Abstract
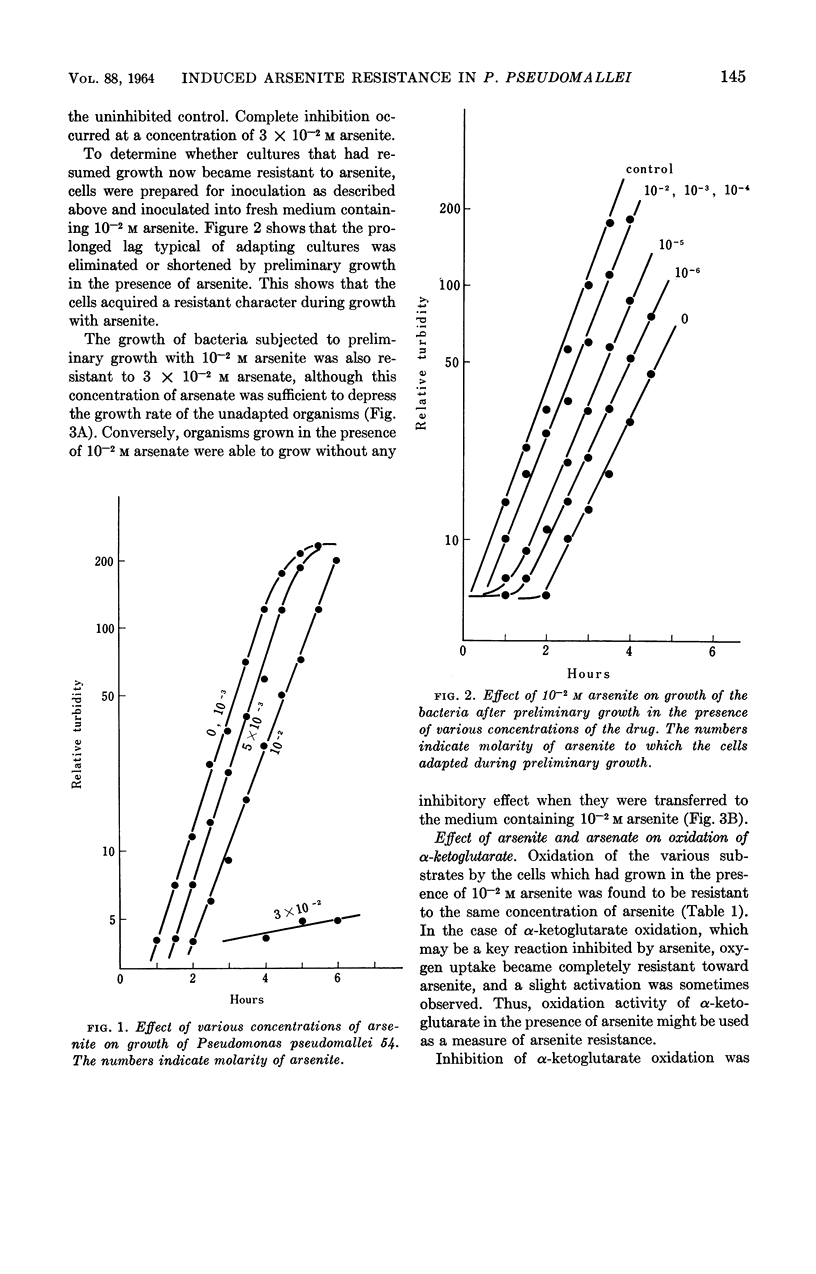
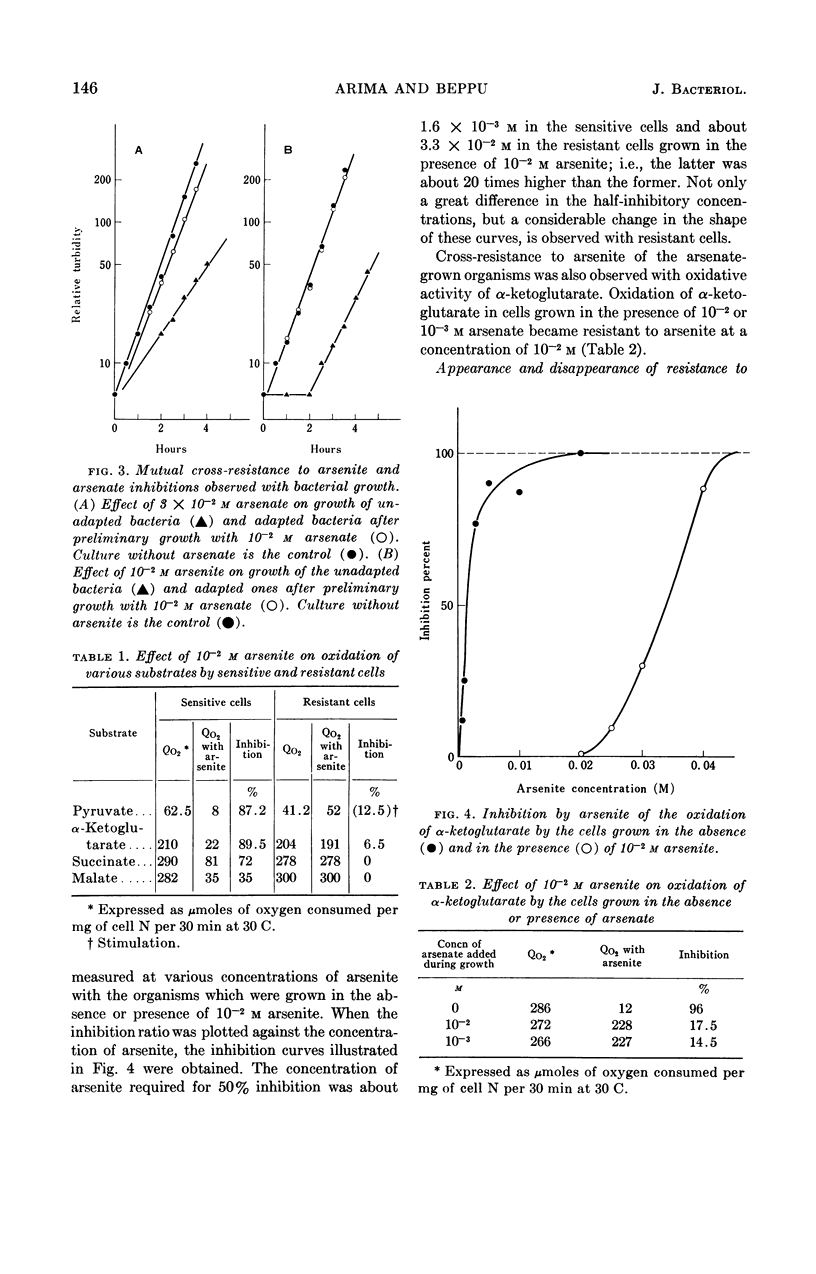
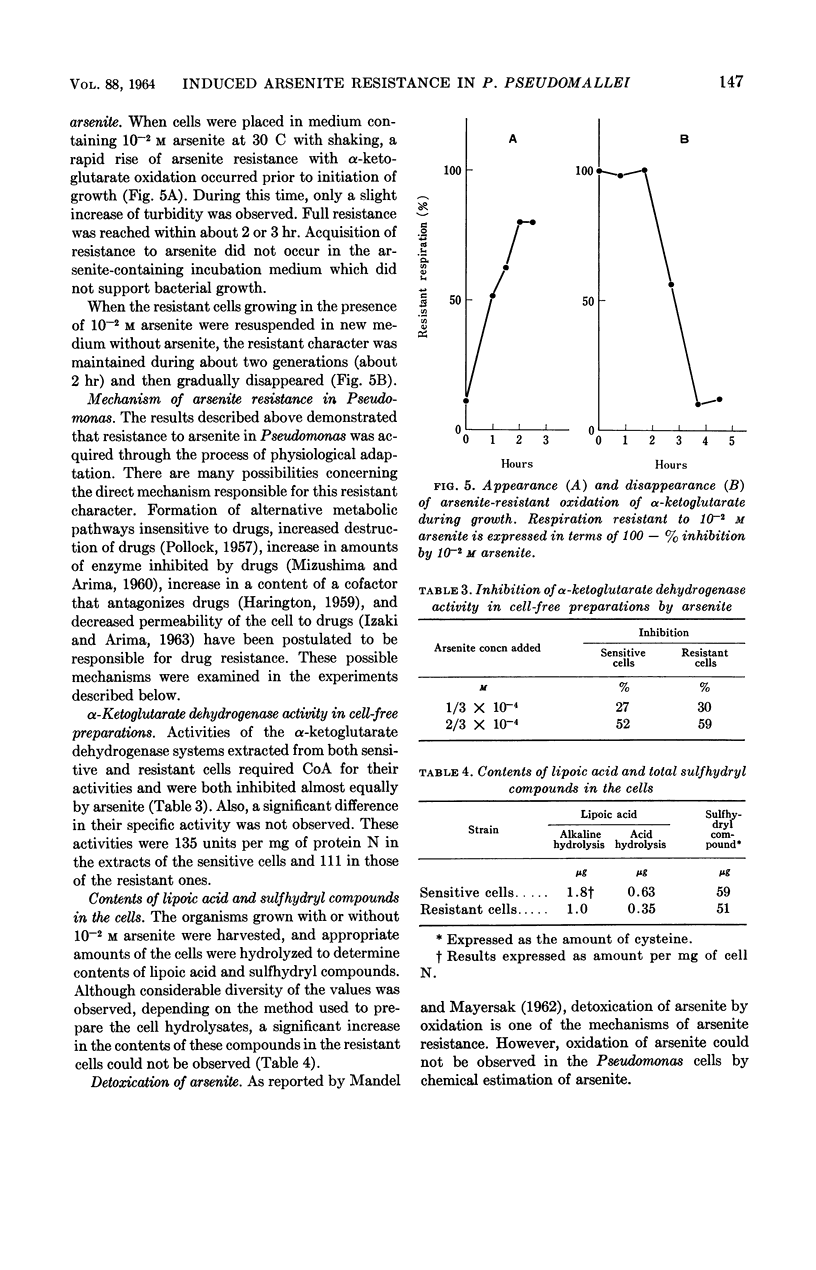
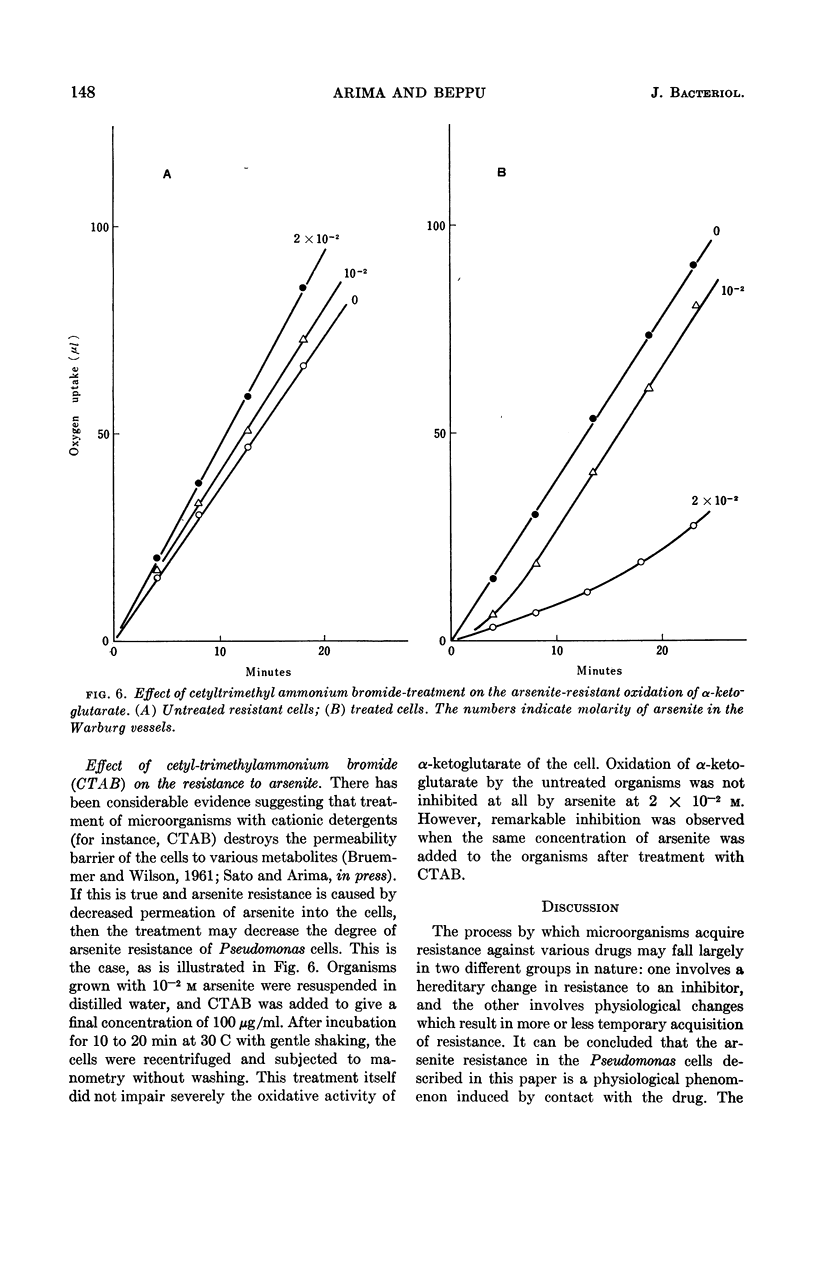
Arima, Kei (University of Tokyo, Tokyo, Japan), and Michiko Beppu. Induction and mechanisms of arsenite resistance in Pseudomonas pseudomallei. J. Bacteriol. 88:143–150. 1964.—Pseudomonas pseudomallei strain 54, able to grow in the presence of 2 × 10−2m arsenite, was isolated from soil. After a short lag period, it grew at a normal growth rate. In the organisms grown with 10−2m arsenite, oxidation of α-ketoglutarate and other substrates proceeded in the presence of the same concentration of the drug. The concentration of arsenite which was half-inhibitory to α-ketoglutarate oxidation was 1.6 × 10−3m in the sensitive bacteria and 3.3 × 10−2m in the resistant ones. Cells capable of