Abstract
CD4C/HIVnef transgenic (Tg) mice express Nef in CD4+ T cells and in the cells of the macrophage/monocyte/dendritic lineage, and they develop an AIDS-like disease similar to human AIDS. In these mice, Nef is constitutively expressed throughout life. To rule out the contribution of any developmental defects caused by early expression of Nef, we generated inducible human immunodeficiency virus type 1 (HIV-1) Nef Tg mice by using the tetracycline-inducible system. Faithful expression of the Nef transgene was induced in (CD4C/rtTA × TRE/HIVNef) or (CD4C/rtTA2S-M2 × TRE/HIVNef) double-Tg mice upon doxycycline (DOX) treatment in drinking water. Long-term treatment of these mice with DOX also led to loss, apoptosis, and activation of CD4+ T cells, this latter phenotype being observed even with low levels of Nef. These phenotypes could be transferred by bone marrow (BM) transplantation, indicating a hematopoietic cell autonomous effect. In addition, in mixed Tg:non-Tg BM chimeras, only Tg and not non-Tg CD4+ T cells exhibited an effector/memory phenotype in the absence of lymphopenia. Finally, the DOX-induced double-Tg mice developed nonlymphoid organ diseases similar to those of CD4C/HIVNef Tg mice and of humans infected with HIV-1. These results show for the first time that adult mice are susceptible to the detrimental action of Nef and that Nef-mediated T-cell activation can be independent of lymphopenia. These Tg mice represent a unique model which is likely to be instrumental for understanding the cellular and molecular pathways of Nef action as well as the main characteristics of immune reconstitution following DOX withdrawal.
Small animal models able to express the entire human immunodeficiency virus (HIV) genome or selected HIV genes have provided useful information on the pathogenesis of AIDS and still represent important research tools toward this goal. Among these models, transgenic (Tg) mice containing intact copies of HIV DNA, defective provirus with the gag and pol genes deleted, or individual HIV-1 genes have been reported to develop various pathologies, some of which resemble those found in human AIDS (2, 3, 8, 9, 16, 17, 18, 24, 27, 29, 30, 38, 44, 45, 46, 49, 51, 52). The cell type context in which the HIV-1 transgene is expressed in these Tg mice appears to play an important role in determining the type of pathological lesions. Tg mice generated in our laboratory and expressing the entire coding sequence of HIV-1 (CD4C/HIVWT) or HIV-1 Nef alone (CD4C/HIVNef) in the relevant target cells of HIV-1, namely, CD4+ T cells, macrophages, and dendritic cells, develop pathologies very similar to those in human AIDS (17, 18). The AIDS-like disease of CD4C/HIVNef Tg mice is characterized by immunodeficiency, loss of CD4+ T cells, thymic atrophy, activation of T cells and pathologies in heart, lungs, and kidneys (18, 53). Similarly, expression of simian immunodeficiency virus (SIV) Nef in Tg mice under the control of the same promoter sequences (CD4C) results in an AIDS-like disease (42). These studies demonstrated that Nef plays an important role in the development of the AIDS-like disease induced by HIV-1 or SIV in Tg mice.
Among the AIDS-like phenotypes of these models, the T-cell activation observed by a number of groups in Tg mice expressing Nef (3, 33, 44, 53) may be of special interest for its resemblance to that of humans or macaques infected with HIV-1 or SIV, respectively. HIV infection results in a state of chronic immune activation which correlates very closely with disease progression in humans (11, 14, 23). Similarly, SIV-infected macaques which develop AIDS show aberrant immune activation (35), while SIV-infected sooty mangabey monkeys, natural hosts of SIV, do not develop immunopathologies and do not show immune activation either (41). Various factors may contribute to this immune activation, including increased plasma lipopolysaccharide levels due to microbial translocation from the gut (4), impaired regulatory T cell function (32), or the action of the HIV-1 gene products themselves, such as Env gp120 and Nef (10, 12, 43). Consistent with this latter scenario, we reported that in CD4C/HIVNef Tg mice the extent of T-cell activation correlates with levels of Nef expression in CD4+ T cells, thus suggesting a direct involvement of Nef in this activation (53). In contrast, Koenen and coworkers reported that T-cell activation in CD2/Nef Tg mice is induced indirectly by lymphophenia (26). In that study, chimeric mice, which were generated from a mixture of non-Tg and Nef Tg bone marrow (BM) cells, were not lymphopenic, and the donor-derived Nef-expressing Tg T cells did not show an activated phenotype. However, the donor Nef Tg T cells constituted only 1 to 2% of peripheral T cells of these chimeric mice (26). Clearly, alternative experimental approaches are needed to study this phenotype in a more physiological context.
