Abstract
Tetherin (CD317/BST-2), an interferon-induced membrane protein, restricts the release of nascent retroviral particles from infected cell surfaces. While human immunodeficiency virus type 1 (HIV-1) encodes the accessory gene vpu to overcome the action of tetherin, the lineage of primate lentiviruses that gave rise to HIV-2 does not. It has been previously reported that the HIV-2 envelope glycoprotein has a Vpu-like function in promoting virus release. Here we demonstrate that the HIV-2 Rod envelope glycoprotein (HIV-2 Rod Env) is a tetherin antagonist. Expression of HIV-2 Rod Env, but not that of HIV-1 or the closely related simian immunodeficiency virus (SIV) SIVmac1A11, counteracts tetherin-mediated restriction of Vpu-defective HIV-1 in a cell-type-specific manner. This correlates with the ability of the HIV-2 Rod Env to mediate cell surface downregulation of tetherin. Antagonism requires an endocytic motif conserved across HIV/SIV lineages in the gp41 cytoplasmic tail, but specificity for tetherin is governed by extracellular determinants in the mature Env protein. Coimmunoprecipitation studies suggest an interaction between HIV-2 Rod Env and tetherin, but unlike studies with Vpu, we found no evidence of tetherin degradation. In the presence of HIV-2 Rod Env, tetherin localization is restricted to the trans-Golgi network, suggesting Env-mediated effects on tetherin trafficking sequester it from virus assembly sites on the plasma membrane. Finally, we recapitulated these observations in HIV-2-infected CD4+ T-cell lines, demonstrating that tetherin antagonism and sequestration occur at physiological levels of Env expression during virus replication.
Various stages of the replication cycle of primate lentiviruses can be targeted by host antiviral restriction factors (reviewed in reference 49). In addition to the well-characterized antiviral effects of members of the APOBEC3 family of cytidine deaminases, particularly APOBEC3G and -3F, and species-specific variants of tripartite motif family 5α, the release of nascent retroviral particles has recently been shown to be a target for a novel restriction factor, tetherin (CD317/bone marrow stromal cell antigen 2 [BST-2]) (31, 46). Tetherin is an interferon-inducible gene that was originally shown to impart a restriction on the release of mutants of human immunodeficiency virus type 1 (HIV-1) that lack a vpu gene (31, 46). In tetherin-positive cells, mature Vpu-defective HIV-1 particles are retained on the cell surface, linked to the plasma membrane (PM) and each other via protease-sensitive tethers, and can be subsequently endocytosed and accumulate in late endosomes (30, 31). Tetherin is not HIV specific and restricts the release of virus-like particles derived from all retroviruses tested (18), as well as those of filoviruses and arenaviruses (18, 19, 39).
Tetherin is a small (181-amino-acid) type II membrane protein with an unusual topology that exists mainly as a disulfide-linked dimer (34). It consists of an N-terminal cytoplasmic tail, a transmembrane anchor, an extracellular domain that includes three cysteine residues important for dimerization, a putative coiled-coil, and finally a glycophosphatidyinosityl-linked lipid anchor (22) that is essential for restriction (31). Tetherin localizes to retroviral assembly sites on the PM (18, 31), and this unusual structure is highly suggestive that tetherin restricts virion release by incorporation into the viral membrane and cross-linking virions to cells. Such a mechanism would make tetherin a powerful antiviral effector that can target an obligate part of most, if not all, enveloped virus assembly strategies. Moreover, since tetherin restriction has no specific requirement for virus protein sequences, to avoid its action, mammalian viruses have evolved to encode several distinct countermeasures that specifically inhibit tetherin's antiviral function.
The Vpu accessory protein antagonizes tetherin-mediated restriction of HIV-1 (31, 46). In the presence of Vpu, tetherin is downregulated from the cell surface (2, 46) and is targeted for degradation (10, 13, 14), although whether these processes are required for antagonism of tetherin function is unclear (27). HIV-1 Vpu displays a distinct species specificity in that it is unable to target tetherin orthologues from rhesus macaques or African green monkeys (14, 25). This differential sensitivity maps to the tetherin transmembrane domain, particularly residues that are predicted to have been under high positive selection pressure during primate evolution (14, 16, 25). This suggests that tetherin evolution may have been driven in part by viral countermeasures like Vpu. Vpu, however, is only encoded by HIV-1 and its direct simian immunodeficiency virus (SIV) lineage precursors. The majority of SIVs, including the SIVsm, the progenitor of both HIV-2 and SIVmac, do not encode a Vpu protein (21). In some of these SIVs, tetherin antagonism was recently shown to map to the nef gene (16, 51). SIV Nef proteins, however, are generally ineffective against human tetherin because they target a (G/D)DIWK motif that was deleted from the human tetherin cytoplasmic tail sometime after the divergence of humans and chimpanzees (51). This raises the question of how HIV-2 is able to overcome human tetherin, as recent data show chronically HIV-2-infected CEM T cells have reduced tetherin levels on their surface (10).
Interestingly, it has long been known that the envelope glycoprotein of certain HIV-2 isolates can stimulate the release of Vpu-defective HIV-1 virions from cells we now know to be tetherin positive (5, 6, 43). HIV and SIV Envs form trimeric spikes of dimers of the surface subunit (SU-gp105 in HIV-2/SIVmac and gp120 in HIV-1) that bind CD4 and the chemokine coreceptor and gp41 (the transmembrane [TM] subunit that facilitates fusion with and entry into the target cell). Envelope precursors (gp140 or gp160) are synthesized in the endoplasmic reticulum, where they become glycosylated and are exported to the surface via the secretory pathway (8). During transit through the Golgi apparatus and possibly in endosomal compartments, the immature precursors are cleaved by furin-like proteases to form mature spikes (15, 29). Multiple endocytosis motifs in the gp41 cytoplasmic tail lead to only minor quantities of Env being exposed at the cell surface at any given time (7, 40). Recent data demonstrated that the conserved GYxxθ motif, a binding site for the clathrin adaptor protein AP-2 (3), in the membrane-proximal region of HIV-2 gp41 is required to promote Vpu-defective HIV-1 release from HeLa cells (1, 32). Based on experiments with HIV-1/HIV-2 chimeric envelopes, an additional requirement in the extracellular component was suggested (1). In this study we set out to examine the Vpu-like activity of HIV-2 envelope in light of the discovery of tetherin. We demonstrate that the HIV-2 Env is a tetherin antagonist, and we provide mechanistic insight into the basis of this antagonism.
MATERIALS AND METHODS
Cells and plasmids.
All adherent cells were maintained in Dulbecco's modified Eagle medium (Invitrogen, United Kingdom) supplemented with 10% fetal calf serum and penicillin-streptomycin; T-cell lines were grown in Roswell Park Memorial Institute medium supplemented with 10% fetal calf serum and penicillin-streptomycin. HEK293T, HeLa, HT1080, and Jurkat cells were obtained from the ATCC. HT1080/THN-HA and 293T/THN-HA are clonal cell lines stably expressing human tetherin with a hemagglutinin (HA) epitope tag inserted at nucleotide 463 in the context of the retroviral vector pLHCX (Clontech) (25). HeLa-TZM, an indicator cell line for HIV/SIV infection that expresses CD4 and CCR5 and carries an HIV-1 long terminal repeat (LTR)-driven β-galactosidase reporter gene, was obtained from the NIH AIDS Research and Reference Reagent Program (ARRP). CEM-G37 cells that express green fluorescent protein (GFP) under the control of an HIV-1 LTR were kindly provided by A. Vyakarnam, King's College London, London, United Kingdom.
Wild-type HIV-1 NL4.3 and a Vpu-defective counterpart have been described previously (30). Envelope-defective clones were derived by filling and ligating a unique NheI site in the envelope reading frame. The HIV-2 molecular clone pRod10 was obtained from the Centre for AIDS Research (NIBSC, Potters Bar, United Kingdom), and the SIVmac1A11 (24) clone was obtained from the ARRP, NIAID, NIH. The HIV-2 RodA Env cDNA was kindly provided by A. McKnight (45). Envelope expression vectors of the HIV-2 RodA envelope, Rod10 envelope, NL4.3 envelope, and SIVmac1A11 envelope and mutants/chimeras thereof created by overlapping PCR were constructed by cloning Env PCR products as EcoRI-NotI (HIV-1/HIV-2 Env) or NotI (SIVmac) fragments into pCRV1-delVpu (kindly provided by P. Bieniasz), an HIV-based expression vector (50). An internal ribosome entry site (IRES)-GFP derived from pMigR1-GFP was cloned downstream of the Env protein in some vectors for cell surface downregulation assays. For delivery by retroviral transduction, envelopes were cloned into pMigR1-IRES-puro, kindly provided M. Malim.
Production of viral and vector stocks.
