.Horsburgh, B. C., M. M. Hubinette, D. Qiang, M. L. MacDonald, and F. Tufaro. 1999. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 6:922-930. [DOI] [PubMed] [Google Scholar]
- 24.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 75:10309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson, L., and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K promotes egress of virus particles. J. Virol. 69:5401-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 69:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutt-Fletcher, L. M. 2007. Epstein-Barr virus entry. J. Virol. 81:7825-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, D. C., R. L. Burke, and T. Gregory. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J. Virol. 64:2569-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato, A., J. Arii, I. Shiratori, H. Akashi, H. Arase, and Y. Kawaguchi. 2009. Herpes simplex virus 1 protein kinase Us3 phosphorylates viral envelope glycoprotein B and regulates its expression on the cell surface. J. Virol. 83:250-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loret, S., G. Guay, and R. Lippe. 2008. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 82:8605-8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9:423-429. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 38.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stannard, L. M., S. Himmelhoch, and S. Wynchank. 1996. Intra-nuclear localization of two envelope proteins, gB and gD, of herpes simplex virus. Arch. Virol. 141:505-524. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torrisi, M. R., C. Di Lazzaro, A. Pavan, L. Pereira, and G. Campadelli-Fiume. 1992. Herpes simplex virus envelopment and maturation studied by fracture label. J. Virol. 66:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warming, S., N. Costantino, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wills, E., F. Mou, and J. D. Baines. 2009. The UL31 and UL34 gene products of herpes simplex virus 1 are required for optimal localization of viral glycoproteins D and M to the inner nuclear membranes of infected cells. J. Virol. 83:4800-4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wisner, T. W., C. C. Wright, A. Kato, Y. Kawaguchi, F. Mou, J. D. Baines, R. J. Roller, and D. C. Johnson. 2009. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 83:3115-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]