In the previously described CD4C/HIVNef Tg mice (18), Nef expression begins early in life and is constitutively expressed throughout the life of the animal. The AIDS-like disease caused by this early expression of Nef best represents a model for pediatric AIDS. However, in these Tg mice, Nef may interfere with normal developmental processes and these latter defects may contribute to some of the phenotypes observed. To assess the effects of Nef in fully mature adult animals, and thus develop a model of adult AIDS, temporal regulation of Nef expression in adult mice using an inducible system is required.
In the present study, we chose the tet-On (rtTA and rtTA2S-M2) system (13, 15, 25, 48) to induce expression of HIV-1 Nef in CD4+ T cells and cells of the macrophage/dendritic lineage of mice using the CD4C tissue-specific regulatory elements. These CD4C sequences were previously used to generate the constitutively Nef-expressing CD4C/HIVNef Tg mice (18). These inducible adult (TRE/HIVNef × CD4C/rtTA) and (TRE/HIVNef × CD4C/rtTA2S-M2) double-Tg (DTg) mice express Nef when treated with doxycycline (DOX) and develop an AIDS-like disease very similar to that seen in constitutively Nef-expressing CD4C/HIVNef Tg mice. We took advantage of this novel biological system to reassess the role of Nef in T-cell activation. Using a mixed chimera made with BM cells from these inducible Nef Tg mice and from non-Tg mice, we could document CD4+ T-cell activation only in donor-derived Nef-expressing Tg cells, but not in non-Tg cells, in the absence of lymphopenia. This result strongly suggests that this CD4+ T-cell activation phenotype is most likely driven by expression of Nef in these cells.
MATERIALS AND METHODS
Mice.
Green fluorescent protein (GFP) reporter mice (tetO/H2B-GFP) were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred on the C3H/HeNHsd background.
Transgene construction and generation of Tg mice.
The CD4C regulatory sequences and the HIVMutG DNA, designated here HIVNef, have been described before (18). The AatII and BamHI fragment of the pRetro-Off plasmid containing the TRE sequences was fused upstream of the HIVNef fragment and with simian virus 40 (SV40) polyadenylation sequences in the Litmus29 vector. The TRE-HIVNef-SV40 fragment was excised from this vector with AatII and cloned in the NotI site of the hypoxanthine phosphoribosyltransferase (HPRT)-targeting vector/pBR322. This construct was linearized with PuvI and used to transfect mouse BK4 embryonic stem (ES) cells, essentially as described previously (5). ES cell clones containing a single copy of Tg inserted at the HPRT locus by homologous recombination were selected and injected into mouse blastocysts to generate TRE/HIVNef Tg mice.
The DNA fragment containing the rtTA gene was PCR amplified with 5′ oligo 1344 (GATGTAAAGAGAGGCACGTGG) and 3′ oligo 2155 (CCTACCCACCGTACTAGTCA) on genomic DNA extracted from the kidney of an hCD2/rtTA Tg mouse (Jackson Laboratory, Bar Harbor, Me) and cloned in the pBluescript KS vector. An EcoRI fragment containing the rtTA sequences was excised from this construct and blunt ended with Klenow. The rtTA2S-M2 sequences (48) were excised from the pUHrT62-1 vector with EcoRI and BamHI and blunt ended with Klenow. The rtTA and rtTA2S-M2 fragments were each ligated downstream of the CD4C promoter followed by SV40 polyadenylation sequences and cloned in the pBR322 vector.
The CD4C/rtTA and CD4C/rtTA2S-M2 DNAs were excised with EcoRI and microinjected into fertilized (C57BL/6 × C3H) F2 oocytes to generate Tg mice, as described before (18). Mice carrying the Tg were bred as heterozygotes on the C3H/HeNHsd background. To generate DTg mice, TRE/HIVNef Tg mice were bred with CD4C/rtTA or CD4C/rtTA2S-M2 Tg mice. To induce Tg expression, DTg progeny were treated with DOX in drinking water at 2 mg/ml, except where indicated. All mice were housed in a specific-pathogen-free environment.