Subconfluent 293T cells were transfected using polyethylenimine (1 mg/ml; Polysciences GmBH, Germany). For retroviral vectors encoding primate tetherins or HIV-2 Envs, cells were transfected with a 5:5:1 ratio of the vector plasmid, pMLV-GagPol, and pCMV-VSV-G, and supernatants were harvested 48 h posttransfection and used to transduce target HT1080 or HT1080/THN-HA cells. For full-length G-protein of vesicular stomatitis virus (VSV-G)-pseudotyped HIV-1, HIV-2, and SIVmac1A11 stocks, 293T cells were transfected with 2 μg of proviral plasmid and 200 ng of pCMV-VSV-G. Forty-eight hours later, viral stocks were harvested and endpoint titers were determined on HeLa-TZM. Two days after infection the cells were fixed, stained for β-galactosidase activity using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (Sigma, United Kingdom), and foci were enumerated to calculate infectious units/ml.
Viral release assays.
For transient-transfection-based virus release assays, subconfluent HeLa or 293T cells were transfected with 500 ng of proviral clone, 100 ng of Vpu/Env expression vector, 25 ng of pCMV-VSVG if required, and for 293T-based assays increasing doses of pCR3.1-tetherin, using Lipofectamine 2000 (Invitrogen, United Kingdom). At 5 h posttransfection the medium was replaced and the cells were cultured for 2 days. Viral supernatants were filtered (0.22 μm) and, for infectious release assays, used to infect HeLa-TZM cells, which were then assayed for relative β-galactosidase activity by using a chemiluminescence Tropix GalactoStar kit (Applied Biosystems) 48 h later. For biochemical analyses, virions were pelleted through 20% sucrose in a benchtop microcentrifuge and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. Cell and virion lysates were then subjected to SDS-PAGE, and Western blot assays were performed for HIV-1 p24CA (monoclonal antibody 183-H12-5C; kindly provided by K. Werhly and B. Cheseboro through the ARRP, NIAID, NIH) or HIV-2/SIVmac p26/p27CA (ARP3601; NIBSC, Potters Bar, United Kingdom) or rabbit anti-Hsp90 (Santa Cruz Biotechnology) as a loading control. Blots were visualized with a Licor apparatus and using anti-mouse 680 and anti-rabbit 700 secondary antibodies.
For single-round virus replication assays, HeLa cells were plated at 105 cells per well of a 12-well dish. The cells were then infected with VSV-G-pseudotyped HIV-1, HIV-2, or SIVmac1A11 stocks at a multiplicity of infection (MOI) of 0.2 as standardized on HeLa-TZM. The cells were washed and the medium was replaced 24 h after infection. Cell lysates and viral supernatants were then harvested the following day and processed as described above. For examining tetherin degradation HT1080/THN-HA cells were treated similarly except that the input inoculum was an MOI of 2 to ensure approximately 90% cell infection, and cell lysates were blotted for tetherin-HA using a rabbit anti-HA polyclonal antibody (Rockwell Biosciences) and analyzed by using a Licor apparatus. For virus release from Jurkat cells, 3 × 105 cells were infected with VSV-G-pseudotyped HIV-2 Rod10 or corresponding envelope mutants were infected at an MOI of 0.2. Forty-eight hours later cell lysates and virions supernatants were harvested and processed as described above.
siRNA-mediated tetherin knockdown.
HeLa cells (50% confluent) seeded in six-well plates were transfected with the small interfering RNA (siRNA) oligonucleotide SmartPool against human tetherin or a control nontargeting pool (Dharmacon) using Oligofectamine (Invitrogen) in Opti-MEM I (Invitrogen) as per the manufacturer's instructions. The following day, the cells were infected with the different viruses described above at an MOI of 0.2. Five hours later, the inoculum was removed and a second transfection with siRNA was performed. At 48 h after infection, cell lysates and viral particles from the supernatant were collected as previously described for virus release Western blot analysis.
Immunoprecipitation.
HeLa cells transfected 48 h previously with pCR3.1-tetherin-HA and either empty vector or pCRV1-RodA Env, pCRV1-RodA CS- Env, or pCRV1 NL4.3 Env were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, Complete protease inhibitors [Roche]) and sonicated. Lysates were incubated with monoclonal mouse anti-HA.11 antibody (Covance, Cambridge Bioscience, United Kingdom) for 1 h at 4°C. Sepharose-protein G beads (Invitrogen, United Kingdom) were added, and samples were incubated overnight at 4°C with shaking. Samples were washed four times in RIPA buffer and were resuspended in 2× loading buffer and subjected to SDS-PAGE. Proteins were analyzed by Western blotting with either rabbit antiserum to HIV-2 gp105 (ARP418; Center For AIDS Reagents, NIBSC, Potter's Bar, United Kingdom) or HIV-1 gp120 (provided by M. Malim) or with anti-HA antibodies.
Flow cytometry.
Six-well dishes of HeLa cells or 293T/THN-HA cells were transfected with 1 μg of pCRV1-envelope/Vpu-IRES-GFP vectors using Lipofectamine. At 48 h after transfection the cells were harvested in phosphate-buffered saline (PBS)-5 mM EDTA and stained for surface tetherin using either an anti-human BST-2 monoclonal antibody (Abnova) or anti-HA monoclonal antibody and a secondary goat anti-mouse Alexa 633 antibody (Molecular Probes). Cells were then analyzed using a FACSCalibur flow cytometer (Beckton Dickinson) and CellQuest software. For CEM-G37, cells were stained for surface tetherin 48 h postinfection, and the GFP+ cell population was analyzed and compared to tetherin expression on uninfected control cells.
Confocal microsocopy.
HeLa cells or 293T/THN-HA cells transiently transfected or HT1080/THN-HA cells retrovirally transduced and selected in puromycin (2 μg/ml), with HIV/SIV envelope expression vectors, were grown on glass coverslips overnight. The cells were then fixed in 4% paraformaldehyde-PBS, quenched in 10 mM glycine-PBS, and permeabilized for 10 min in PBS-1% bovine serum albumin-0.1% Triton X-100. The cells were then stained for tetherin (either mouse anti-human BST-2 polyclonal antibody [Abnova] or mouse anti-HA.11 [Covance]), rabbit anti-HIV-2/HIV-1/SIVmac envelope, and if required, sheep-anti-human TGN46 (Serotec), followed by secondary goat or donkey anti-primary antibodies conjugated to Alexa 488, 594, or 633 (Molecular Probes, Invitrogen, United Kingdom), and mounted on slides using AntiFade-4′,6-diamidino-2-phenylindole (DAPI) mounting solution (Molecular Probes, Invitrogen, United Kingdom). Cells were then visualized with a Leica DM-IRE2 confocal microscope. For imaging infected T cells, Jurkat cells were infected with VSV-G-pseudotyped viral stocks at an MOI of 1. Forty-eight hours later the infected cells were allowed to adhere to polylysine-coated coverslips for 2 h, fixed, and processed as above.
RESULTS
Cell-type-specific antagonism of tetherin by HIV-2 Env.
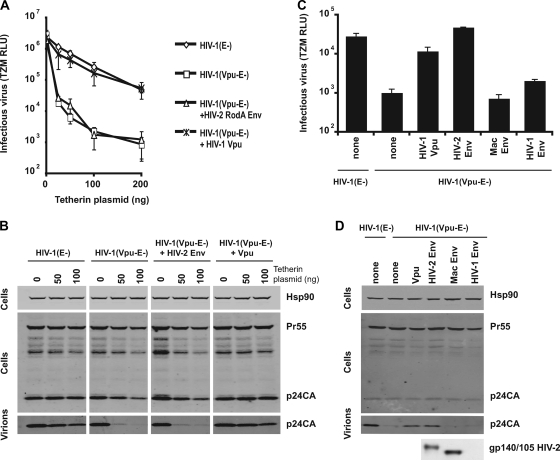
Since the HIV-2 Env has been reported to have a Vpu-like function, we first sought to demonstrate that it was capable of rescuing the release of Vpu-defective HIV-1 from tetherin restriction. Human 293T cells, which lack constitutive tetherin expression (31, 46), were transfected with HIV-1 NL4.3 Env− [HIV-1 (E-)] or HIV-1 NL4.3 Env− Vpu− [HIV-1 (Vpu-E-)] proviruses in the presence of increasing plasmid doses of a human tetherin expression vector, with either an expression vector encoding the HIV-2 RodA Env (45) or HIV-1 Vpu. Twenty-five nanograms of a VSV-G-encoding plasmid was added to each transfection mixture to allow the titration of the produced viruses on HeLa-TZM indicator cells. At 48 h after transfection, cell lysates and viral supernatants were harvested and prepared for Western blotting and/or titrated on HeLa-TZM. As expected (31), despite having no effect on viral gene expression, increasing levels of tetherin selectively restricted the release of the Vpu− virus, at both the level of infectious virus release (Fig. 1A) and physical particle yield as measured by p24-CA Western blotting of sucrose-pelleted virions (Fig. 1B). Furthermore, expression of Vpu in trans fully restored HIV-1 (Vpu-E-) virus production to wild-type levels. To our surprise, however, expression of HIV-2 RodA Env was unable to rescue Vpu-defective virus release, even at the lowest inputs of tetherin plasmid. We therefore reexamined the effects of HIV-2 RodA Env in HeLa cells that were constitutively tetherin positive. Again, as expected, Vpu-defective HIV-1 release was restricted 20-fold compared to the wild-type virus, and this was rescued by Vpu expression in trans (Fig. 1C and D). By contrast to 293T cells, however, expression of HIV-2 RodA Env was also capable of rescuing HIV-1 (Vpu−) to wild-type levels, in terms of both virus infectivity and physical particle yield. This effect was specific to HIV-2 RodA Env, as expression of either the HIV-1 NL4.3 Env or the related SIVmac1A11 envelope failed to similarly rescue HIV-1 (Vpu-E-) release (Fig. 1C and D). Thus, we could confirm that the HIV-2 RodA Env has an intrinsic Vpu-like activity in promoting virus release. Therefore, the HIV-2 RodA Env, but not a related SIVmac nor an HIV-1 Env, was capable of antagonizing tetherin in HeLa cells. Unlike Vpu-mediated tetherin antagonism, the HIV-2 RodA Env displays a degree of cell type specificity, suggesting mechanistic differences between the two proteins’ modes of action.