oxidizing α-ketoglutarate in the presence of arsenite were induced rapidly by contact with arsenite in growing cultures; when the drug was removed from the cultures, resistance was maintained for about two generations and then gradually disappeared. From the data presented, it was concluded that resistance in this organism is a physiological change and not a hereditary one. Further studies were carried out to investigate the arsenite-resistance mechanisms. α-Ketoglutarate dehydrogenase activity in the cell-free extracts of the resistant bacteria was sensitive to arsenite. An increase in the contents of this enzyme and sulfhydryl compounds, involving lipoic acid, was not observed in the resistant bacteria. The possibility of detoxication of arsenite was ruled out. Treatment of the resistant cells with cetyl-trimethylammonium bromide made them susceptible to 2 × 10−2m arsenite, although untreated cells were resistant to the same concentration of the drug. These data suggest the decreased permeability to arsenite of the resistant bacteria as a main mechanism of resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS R. J., HUTNER S. H., SEAMAN G. R., STOKSTAD E. L. Assay of thioctic acid. Methods Biochem Anal. 1956;3:23–47. doi: 10.1002/9780470110195.ch2. [DOI] [PubMed] [Google Scholar]
- HARINGTON J. S. Contents of cystine-cysteine, glutathione and total free sulphydryl in arsenic-resistant and sensitive strains of the blue tick, Boophilus decoloratus. Nature. 1959 Nov 28;184(Suppl 22):1739–1740. doi: 10.1038/1841739b0. [DOI] [PubMed] [Google Scholar]
- IZAKI K., ARIMA K. DISAPPEARANCE OF OXYTETRACYCLINE ACCUMULATION IN THE CELLS OF MULTIPLE DRUG-RESISTANT ESCHERICHIA COLI. Nature. 1963 Oct 26;200:384–385. doi: 10.1038/200384a0. [DOI] [PubMed] [Google Scholar]
- Newcombe H. B., Hawirko R. SPONTANEOUS MUTATION TO STREPTOMYCIN RESISTANCE AND DEPENDENCE IN ESCHERICHIA COLI. J Bacteriol. 1949 May;57(5):565–572. doi: 10.1128/jb.57.5.565-572.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHRIFT A., KELLY E. Adaptation of Escherichia coli to selenate. Nature. 1962 Aug 18;195:732–733. doi: 10.1038/195732a0. [DOI] [PubMed] [Google Scholar]
- TURNER A. W. Bacterial oxidation of arsenite. I. Description of bacteria isolated from arsenical cattle-dipping fluids. Aust J Biol Sci. 1954 Nov;7(4):452–478. doi: 10.1071/bi9540452. [DOI] [PubMed] [Google Scholar]
- TURNER A. W., LEGGE J. W. Bacterial oxidation of arsenite. II. The activity of washed suspensions. Aust J Biol Sci. 1954 Nov;7(4):479–495. doi: 10.1071/bi9540479. [DOI] [PubMed] [Google Scholar]