Northern blot analysis.
RNA was isolated from the thymus using the TRIzol reagent (Invitrogen) following the manufacturer's instructions. RNA (15 μg) was separated on a formaldehyde agarose gel, transferred to nylon membrane, and hybridized with 32P-labeled HIV-1 probe and quantitated, as described before (18, 50). The membranes were washed and rehybridized to an actin probe.
Western blot analysis.
Protein extracts were prepared from different tissues using radioimmunoprecipitation assay buffer in the presence of protease inhibitors, as described previously (36, 50). The amount of protein in the lysates was determined using a micro-BCA assay (Sigma-Adrich). For thymocyte activation, the hamster anti-mouse CD3ɛ (145-2C11) monoclonal antibody (MAb) produced from the hybridoma was purified on a protein G affinity column. Thymocytes were stimulated with anti-CD3 antibody, and protein extracts were prepared in the presence of protease and phosphatase inhibitors, as described previously (18, 50). Proteins (100 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to membrane, and detected with polyclonal rabbit anti-Nef antibodies and goat Alexa 680-conjugated anti-rabbit secondary antibodies (Molecular Probes).
Phosphoproteins were detected with mouse anti-phosphotyrosine MAb (4G10)/Alexa 680-conjugated anti-mouse secondary antibodies. Membranes were washed and then reacted with rabbit antiactin antibodies (Sigma A2066) to estimate levels of proteins in each extract after transfer. Detection was done with the Odyssey infrared detector, and quantification was performed as previously described (50).
Preparation of peritoneal macrophages.
Peritoneal cells were harvested by washing the peritoneal cavity twice with 5 ml RPMI medium containing 10% heat-in activated fetal bovine serum. Cells were collected in petri dishes (Falcon), and macrophages were allowed to attach to the bottom of the plates overnight. Macrophages were washed and collected for protein extraction.
Flow cytometry.
Cell suspensions were prepared from lymphoid organs and stained with antibodies, as described previously (18, 53). Fluorescein isothiocyanate-, phycoerythrin-, and allopycocyanin-labeled MAbs, including anti-CD4, CD8, TcRαβ, CD2, CD5, CD69, CD25, CD44, CD45RB, CD62L, CD45.1, and CD45.2 were purchased from Cederlane or BD-Pharmingen. Irrelevant rat immunoglobulin G1 (IgG1), rat IgG2a, rat IgG2b, and Armenian hamster IgG1 were used as isotype controls. Annexin V-fluorescein isothiocyanate and 7-aminoactinomycin D (7-AAD) were purchased from Cedarlane, and propidium iodide (PI) was from Sigma. Staining to evaluate apoptosis was performed with Annexin V, PI, and 7-AAD, as described before (37). Cytometric analysis was done with LSR-1 and FACSCalibur flow cytometers (BD Biosciences, San Jose, CA), and CellQuest software (Becton Dickinson) was used for analysis.
Microscopic analysis.
Organs were fixed in 3.7% formaldehyde and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin. All sections were assessed blindly using bright-field microscopy, essentially as previously described (20).
Bone marrow transplantation.
Tibias and femurs of CD45.2+ Tg and CD45.1+CD45.2+ non-Tg donor mice were flushed with Iscove's medium to obtain the bone marrow. T cells were depleted using rat monoclonal antibodies against mouse CD4 (GK1.5), CD8 (53-6.72), and Thy1.2 (30H12), followed by magnetic separation with sheep anti-rat IgG Dynabeads (Invitrogen). The CD45.1+ congenic C3H recipient mice were irradiated with a lethal dose of 950 rads. A total of 5 × 106 BM cells were transferred to the recipient mice through intravenous injection. Immune reconstitution and chimerism were monitored by fluorescence-activated cell sorter (FACS) analysis. To induce Nef expression, chimeras were treated with DOX at 2 mg/ml in drinking water.
Statistical analysis.
Statistical analyses were performed as previously described (37, 53).
RESULTS
Construction of Tg mice.