FIG. 1.
Cell-type-dependent antagonism of tetherin restriction by the HIV-2 RodA envelope. (A) 293T cells were transfected with envelope-defective HIV-1 (E-) or a Vpu-defective counterpart, HIV-1 (Vpu-E-) molecular clones, in the presence of HIV-2 RodA Env or HIV-1 Vpu expression vectors and increasing doses of a human tetherin expression vector and pseudotyped with VSV-G. Supernatants were harvested 48 h later and used to infect HeLa-TZM indicator cells. Infectious virus release is plotted as β-galactosidase activity in relative light units (RLU) based on a commercial chemiluminescence assay. Error bars represent standard deviations of the means of three independent experiments. (B) Cell lysates of 293T cells in panel A and virions pelleted from the corresponding supernatants were subjected to SDS-PAGE and Western blotting for HIV-1 Gag or cellular Hsp90 and revealed by Licor fluorescent secondary antibodies. (C) A similar experiment as that in panel A was performed in HeLa cells, which express tetherin endogenously. In this case expression constructs for the HIV-1 NL4.3 Env and SIVmac1A11 Env were also used. Error bars represent standard deviations of the means of three independent experiments. (D) Western blots corresponding to results in panel C for HIV-1 p24-CA and HIV-2 Env gp140/105 (in cell lysates).
Expression of HIV-2 Env leads to downregulation of cell surface tetherin levels.
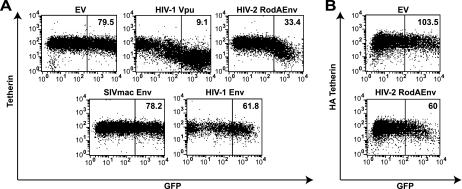
Expression of Vpu has been previously shown to lead to cell surface downregulation of tetherin levels (26, 46). To examine whether this was similar for HIV-2 RodA Env, we constructed expression vectors in which HIV-2 RodA Env and mutants thereof, SIVmac1A11, HIV-1 NL4.3 Env, and Vpu open reading frames, were linked to an enhanced GFP gene via a downstream IRES. HeLa cells were transfected with these constructs and analyzed 48 h later for surface tetherin expression by flow cytometry using a monoclonal antibody specific for human tetherin. Expression of GFP alone had no effect on tetherin surface levels, whereas Vpu expression led to a profound downregulation of tetherin expression in the GFP+ population (Fig. 2A). HIV-2 RodA Env− expression also led to cell surface downregulation of tetherin. While this downregulation was clearly less efficient than that induced by Vpu, the effect was specific, since neither SIVmac1A11 nor HIV-1 Env expression could mediate any difference in tetherin levels. We also examined the ability of HIV-2 RodA Env to downregulate tetherin from 293T cells stably expressing a tetherin molecule with an HA tag embedded in its extracellular domain (Fig. 2B). In this case little downregulation was observed, consistent with a defect in antagonism of tetherin in these cells. Thus, like Vpu, HIV-2 RodA Env is capable of lowering cell surface levels of tetherin in cells where it can antagonize its antiviral function.
FIG. 2.
HIV-2 RodA Env expression mediates cell surface tetherin downregulation in HeLa cells. HeLa (A) or 293T/THN-HA (B) cells were transfected with a control empty vector (EV) or vectors encoding the indicated viral proteins linked to enhanced GFP via an IRES. At 48 h posttransfection the cells were stained for cell surface tetherin levels with a monoclonal anti-BST-2 antibody (A) or a monoclonal anti-HA (B) antibody and then a secondary goat-anti-mouse Alexa 633 antibody. The cells were then analyzed by flow cytometry. Median fluorescence intensities are indicated for the boxed regions.
Antagonism and downregulation of tetherin requires both the conserved GYxxθ endocytosis signal in the cytoplasmic tail of gp41 and a specificity determinant in the extracellular domain of the mature Env protein.
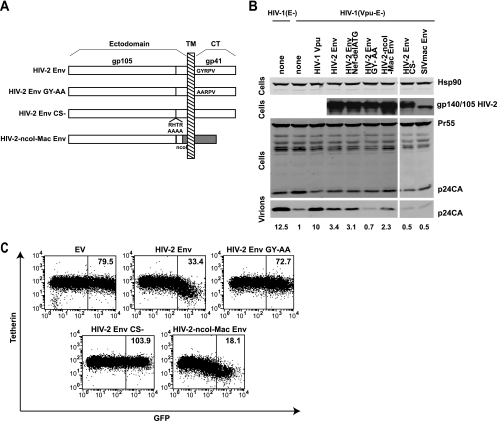
Previous studies have shown that the majority of the cytoplasmic tail of HIV-2 gp41 is dispensable for its Vpu-like function, but a membrane-proximal GYxxθ located 8 amino acids from the transmembrane domain plays a role in promoting virus release (1). This motif, a binding site for the clathrin adaptor protein AP-2 (32), is a major determinant of HIV/SIV envelope endocytosis (40). However, it has also been suggested that extracellular domains of Env contribute to the enhanced release of virions from HeLa cells (1). Since we had two related envelopes (HIV-2 RodA and SIVmac1A11) with different abilities to counteract tetherin-mediated restriction and promote its cell surface downregulation, we constructed several mutants and chimeric envelopes to examine the determinants of tetherin antagonism by HIV-2 Env (Fig. 3A). We first made an inactivating mutation in the GYxxθ motif (GY 719/720 AA). As expected, despite equivalent expression levels, this mutant envelope was compromised in its abilities to pseudotype Env− HIV-1 (Fig. 3B and data not shown), consistent with published data concerning the requirements for this endocytic motif in the Env CT-Gag interactions that effect particle infectivity (48). The GY-AA mutant was defective in its ability to rescue HIV-1 (Vpu-E-) from HeLa cells (Fig. 3B). Furthermore, in contrast to the wild-type Env, HIV-2 RodA Env GY-AA was also unable to mediate cell surface downregulation of tetherin (Fig. 3C). The membrane-proximal GYxxθ is highly conserved in the envelopes of primate immunodeficiency viruses (23), including both HIV-1 NL4.3 and SIVmac1A11, which were shown above to have no Vpu-like function. In addition, given the overlap of the nef open reading frame with downstream gp41 sequences, we silently mutated the nef start codon (Nef-delATG) to ensure that no effect we were observing could be attributed to expression of N-terminal Nef fragments from our constructs (Fig. 3B). Thus, the GYxxθ endocytic motif is essential for the ability of the HIV-2 RodA Env to antagonize tetherin and for cell surface downregulation but does not in itself confer specificity for human tetherin.
FIG. 3.
Determinants of tetherin antagonism in HIV-2 Env. (A) HIV-2 RodA Env constructs were made with inactivating mutations in the GYxxθ motif (GY 719/720 AA): RodA GY-AA, disruption of the SU-TM cleavage site RTHR, RodA-CS-, and a chimeric envelope in which the RodA and SIVmac1A11 Envs were fused at a conserved NcoI site in the extracellular gp41 coding sequence (Rod-NcoI-Mac). (B) The ability of these Envs to promote Vpu− virus release from HeLa cells was tested as described for Fig. 1, and Western blot assays were performed on cell lysates and virions for HIV-1 p24-CA and HIV-2 Env. Numbers represent the fold increase in virus release (p24 band intensity) compared to the Vpu-defective control. (C) HeLa cells were transfected with Env mutant and chimera IRES-GFP constructs, and cell surface staining for tetherin was analyzed by flow cytometry and compared to that for the control empty vector (EV) or wt RodA Env (reproduced from Fig. 2). Median fluorescence intensities are indicated for the boxed regions.
We then asked whether correct envelope cleavage was required for tetherin antagonism, since recent evidence shows that Ebola virus G protein tetherin antagonism is unaffected by inhibiting its proteolytic maturation (19). We therefore mutated the furin-like cleavage site (RHTR) at the gp105/gp41 junction (Fig. 3A) and found that gp140 was not processed in transfected HeLa cells, nor could it functionally pseudotype Env− HIV-1 (data not shown). In contrast to the Ebola virus G protein, however, mutation of the cleavage site completely abolished the ability of HIV-2 RodA Env to antagonize tetherin restriction in HeLa cells (Fig. 3B) and downregulate tetherin surface levels (Fig. 3C). Thus, to antagonize tetherin, the HIV-2 RodA Env must be able to be processed correctly into mature envelope trimers. This suggested to us that extracellular motifs in HIV-2 RodA Env may play a role in tetherin specificity.
The SIVmac1A11 envelope has a truncated gp41 (gp36) cytoplasmic tail, stopping 9 amino acids after the GYxxθ motif (24). To determine where specificity for tetherin lies in HIV-2 RodA Env, we constructed a Rod-Mac chimeric envelope by fusing the N-terminal HIV-2 Rod Env and the C-terminal SIVmac1A11 Env at a conserved NcoI site within the coding sequence of the C-terminal heptad repeat in the extracellular fusion mechanism of gp41/gp36. This chimera was expressed as a functional envelope and could both partially rescue HIV-1 (Vpu-E-) particle release form HeLa cells and mediate cell surface tetherin downregulation (Fig. 3B and C). Thus, the TM domain and GYxxθ motif of SIVmac1A11 Env were capable of substituting for those in the RodA Env in tetherin antagonism and cell surface downregulation, thereby implicating extracellular parts of the mature HIV-2 Env in determining tetherin specificity.
The envelope is responsible for HIV-2 evasion of tetherin in HeLa cells.
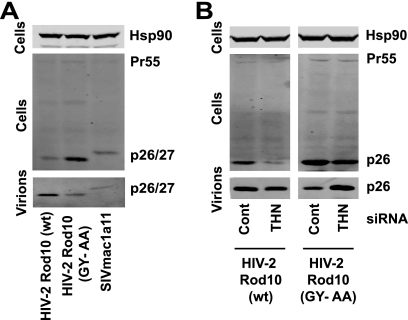
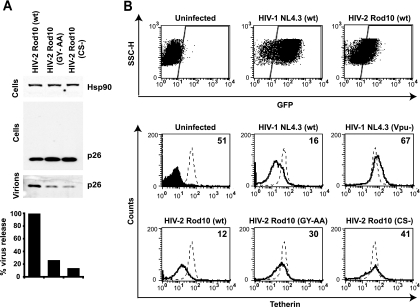
We then sought to demonstrate that our observations of the requirements for HIV-2 Rod Env to rescue HIV-1 (Vpu-E-) in trans were similarly required in the context of a full-length HIV-2 molecular clone to avoid tetherin restriction. The HIV-2 Rod10 molecular clone was therefore modified to bear the GY-AA mutation in the gp41 cytoplasmic tail. VSV-G-pseudotyped stocks were produced and used to infect HeLa cells at a multiplicity of infection of 0.2. Forty-eight hours later the viral supernatants were harvested, and cell lysates and virions were analyzed by Western blotting for capsid proteins using an anti-SIVmac p27-CA antibody. HIV-2 Rod10 (GY-AA) virion release from HeLa cells was impaired compared to the wild-type virus (Fig. 4A). This was reflected by a concomitant increase of mature p26-CA in cell lysates, which can often be seen in tetherin-induced virus retention (30). Interestingly, the GY-AA particle release was similar to that released from SIVmac1A11-infected cells, whose envelope cannot antagonize tetherin in trans. We then showed that RNA interference depletion of tetherin, but not a control siRNA oligonucleotide pool, from HeLa cells infected with HIV-2 Rod10 (GY-AA) was sufficient to restore virus release to wild-type levels (Fig. 4B). Thus, HIV-2 Rod Env antagonizes human tetherin in the context of the wild-type virus, and the requirement for the GYxxθ endocytic motif is functionally relevant. Moreover, these data demonstrate Env is the major determinant of HIV-2 Rod10 resistance to tetherin.
FIG. 4.
Effects of Env on tetherin-mediated particle release of HIV-2 Rod10 virions. (A) HeLa cells were infected with VSV-G-pseudotyped HIV-2 Rod10, HIV-2 Rod10 (GY-AA), or SIVmac1A11 at an MOI of 0.2 as standardized on HeLa-TZM. Forty-eight hours later virions and cell lysates were analyzed by Western blotting for HIV-2/SIVmac p26/p27 CA. (B) HeLa cells were treated with siRNA oligonucleotides directed at human tetherin or a control pool and then infected with VSV-G-pseudotyped HIV-2 Rod10 and HIV-2 Rod10 (GY-AA) and processed as described for panel A.
Coimmunoprecipitation of HIV-2 Env with tetherin.
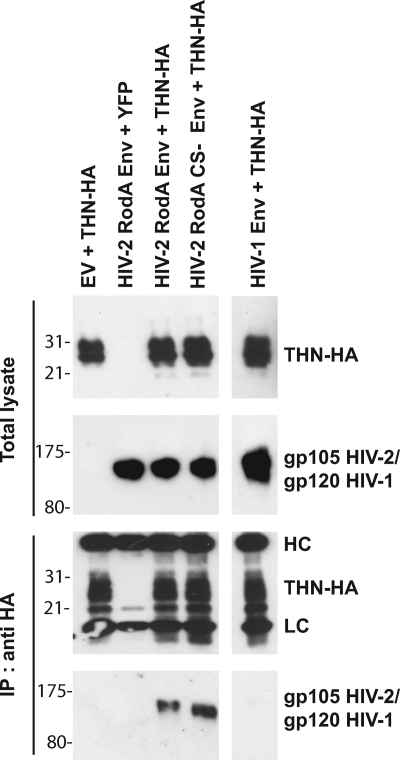
To examine some of the mechanistic processes that might underlie the antagonism of tetherin by HIV-2 RodA Env, we first sought to determine if tetherin and HIV-2 RodA Env physically interact in HeLa cells. HeLa cells were transfected with a human tetherin expression vector where the tetherin bears an HA tag embedded within its extracellular domain, C-terminal to the predicted coiled-coil (18). The cells were also cotransfected with empty vector, HIV-2 RodA Env, or HIV-1 NL4.3 Env. After 48 h the cells were lysed and tetherin immunoprecipitated with an anti-HA monoclonal antibody. Immunoprecipitates were then blotted for either HIV-2 or HIV-1 Env (Fig. 5). Despite equivalent expression levels, immunoprecipitation of tetherin-HA led to selective pulldown of the HIV-2 Env protein but not the HIV-1 Env, suggesting that tetherin and HIV-2 Env can directly or indirectly interact in cells where it can antagonize tetherin activity. Since the cleavage mutant of the HIV-2 envelope was unable to antagonize tetherin, we also determined whether it too could coimmunoprecipitate. Interestingly, the HIV-2 RodA CS- Env retained the ability to coprecipitate with tetherin, suggesting that while RodA Env specifically interacts with tetherin, proper envelope processing is required for subsequent antagonism of its antiviral activity, and therefore interaction between Env and tetherin is insufficient to explain antgonism.
FIG. 5.
Coimmunoprecipitation of HIV-2 RodA Env with tetherin. HeLa cells were transfected with the indicated plasmid vectors with empty vector (EV) or yellow fluorescent protein (YFP) vector replacing Env or tetherin-HA, respectively, as negative controls. Forty-eight hours later, tetherin was immunoprecipitated (IP) from cell lysates and subjected to SDS-PAGE. Total lysates and immunoprecipitates were then Western blotted for tetherin-HA and HIV-2 or HIV-1 Env. HC and LC signify the heavy and light chains of the immunoprecipitating antibody, respectively.
Tetherin is degraded in HIV-1-infected but not HIV-2-infected cells.