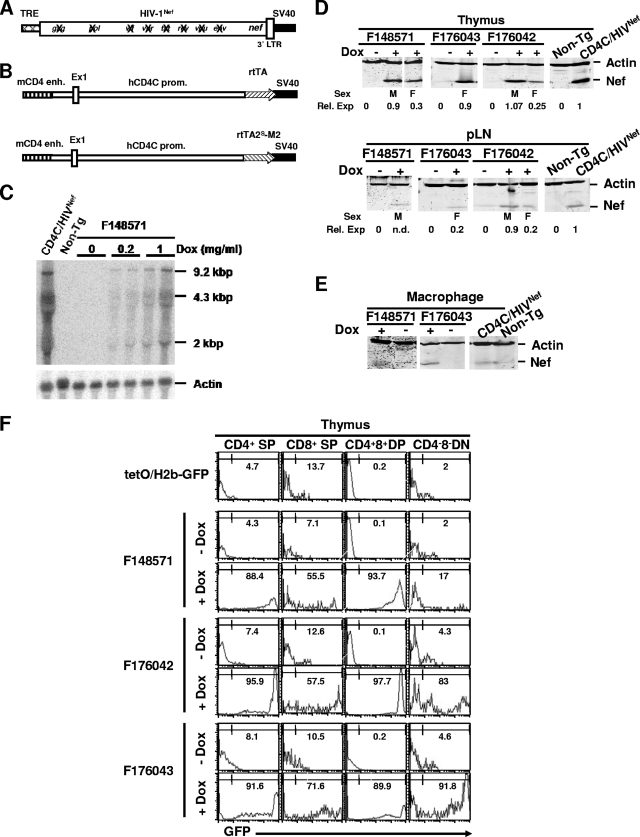
To construct the inducible TRE/HIVNef Tg line, the NL-4-3 HIV-1MutG viral genome (18) (designated here HIVNef) was used. This construct harbors mutations of each of the HIV-1 open reading frames (gag, pol, env, vif, vpu, rev, tat, and vpr) except nef, thus coding only for the Nef protein. The HIVNef DNA fragment, with its downstream SV40 polyadenylation signal, was ligated downstream of the tetracycline-responsive regulatory element (TRE) in an HPRT targeting vector (Fig. 1A). This construct was used to transfect ES cells and to generate Tg mice with a single copy of the transgene integrated at the HPRT locus in the X chromosome. This founder (F) TRE/HIVNef Tg line was designated F130391. The CD4C/rtTA and CD4C/rtTA2S-M2 transactivator lines were generated by ligating the transactivator rtTA or rtTA2S-M2 fragment, respectively, downstream of CD4C regulatory sequences (Fig. 1B). The rtTA2S-M2 gene is a mutated version of the rtTA gene found to exhibit lower basal activity and higher sensitivity to DOX (48). Southern blot analysis of all the Tg mice founders showed a grossly intact transgene structure (data not shown). One CD4C/rtTA founder (F148571) and two CD4C/rtTA2S-M2 founder (F176042 and F176043) Tg lines were used in the experiments. All Tg lines were maintained on the C3H/HeNHsd background, and progeny were routinely genotyped.
FIG. 1.
Transgene structure and DOX-dependent transgene expression. (A) TRE/HIVNef. Crossed thin bar, TRE promoter sequences; thick bar, HIV-1Nef sequences in which all HIV-1 genes except nef are disrupted (X); dark bar, SV40 poly(A) sequences. (B) CD4C/rtTA and CD4C/rtTA2S-M2. The human CD4 promoter (thin bar) was fused to the mouse enhancer (striped bar). Stripted arrow, rtTA or rtTA2S-M2; dark bar, SV40 poly(A) sequences. (C) Northern blot analysis of HIV-1 RNA of thymus from mice treated for 1 week with different concentrations of DOX in drinking water. Total RNA (15 μg) extracted from thymuses was hybridized with 32P-labeled HIV-1-specific probe. RNA from CD4C/HIVNef (F27367) mice was used as a positive control. To control sample loading, blots were washed and rehybridized with actin-specific probe. (D and E) Western blot analysis of Nef protein of thymuses and peripheral LN (D) and of peritoneal macrophages (E) of different transactivator founder lines treated with DOX (2 mg/ml) in drinking water for 1 week. Protein extracts (100 μg) from different organs were blotted with anti-Nef serum and antiactin antibody. Protein samples from CD4C/HIVNef (F27367) mice were used as positive controls. (F) Histogram plots of GFP expression detected by FACS analysis in thymic populations of representative mice treated with DOX (2 mg/ml) for 1 week and untreated controls. The percentages of the indicated gated GFP+ cells are indicated.