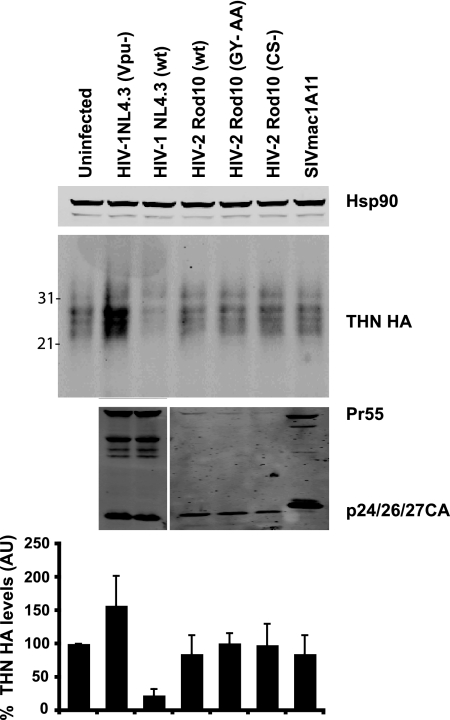
Since Vpu expression can lead to either a endolysozomal (10) or proteasomal (13) degradation of tetherin in infected cells, we next asked whether HIV-2-infected cells showed any evidence of tetherin degradation. HIV-1 or HIV-2 stocks pseudotyped with VSV-G were used to infect HT1080/THN-HA cells, which stably express a human tetherin bearing an HA tag C-terminal to the extracellular coiled-coil domain (18), at an MOI of 2, and 48 h later cell lysates were analyzed for tetherin expression (Fig. 6). As expected in cells infected with HIV-1 (wild type [wt]), tetherin immunoreactivity was substantially diminished compared to uninfected cells. By contrast, HT1080/THN-HA cells infected with high-MOI doses of HIV-2 (wt) or HIV-2 Rod10 (CS-), HIV-2 Rod10 (GY-AA), or SIVmac1A11 showed no reduction in total cellular content of tetherin. Cells infected with HIV-1 (Vpu-) showed a 1.5-fold increase in tetherin levels, perhaps reflecting accumulation of tetherin in the dramatic sheets of tethered particles seen in these cells (31). Thus, while HIV-2 Env downregulates tetherin levels from the cell surface, in contrast to Vpu, this does not appear to be accompanied by substantial degradation of tetherin in cell lysates.
FIG. 6.
Tetherin is not degraded in HIV-2-infected cells. HT1080 cells stably expressing tetherin-HA (HT/THN-HA) were infected with VSV-G-pseudotyped HIV-1 (wt), HIV-1 (Vpu-), HIV-2 (wt), HIV-2 (GY-AA), HIV-2 (CS-), or SIVmac1A11 at an MOI of 2 as standardized on HeLa-TZM cells to ensure approximately 90% of the cells were infected. At 48 h after infection total cell lysates were subjected to SDS-PAGE and Western blotted for tetherin-HA, HIV-1/HIV-2 CA, and Hsp90. Corresponding tetherin band intensities were corrected for Hsp90 levels and plotted as a percentage of tetherin-HA levels relative to the uninfected cells. Error bars represent standard deviations of three independent experiments. AU, arbitrary units.
HIV-2 Env induces accumulation of tetherin in the trans-Golgi network.
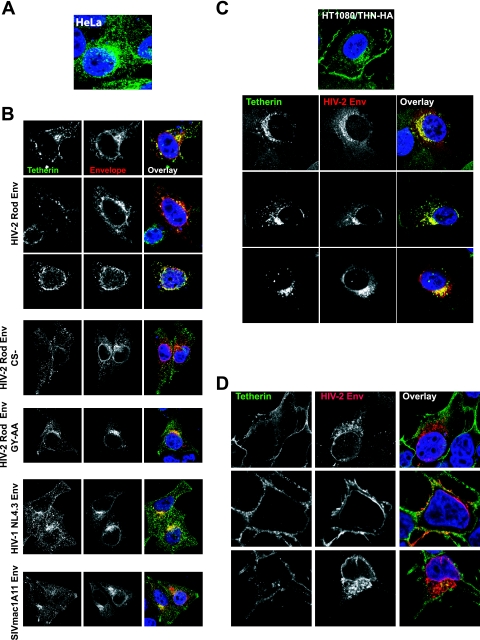
Since we could observe no apparent degradation of tetherin despite cell surface downregulation, we hypothesized that HIV-2 Env might mediate sequestration of tetherin in intracellular compartments. We therefore examined the effects of HIV-2 Env on tetherin localization. In HeLa cells, tetherin localizes to multiple peripheral membrane compartments in addition to the PM (Fig. 7A). Upon expression of the HIV-2 RodA Env, tetherin immunoreactivity was redistributed from the periphery and the PM to perinuclear compartments that stained positive for Env (Fig. 7B). Such a relocalization of tetherin was not observed in the presence of either GY-AA or CS- mutants of RodA Env, nor was it seen when the HIV-1 NL4.3 of SIVmac1A11 Envs were expressed (Fig. 7B). A similar result was observed in HT1080/THN-HA cells (Fig. 7C). Here tetherin localizes more prominently to the PM. When the HIV-2 RodA envelope was expressed in these cells by retroviral transduction, cell surface tetherin was again reduced but, consistent with the lack of degradation observed in these cells (Fig. 6), it was still readily observable in the perinuclear area, where it colocalized with HIV-2 Env. By contrast, and in line with our observations that 293T cells do not support HIV-2 RodA Env-mediated tetherin antagonism, we did not observe any obvious relocalization of tetherin from the cell surface of 293T/THN-HA cells (Fig. 7D). Thus, the ability of HIV-2 RodA Env to antagonize tetherin function appeared to be related to an induction of intracellular accumulation of tetherin.
FIG. 7.
Localization of tetherin in HeLa, HT1080, and 293T cells in response to envelope expression. (A) Localization of tetherin in HeLa cells stained with a mouse polyclonal anti-human BST-2 antibody (green). (B) Effects of envelope expression on endogenous tetherin localization in HeLa cells. Cells were transfected with the indicated envelope and 24 h later fixed and stained for tetherin (green) and envelope (red). (C) Tetherin-HA localization in HT1080/THN-HA cells expressing HIV-2 RodA Env introduced by retroviral vector transduction. Tetherin was stained with anti-HA (green) and envelope was costained (red). (D) 293T-THN/HA cells transfected with HIV-2 RodA Env were stained as described above. Nuclei were counterstained with DAPI (blue).
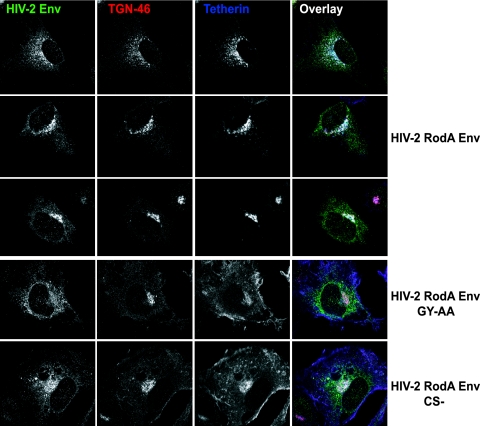
Since tetherin was originally identified as a marker of the TGN (22), we next examined whether these perinuclear accumulations of tetherin and HIV-2 Env localized to this compartment. Costaining the cells for the TGN marker TGN46 revealed that this was indeed the case (Fig. 8). While little or no tetherin could be seen at the PM of HIV-2 RodA Env-expressing cells, both proteins localized predominantly to TGN46-positive compartments. By contrast, the HIV-2 RodA GY-AA mutant appeared more reticular in these cells than wild-type protein and failed to affect this change in tetherin localization. Similarly, the HIV-2 Rod Env cleavage site mutation also failed to relocalize tetherin from the PM, despite localizing similarly to the wild-type protein. Taken together with the lack of tetherin degradation induced by HIV-2 in cell lysates and the coimmunoprecipitation of tetherin with HIV-2 Env, these microscopy studies strongly suggest that the HIV-2 envelope protein promotes the sequestration of tetherin in the TGN, preventing tetherin's recycling and/or trafficking to the PM, and that this subcellular relocalization correlates with the antagonism of tetherin function.
FIG. 8.
HIV-2 Env induces tetherin sequestration in the trans-Golgi network. HT1080/THN-HA cells expressing HIV-2 RodA Env or the indicated mutant were stained for HIV-2 Env (green), the TGN marker TGN46 (red), and tetherin-HA (blue).
Antagonism of tetherin by HIV-2 in CD4+ T cells.
All the preceding studies of HIV-2 Env-mediated tetherin antagonism were performed in adherent tumor cell lines. We therefore tested whether our observations were reproducible in more relevant cellular targets, namely, CD4+ T-cell lines. Jurkat T cells, which express tetherin (31), were infected with VSV-G-pseudotyped wild-type HIV-2 Rod10, HIV-2 Rod10 (GY-AA), or HIV-2 Rod10 (CS-) at an MOI of 0.2. Forty-eight hours later viral supernatants and cell lysates were analyzed by Western blotting as described above (Fig. 9A). Both GY-AA and CS- mutants released 5- to 10-fold fewer particles into the supernatant and displayed a concomitant increase in cell-associated mature p26-CA, indicating their release was inhibited in a manner consistent with tetherin-mediated restriction.
FIG. 9.
Antagonism and cell surface downregulation of tetherin by HIV-2 in CD4+ T-cell lines. (A) Jurkat cells were infected with the indicated HIV-2 Rod10 virus pseudotyped with VSV-G at an MOI of 0.2. Cell lysates and pellet virions were analyzed by Western blotting 48 h later, and virus release was quantified as the supernatant p26-CA band intensity, as a percentage of the wild-type control. (B) CEM-G37 cells were infected with the indicated VSV-G-pseudotyped viral stock at an MOI of 1. Forty-eight hours later cells were stained for surface tetherin expression and analyzed by flow cytometry. GFP+ infected cells were gated, and surface tetherin levels (solid lines) were compared to those of uninfected CEM-G37 cells (dotted lines). Numbers indicate median fluorescence intensities of surface tetherin on the infected cells. The solid peak in the upper left histogram represents the binding of the isotype control.
To examine the effect of viral infection on surface levels of tetherin in CD4+ T cells, we infected CEM-G37 with VSV-G pseudotypes of HIV-1 NL4.3 or HIV-2 Rod10 and mutants thereof. These cells encode GFP under the control of an HIV-1 LTR promoter, and thus cells fluoresce when infected. At 48 h after infection, the cells were stained for surface tetherin expression and analyzed by flow cytometry (Fig. 9B). As expected GFP-positive cells infected with wild-type HIV-1, but not HIV-1 (Vpu-), had strongly downregulated surface tetherin. HIV-2 Rod10-infected cells also had similarly reduced tetherin expression levels. By contrast cells infected with either HIV-2 Rod10 (GY-AA) or HIV-2 Rod10 (CS-) displayed only a minor reduction in tetherin surface levels (less than a twofold decrease in median fluorescence intensities). Thus, tetherin is downregulated by HIV-1 Vpu and HIV-2 Rod10 Env on infected CD4+ T cells, and both the GYxxθ motif and proper Env maturation are required for this process.
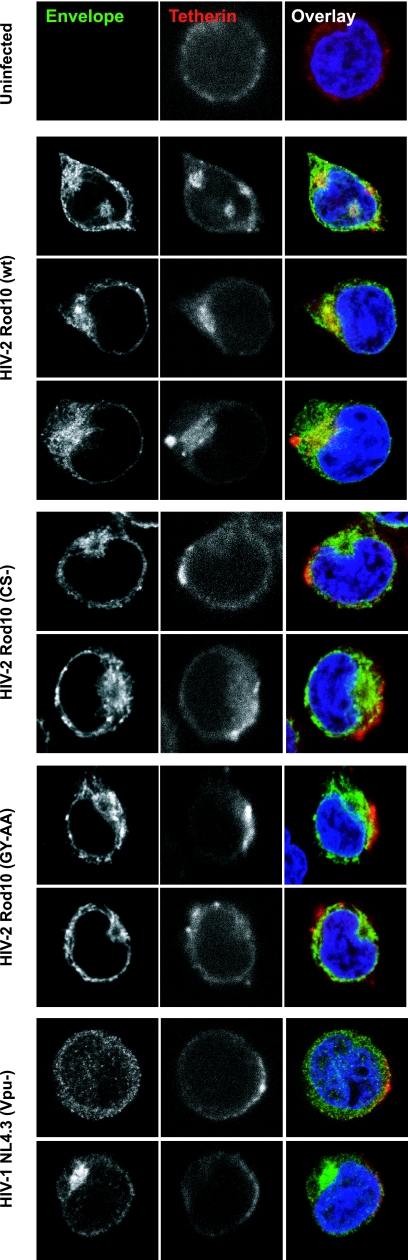
Finally we examined the localization of tetherin in HIV-2-infected Jurkat cells (Fig. 10). In uninfected Jurkat cells, tetherin was detected as a predominantly surface stain. Consistent with our earlier data in HeLa and HT1080 cells (Fig. 7), in Jurkat cells infected with HIV-2 Rod10, tetherin appeared to be relocalized to perinuclear compartments, where it colocalized with Env. Again this relocalization was not observed for Jurkat cells infected with either HIV-2 Rod10 (CS-), HIV-2 Rod10 (GY-AA), or HIV-1 (Vpu-). In these cells, tetherin remained visible at the cell surface but often formed striking patches on the PM, suggestive of accumulation in sheets of surface-tethered virions that we have observed previously (31). In cells infected with wild-type HIV-1, tetherin staining was reduced to background levels, consistent with induction of its degradation rather than sequestration (data not shown). Thus, in infected CD4+ T cells, HIV-2 Env induces cell surface downregulation of tetherin and intracellular sequestration similar to that seen in adherent cell lines.
FIG. 10.
Intracellular sequestration of tetherin in HIV-2-infected Jurkat cells. Jurkat cells were infected with the indicated VSV-G-pseudotyped viral stocks at an MOI of 1. Forty-eight hours later the cells were allowed to adhere to polylysine-coated coverslips, fixed, permeabilized, and stained for HIV-2/HIV-1 Env (green) or tetherin (red) using Alexa 488 and 633 secondary antibodies, respectively. Nuclei were counterstained with DAPI (blue), and the cells were examined by confocal microscopy.
DISCUSSION
In this study we showed that the HIV-2 Rod envelope glycoprotein is a bona fide tetherin antagonist. Like HIV-1 Vpu (31, 46) and SIV Nef (16, 51) proteins, HIV-2 Env can stimulate the release of lentiviral particles from tetherin-expressing cells. This antagonism of particle release restriction appears to closely correlate with the ability of the HIV-2 Env to mediate cell surface downregulation of tetherin expression. However, in contrast to Vpu, expression of HIV-2 Env does not ultimately lead to a reduction in overall cellular tetherin levels. Rather, tetherin is sequestered away from the cell surface, predominantly localizing in the TGN. Coupled with coimmunoprecipitation studies that indicate that HIV-2 Env interacts with tetherin, these data suggest a model whereby Env modulates tetherin trafficking or recycling to the PM from the TGN, thus removing tetherin from the cellular locale where nascent retroviral particles assemble. Importantly, all of our virological and cell biology observations of HIV-2 Env's antitetherin function could be recapitulated in infected CD4+ T-cell lines.
The HIV-2 Rod Env had previously been shown to harbor a Vpu-like activity, promoting Vpu-negative HIV-1 particle release from HeLa cells and CD4+ T cells (5, 6). The determinants of this effect were multiple and not well defined in the absence of an identified target. The cytoplasmic tail of HIV/SIV contains a major tyrosine-based endocytic motif (GYxxθ) in the membrane-proximal region that binds AP-2 and is required for the rapid clathrin-mediated endocytosis of Env from the surface of infected cells and its recycling through the Golgi apparatus (3, 23, 33, 38). This sequence was required to effect particle release, while the rest of the cytoplasmic tail was dispensable (1, 32). Here we confirm that HIV-2 Env lacking this sequence is defective for promoting particle release and is also unable to promote tetherin downregulation from the cell surface, suggesting that transit of Env via the cell surface is required to antagonize tetherin. However, this motif, which is required for SIVmac pathogenesis (12), is conserved across divergent primate immunodeficiency virus envelope proteins (40), including those described herein, indicating that it cannot in itself determine specificity for human tetherin. Moreover, fusion of a C-terminal fragment of SIVmac1A11 gp41 to the HIV-2 RodA envelope at a conserved sequence in the C-terminal heptad repeat domain of gp41 resulted in a chimeric protein that partially retained tetherin antagonism and promoted its downregulation from the surface. Thus, the GYxxθ sequence in SIVmac1A11 is able to function in tetherin antagonism in the context of the HIV-2 Env and, importantly, specificity for human tetherin must therefore be determined by an extracellular domain in the HIV-2 Env. These data are further strengthened by our observation that mutating the furin-like protease cleavage site between gp105 and gp41 abolishes HIV-2 RodA Env's tetherin antagonism and cell surface downregulation, suggesting that proper mature envelope conformation is critical for this function. The N-terminal region of gp41 contains the membrane fusion mechanism and is buried within the trimer of the surrounding mature SU subunits (37). Due to this likely occlusion and the high degree of homology between HIV-2 and SIVmac Envs in this area, we think it less likely that the N terminus of gp41 contains the determinants for tetherin specificity. Thus, we suggest that these determinants probably reside in the highly variable surface subunit, gp105.