DTg mice were generated by breeding the TRE/HIVNef Tg mice with mice of either transactivator Tg line.
DOX-dependent expression of HIV-1 in DTg mice.
The levels of HIV-1 transgene expression were first determined by Northern (Fig. 1C) and Western (Fig. 1D and E) blot analysis of, respectively, RNA and proteins from lymphoid organs of (TRE/HIVNef × CD4C/rtTA) and (TRE/HIVNef × CD4C/rtTA2S-M2) DTg mice fed with DOX in drinking water for 1 week. All untreated DTg mice showed no or very low levels of HIV expression, indicating low leakiness. Transgene induction was DOX and dose dependent with each transactivator Tg line. The three main transcripts of HIV-1 (9.2 kb, 4.3 kb, and 2 kb) could be detected by Northern blot analysis (Fig. 1C). Moreover, the rtTA and rtTA2S-M2 transgenes were not expressed in nonlymphoid organs of the Tg lines, as assessed by Northern blot analysis (data not shown). Western blot analysis with anti-Nef antibodies showed expression of Nef in lymphoid tissues and peritoneal macrophages (Fig. 1D and E), as expected. The expression levels varied between different founder lines, most likely reflecting the positional effect at the site of CD4C/rtTA or CD4C/rtTA2S-M2 transgene integration. We also noticed that Tg expression was lower in female than in male DTg mice of all founder lines, most likely reflecting the X chromosome inactivation of TRE/HIVNef DNA integrated in the X chromosome. At the highest dose of DOX used (2 mg/ml for 1 week), Nef expression was comparable to that of CD4C/HIVNef (F27367) Tg mice, which were previously shown to develop an AIDS-like disease (18). Therefore, a DOX concentration of 2 mg/ml in drinking water was used for all subsequent experiments.
To further determine the specificity of transgene expression and to monitor the identity of the cells expressing HIV-1 in DTg mice, we utilized a GFP reporter mouse, tetO/H2B-GFP, which expresses histone H2B-GFP fusion protein under the control of TRE promoter sequences (47) after induction by rtTA or rtTA2S-M2 transactivator. FACS analysis of GFP expression in the thymus of (CD4C/rtTA × tetO/H2B-GFP) and (CD4C/rtTA2S-M2 × tetO/H2B-GFP) DTg mice is shown in Fig. 1F. Double Tg mice not receiving DOX and single tetO/H2B-GFP Tg mice were used as controls to monitor leaky expression. In the thymus, only a low level of leakiness was observed. After DOX treatment, most CD4+ CD8+ double-positive (DP) and CD4+ CD8− single-positive (SP) thymocytes of the three founder transactivator Tg lines expressed high levels of GFP, as expected (Fig. 1F; Table 1). Most CD4− CD8+ SP thymocytes of the three founders did not express high levels of GFP, but a significant proportion expressed moderate levels. Interestingly, a significant percentage of CD4− CD8− double-negative (DN) thymocytes of two (F176042 and F176043) of the three founder lines were GFP positive (Fig. 1F; Table 1), most likely reflecting the expression of human CD4 in human immature thymocytes (34).
TABLE 1.
Expression of reporter GFP induced in DOX-treated (CD4C/rtTA × tetO/H2B-GFP) and (CD4C/rtTA2S-M2 × tetO/H2B-GFP) DTg mice
| Mouse or founder line | Dox treatmenta | Mean (±SD) % GFPhigh thymocytes in subpopulation |
|||
|---|---|---|---|---|---|
| CD4+ SP | CD8+ SP | CD4+ CD8+ DP | CD4− CD8− DN | ||
| tetO/H2B-GFP | No | 4.7 ± 1.6 | 13.7 ± 3.9 | 0.18 ± 0.16 | 2.7 ± 0.7 |
| F148571b | No | 4.3 ± 2.1 | 7.1 ± 2.1 | 0.14 ± 0.14 | 2 ± 3.2 |
| Yes | 88.3 ± 5.5 | 55.5 ± 8.3 | 93.7 ± 8.4 | 17.8 ± 12.1 | |
| F176042c | No | 7.4 ± 1.1 | 12.6 ± 3.9 | 0.15 ± 0.07 | 4.3 ± 5.7 |
| Yes | 95.9 ± 1.6 | 57.5 ± 7.9 | 97.7 ± 1.9 | 83 ± 23 | |
| F176043c | No | 8.1 ± 3.9 | 10.5 ± 0.9 | 0.2 ± 0.02 | 4.6 ± 2.9 |
| Yes | 91.6 ± 7.1 | 71.6 ± 8.3 | 89.8 ± 11.3 | 91.7 ± 5.6 | |
DOX treatment was 2 mg/ml for 1 week.
(CD4C/rtTA × tetO/H2B-GFP) DTg mice.
(CD4C/rtTA2S-M2 × tetO/H2B-GFP) DTg mice.
These expression results are consistent with our previous work on the expression of various surrogate genes with these CD4C regulatory elements (17, 18, 21, 22).
T-cell phenotypes after early induction of HIV-1 Nef expression.
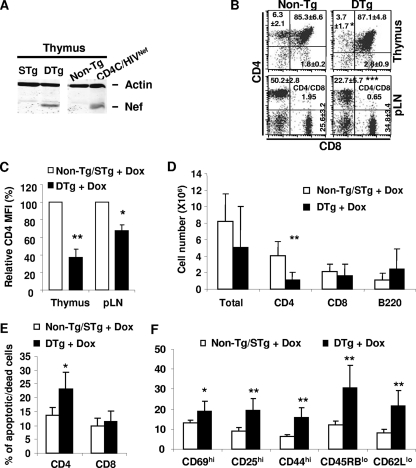
To mimic early Nef expression as reported earlier for CD4C/HIVNef Tg mice (18), (CD4C/rtTA × TRE/HIVNef) DTg pregnant females (F148571) were treated with DOX in drinking water during the second week of pregnancy and maintained on this regimen throughout the weaning period. Pups born from these females were maintained on DOX treatment during all their adult life (up to 6 months of age). The Nef protein was detected in the thymi of 2-week-old pups born to DOX-treated mothers (Fig. 2A). FACS analysis performed on lymphoid tissues showed downregulation of surface CD4 and CD4+ T-cell loss in all male (TRE/HIV × CD4C/rtTA) DTg mice compared to their CD4C/rtTA or TRE/HIV single-Tg and non-Tg littermates on DOX treatment (Fig. 2B to D).
FIG. 2.
Early and continuous induction of Nef expression in Tg mice. Pregnant mice (F148571) were fed with DOX (2 mg/ml) in the drinking water starting at embryonic day E10 and until weaning. After weaning, the pups were kept on DOX up to the age of 6 months. (A) Western blot analysis of Nef protein in thymuses of 2-week-old single-Tg (STg) or DTg pups born to female mice on DOX treatment. Total thymus protein extracts (100 μg) were blotted with anti-Nef serum and antiactin antibody. A protein sample from CD4C/HIVNef mice was used as a positive control. STg, single Tg mice for either TRE/HIV or CD4C/rtTA; DTg, double (TRE/HIVNef × CD4C/rtTA) Tg mice. (B) FACS analysis of thymic and pLN cell populations from 6-month-old male non-Tg or DTg mice. The percentages of cells and standard deviations are indicated in each quadrant. The CD4/CD8 ratios in pLN are also indicated. (C) Bar graph showing relative downregulation of cell surface CD4 expression on thymic and pLN cells from mice expressing or not expressing Nef. (D) Bar graph showing pLN cell numbers in DTg mice as well as in non-Tg and STg littermates treated with DOX (2 mg/ml). (E) Bar graph showing percentages of apoptotic/dead cells among CD4+ T cells from non-Tg/STg and DTg mice treated with DOX. (F) Bar graph representing the proportion of CD4+ T cells expressing the indicated markers in non-Tg/STg and DTg mice treated with DOX. Statistical analysis was performed with Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As expected, peripheral CD4+ T cells of the male DTg mice with early Tg induction also exhibited an increased cell death (Fig. 2E) and an effector/memory-like phenotype (Fig. 2F). Similar FACS analysis on DTg mice from the F176042 and F176043 founder lines showed similar results, except for the additional loss of SP CD8+ thymocytes in these mice (see Fig. S1 in the supplemental material). This SP CD8+ T-cell loss was most likely the result of Nef expression in these cells, as documented above in the GFP reporter mice (Fig. 1F). Moreover, mice from these latter two founders exhibited proteinuria (see Fig. S1 in the supplemental material) and kidney disease (data not shown). Together, these results demonstrate that the inducible HIV-1 Nef Tg mice develop T-cell phenotypes and kidney disease similar to those seen in the constitutively Nef-expressing CD4C/HIVNef Tg mice when Nef expression is induced early in life.