The implication of extracellular determinants of tetherin specificity in the HIV-2 Env suggests that if the proteins directly interact, the ectodomain of tetherin is the target. Sensitivity of human tetherin to HIV-1 Vpu is determined by the TM domain (14, 16, 25), whereas SIV Nef proteins target nonhuman primate tetherins via a (G/D)DIWK motif in the protein's cytoplasmic tail (16, 51). Both these areas of tetherin have been under high positive selection during primate evolution (14, 25), suggesting that viral countermeasures that target these domains have exerted considerable evolutionary pressure on tetherin. The extracellular domain of tetherin by contrast is more conserved, although sequence analyses do identify areas in this part of the protein with high positive selection signatures, particularly around the glycophosphatidylinositol anchor. The spectrum of primate tetherins that HIV-2 Rod Env can antagonize remains to be determined, but it is an interesting notion that if tetherin antagonism by envelope glycoproteins were a widespread strategy among mammalian viruses, these areas might reflect common targets for disrupting tetherin function.
Our observation that not all human cells can support HIV-2 Env-mediated tetherin antagonism and intracellular sequestration raises the question of whether additional cellular factors are required for this effect. Several years ago, the Strebel group identified a mutant Rod Env (ROD14) that was defective for promoting virus release (4). Two amino acids were identified as conferring this phenotype (4); one (A598) within the highly conserved core of gp41 is invariant in wild-type HIV-2/SIVmac envelopes and is present in SIVmac1A11, which is unable to antagonize tetherin. The second (K422R) appeared to disrupt the ROD Env's ability to promote virus release in H9 T cells. This polymorphism is common among HIV-2 clade A sequences (see http://hiv-web.lanl.gov/content/hiv-db) and is variable between Rod10 and the RodA envelope we have used for most of this study. Both Rod10 and RodA Envs antagonize tetherin equivalently in HeLa cells, and neither functions in 293T cells (data not shown), indicating that this position is not responsible for the cell-type-specific effect we observed. A requirement for additional cellular factors in the HIV-2 Env-mediated tetherin antagonism is thus a possibility we are interested in exploring further.
Both HIV-1 Vpu (46) and SIVmac Nef (16) have been shown to downregulate cell surface levels of tetherin. In the case of Vpu, this downmodulation is accompanied by enhanced degradation of tetherin. There is controversy as to whether this degradation is mediated by the 20S proteasome (13, 14) or takes place in an endolysozomal compartment (10, 26), but it can be readily observed in several infected cell types (27). By contrast, we observed that in cells infected with HIV-2 there is little change in the steady-state levels of tetherin despite the ability of the envelope to induce cell surface downregulation.
Upon examining cells expressing the HIV-2 RodA Env we noted that tetherin was relocalized from the surface to perinuclear compartments that stained positive for the TGN marker TGN46 and the envelope protein itself. These data suggest that reduction of tetherin levels at the PM is a consequence of the envelope sequestering tetherin in the TGN. Since our coimmunpreciptation data indicate a physical interaction between Env and tetherin, this raises the possibility of two scenarios. First, since all plasma membrane proteins pass through the TGN en route to the cell surface, Env may simply inhibit tetherin trafficking out of the TGN. Second, Env could interact with tetherin during transit to the PM and either promote its removal from the surface by endocytosis or prevent the recycling of tetherin. This second scenario is more consistent with some of the known cell biology of HIV/SIV envelopes and tetherin. Tetherin has been reported to cycle from the PM to the TGN via clathrin/AP-2-mediated endocytosis (36). HIV/SIV envelope proteins are well known to be rapidly endocytosed from the cell surface and recycle via the sorting endosome and TGN (3, 23, 33, 38). Mutation of endocytic signals in the Env cytoplasmic tail increase cell surface envelope levels, but this has a detrimental effect on virus infectivity, and there is evidence to suggest that Env-Gag interactions during viral assembly are regulated through the occlusion of Env endocytic signals (48). Alternatively, rapid Env endocytosis may minimize surface viral antigen exposure to antibodies and perhaps be linked to the maturation of Env precursors to gp120/105 and gp41, which is achieved by furin-like proteases, which themselves recycle between the TGN and the cell surface (28). Since mutation of the GYxxθ signal in the Rod Env cytoplasmic tail abolishes its ability to relocalize tetherin from the PM, and previous data from the Spearman group demonstrated a requirement for the recycling endosome in Vpu and HIV-2 Env function (47), tetherin trapping in the TGN is likely to occur after removal from the cell surface, but whether HIV-2 Env actively promotes this removal or whether its own trafficking intersects with tetherin in the endosomal/TGN compartments remains to be determined. Furthermore, our observation that the CS- mutant of Env still retains the ability to coimmunoprecipitate with tetherin suggests that this interaction is not sufficient to explain antagonism and sequestration of tetherin. This suggests that tetherin antagonism/sequestration is intimately linked to the processing of Env. Furin cleavage therefore may result in release of mature Env from tetherin. How such a process might sequester tetherin in the TGN is not clear, but further studies to identify the determinants of Env sensitivity in tetherin will likely shed light on this mechanism. It is interesting that the ability of Vpu to counteract tetherin has been linked to its localization to the TGN (11), perhaps suggesting a common mechanism of antagonism that results in TGN trapping, which is augmented by the induction of degradation in the case of Vpu.
In the HIV-1 Env, the GYxxθ also appears to be responsible for the targeting of the protein to the basolateral surface of polarized cells (3). Tetherin itself localizes to the apical surface of similarly polarized cells where it is linked to the underlying actin cytoskeleton by RICH2 (35). Although the majority of cellular targets of HIV/SIV are not classically polarized in this way, it is interesting to speculate that interactions between HIV-2 Env and tetherin could affect tetherin's ability to access virus assembly sites on the plasma membrane. Since HIV transfer between primary T cells appears to occur via cell-to-cell contact across a polarized synapse (17), a major mechanism of tetherin antagonism by all the known lentiviral countermeasures may be to disrupt its trafficking to specific areas of the PM.
Another issue that should be noted is whether cell surface downregulation and TGN trapping are a cause or a consequence of HIV-2 Env antagonism. It is clear from recent studies that downregulation of cell surface tetherin levels is insufficient to explain antagonism of restriction by Vpu, with mutants of Vpu that cannot modulate tetherin surface levels still retaining partial function (27, 41). Thus, it is conceivable that HIV-2 Env may disrupt tetherin function prior cell surface removal. However, we have yet to find an Env mutant or chimera that can antagonize tetherin without promoting cell surface downmodulation that would allow such a discrimination to be made.
Tetherin has a broad restrictive capability on enveloped virus particle release (18, 39), and notably, the glycoprotein spike protein of Ebola virus (EBOV-GP) has tetherin-antagonizing activity (20). In this case EBOV-GP needs to be anchored to the membrane, but the mucin domains that mediate the removal of other cell surface proteins (44) appear to be dispensable (20). Like our observations with HIV-2 RodA Env, tetherin antagonism by EBOV-GP does not result in a gross degradation of tetherin (20). However, in contrast to our data, cleavage of the GP precursor from GP0 to GP1/GP2 is not required for tetherin antagonism by EBOV. Whether tetherin sequestration in the TGN is observed with EBOV-GP is unknown at present.
Since the Nef proteins of SIVs that lack a vpu gene appear to govern these viruses’ abilities to antagonize tetherin through a sequence in the cytoplasmic tail (G/DDIWK) of the protein that has been deleted sometime after divergence of humans and chimpanzees (16, 25, 51), antagonism of tetherin restriction by HIV-2 Env raises important questions about the zoonotic origins of this virus and its pathogenesis. Originally derived from SIVsm, HIV-2 appears less virulent than HIV-1, with a higher incidence of long-term nonprogression and an epidemic limited predominantly to West Africa (9). How widespread is human tetherin antagonism by primary isolates of HIV-2? Given the powerful restrictive effects of human tetherin on SIV replication in culture (16, 51), this may suggest that upon zoonotic transfer to humans, the HIV-2 Nef was unable to adapt to human tetherin. Was this sufficient selective pressure for Env to develop a tetherin-antagonizing function, or is this attribute more widespread among SIV/HIV envelopes? It is interesting that at least one HIV-1 isolate, AD8, encodes an envelope that may have Vpu-like function (42). Given the lack of tetherin degradation we observe with HIV-2 Rod Env, does this mean that it is intrinsically a weaker countermeasure in primary target cells? The importance of tetherin antagonism in the pathogenesis of primate immunodeficiency viruses remains to be determined, but the identification of this attribute associated with three different primate lentiviral proteins (Vpu, Nef, and Env) underscores the potential of this innate antiviral factor to exert a powerful limiting effect on HIV/SIV spread in vivo.
Acknowledgments
We thank Michael Malim, Juan Martin-Serrano, their respective labs, and members of the Neil group for helpful advice and assistance.
The study was conceived and designed by A.L.T. and S.J.D.N. All the experiments were performed by A.L.T. A.L.T. and S.J.D.N. analyzed the data and wrote the paper.
This work was funded by Wellcome RCDF WT082774MA to S.J.D.N.
Footnotes
Published ahead of print on 9 September 2009.
REFERENCES
- 1.Abada, P., B. Noble, and P. M. Cannon. 2005. Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J. Virol. 79:3627-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartee, E., A. McCormack, and K. Fruh. 2006. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 4.Bour, S., H. Akari, E. Miyagi, and K. Strebel. 2003. Naturally occurring amino acid substitutions in the HIV-2 ROD envelope glycoprotein regulate its ability to augment viral particle release. Virology 309:85-98. [DOI] [PubMed] [Google Scholar]
- 5.Bour, S., U. Schubert, K. Peden, and K. Strebel. 1996. The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release: a Vpu-like factor? J. Virol. 70:820-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bour, S., and K. Strebel. 1996. The human immunodeficiency virus (HIV) type 2 envelope protein is a functional complement to HIV type 1 Vpu that enhances particle release of heterologous retroviruses. J. Virol. 70:8285-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers, K., A. Pelchen-Matthews, S. Honing, P. J. Vance, L. Creary, B. S. Haggarty, J. Romano, W. Ballensiefen, J. A. Hoxie, and M. Marsh. 2000. The simian immunodeficiency virus envelope glycoprotein contains multiple signals that regulate its cell surface expression and endocytosis. Traffic 1:661-674. [DOI] [PubMed] [Google Scholar]
- 8.Braakman, I., and E. van Anken. 2000. Folding of viral envelope glycoproteins in the endoplasmic reticulum. Traffic 1:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silva, T. I., M. Cotten, and S. L. Rowland-Jones. 2008. HIV-2: the forgotten AIDS virus. Trends Microbiol. 16:588-595. [DOI] [PubMed] [Google Scholar]
- 10.Douglas, J. L., K. Viswanathan, M. N. McCarroll, J. K. Gustin, K. Fruh, and A. V. Moses. 2009. Vpu directs the degradation of the HIV restriction factor BST-2/tetherin via a βTrCP-dependent mechanism. J. Virol. 83:7931-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube, M., B. B. Roy, P. Guiot-Guillain, J. Mercier, J. Binette, G. Leung, and E. A. Cohen. 2009. Suppression of tetherin-restricting activity on HIV-1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83:4574-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fultz, P. N., P. J. Vance, M. J. Endres, B. Tao, J. D. Dvorin, I. C. Davis, J. D. Lifson, D. C. Montefiori, M. Marsh, M. H. Malim, and J. A. Hoxie. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goffinet, C., I. Allespach, S. Homann, H. M. Tervo, A. Habermann, D. Rupp, L. Oberbremer, C. Kern, N. Tibroni, S. Welsch, J. Krijnse-Locker, G. Banting, H. G. Krausslich, O. T. Fackler, and O. T. Keppler. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285-297. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, R. K., S. Hue, T. Schaller, E. Verschoor, D. Pillay, and G. J. Towers. 2009. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 5:e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallenberger, S., V. Bosch, H. Angliker, E. Shaw, H. D. Klenk, and W. Garten. 1992. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360:358-361. [DOI] [PubMed] [Google Scholar]
- 16.Jia, B., R. Serra-Moreno, W. Neidermyer, A. Rahmberg, J. Mackey, I. B. Fofana, W. E. Johnson, S. Westmoreland, and D. T. Evans. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jouvenet, N., S. J. Neil, M. Zhadina, T. Zang, Z. Kratovac, Y. Lee, M. McNatt, T. Hatziioannou, and P. D. Bieniasz. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaletsky, R. L., J. R. Francica, C. Agrawal-Gamse, and P. Bates. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. USA 106:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaletsky, R. L., G. Simmons, and P. Bates. 2007. Proteolysis of the Ebola virus glycoproteins enhances virus binding and infectivity. J. Virol. 81:13378-13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhoff, F. 2009. Is the high virulence of HIV-1 an unfortunate coincidence of primate lentiviral evolution? Nat. Rev. Microbiol. 7:467-476. [DOI] [PubMed] [Google Scholar]
- 22.Kupzig, S., V. Korolchuk, R. Rollason, A. Sugden, A. Wilde, and G. Banting. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694-709. [DOI] [PubMed] [Google Scholar]
- 23.LaBranche, C. C., M. M. Sauter, B. S. Haggarty, P. J. Vance, J. Romano, T. K. Hart, P. J. Bugelski, M. Marsh, and J. A. Hoxie. 1995. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 69:5217-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luciw, P. A., K. E. Shaw, R. E. Unger, V. Planelles, M. W. Stout, J. E. Lackner, E. Pratt-Lowe, N. J. Leung, B. Banapour, and M. L. Marthas. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res. Hum. Retrovir. 8:395-402. [DOI] [PubMed] [Google Scholar]
- 25.McNatt, M. W., T. Zang, T. Hatziioannou, M. Bartlett, I. B. Fofana, W. E. Johnson, S. J. Neil, and P. D. Bieniasz. 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, R. S., C. Katsura, M. A. Skasko, K. Fitzpatrick, D. Lau, A. Ruiz, E. B. Stephens, F. Margottin-Goguet, R. Benarous, and J. C. Guatelli. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyagi, E., A. J. Andrew, S. Kao, and K. Strebel. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. USA 106:2868-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molloy, S. S., L. Thomas, J. K. VanSlyke, P. E. Stenberg, and G. Thomas. 1994. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 13:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulard, M., S. Hallenberger, W. Garten, and H. D. Klenk. 1999. Processing and routage of HIV glycoproteins by furin to the cell surface. Virus Res. 60:55-65. [DOI] [PubMed] [Google Scholar]
- 30.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 32.Noble, B., P. Abada, J. Nunez-Iglesias, and P. M. Cannon. 2006. Recruitment of the adaptor protein 2 complex by the human immunodeficiency virus type 2 envelope protein is necessary for high levels of virus release. J. Virol. 80:2924-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305-315. [DOI] [PubMed] [Google Scholar]
- 34.Ohtomo, T., Y. Sugamata, Y. Ozaki, K. Ono, Y. Yoshimura, S. Kawai, Y. Koishihara, S. Ozaki, M. Kosaka, T. Hirano, and M. Tsuchiya. 1999. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 258:583-591. [DOI] [PubMed] [Google Scholar]
- 35.Rollason, R., V. Korolchuk, C. Hamilton, M. Jepson, and G. Banting. 2009. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Biol. 184:721-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollason, R., V. Korolchuk, C. Hamilton, P. Schu, and G. Banting. 2007. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 120:3850-3858. [DOI] [PubMed] [Google Scholar]
- 37.Roux, K. H., and K. A. Taylor. 2007. AIDS virus envelope spike structure. Curr. Opin. Struct. Biol. 17:244-252. [DOI] [PubMed] [Google Scholar]
- 38.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J. Immunol. 155:473-488. [PubMed] [Google Scholar]
- 39.Sakuma, T., T. Noda, S. Urata, Y. Kawaoka, and J. Yasuda. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schubert, U., S. Bour, A. V. Ferrer-Montiel, M. Montal, F. Maldarell, and K. Strebel. 1996. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol. 70:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert, U., S. Bour, R. L. Willey, and K. Strebel. 1999. Regulation of virus release by the macrophage-tropic human immunodeficiency virus type 1 AD8 isolate is redundant and can be controlled by either Vpu or Env. J. Virol. 73:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubert, U., K. A. Clouse, and K. Strebel. 1995. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 69:7699-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, E. R., C. Shotton, R. A. Weiss, P. R. Clapham, and A. McKnight. 2003. CD4-dependent and CD4-independent HIV-2: consequences for neutralization. AIDS 17:291-300. [DOI] [PubMed] [Google Scholar]
- 46.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varthakavi, V., R. M. Smith, K. L. Martin, A. Derdowski, L. A. Lapierre, J. R. Goldenring, and P. Spearman. 2006. The pericentriolar recycling endosome plays a key role in Vpu-mediated enhancement of HIV-1 particle release. Traffic 7:298-307. [DOI] [PubMed] [Google Scholar]
- 48.West, J. T., S. K. Weldon, S. Wyss, X. Lin, Q. Yu, M. Thali, and E. Hunter. 2002. Mutation of the dominant endocytosis motif in human immunodeficiency virus type 1 gp41 can complement matrix mutations without increasing Env incorporation. J. Virol. 76:3338-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf, D., and S. P. Goff. 2008. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 42:143-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zennou, V., D. Perez-Caballero, H. Gottlinger, and P. D. Bieniasz. 2004. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J. Virol. 78:12058-12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, F., S. J. Wilson, W. C. Landford, B. Virgen, D. Gregory, M. C. Johnson, J. Munch, F. Kirchhoff, P. D. Bieniasz, and T. Hatziioannou. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]