Survival of DOX-treated adult Tg mice.
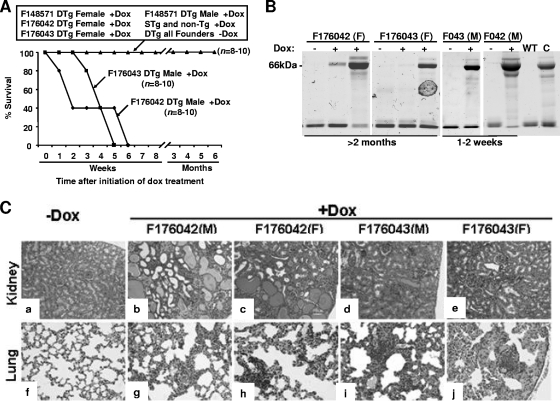
In order to study the long-term effects of the induced HIV Nef on adult mice, the (TRE/HIVNef × CD4C/rtTA) and (TRE/HIVNef × CD4C/rtTA2S-M2) DTg mice were started on DOX treatment (2 mg/ml) at the age of 1 to 1.5 months, and groups of mice (n = 8 to 10) were kept on this regimen for ∼6 months. Controls represent DOX-treated TRE/HIVNef, CD4C/rtTA, CD4C/rtTA2S-M2 STg, and non-Tg mice, as well as untreated DTg mice. All these control mice remained in apparent good health, free of proteinuria (data not shown), throughout the experiment, up to 6 months. All DOX-treated DTg mice generated from the F148571 line and female mice generated from the F176042 and F176043 transactivator founders also remained clinically healthy, although mice from these latter two lines showed proteinuria (Fig. 3B). In contrast, DOX-treated DTg male mice from the F176042 and F176043 transactivator lines had a much shorter survival than their untreated control DTg littermates (Fig. 3A). This shorter survival likely reflects the very severe renal disease developing in these mice, as documented histologically (see below) and by the heavy proteinuria already present after 10 days following initiation of DOX treatment (Fig. 3B).
FIG. 3.
Pathology of DOX-treated adult DTg mice. (A) Cumulative incidence of mortality in DOX-treated DTg mice from different transactivator founder lines. Mice (n = 8 to 10) were treated with DOX (2 mg/ml) for up to 6 months, and the cumulative incidence of mortality was plotted as the percentage of surviving mice. (B) Commassie blue-stained sodium dodecyl sulfate-polyacrylamide gel showing proteinuria, apparent as 66-kDA serum albumin in urine from DOX-treated mice. F, female; M, male; WT, wild-type; C, control CD4C/HIVNef. (C) Histological sections of lungs and kidneys of DOX-treated and untreated DTg mice from the F176042 and F176043 transactivator founder lines, shown at low magnification. Note the glomerulosclerosis and tubular atrophy and dilation in kidneys as well as the lymphocytic interstitial infiltration in lungs of DOX-treated DTg mice.
At autopsy, gross examination confirmed the presence of severe kidney disease (smaller size, irregular surface, pale), as well as severe atrophy of the thymus and lymph nodes (LN) in DOX-treated DTg male mice of the F176042 and F176043 founder lines. Histological examination revealed in each of these organs the same typical AIDS-like pathological changes that we previously described in CD4C/HIVNef Tg mice (18): thymic atrophy, loss of LN, thymus, and spleen architecture, cardiomyopathy (data not shown), lymphocytic interstitial pneumonitis, and focal segmental glomerulosclerosis with tubular cystic dilatation (Fig. 3C). However, lymphocytic interstitial pneumonitis was in general less severe than that of CD4C/HIVNef Tg mice studied previously (18). In DTg female mice from F176042 and F176043 founder lines, the same lesions were present but they were much less severe (Fig. 3C), consistent with the lower level of Nef expression in these female mice. DOX-treated DTg mice from F148571 developed no organ disease (data not shown).
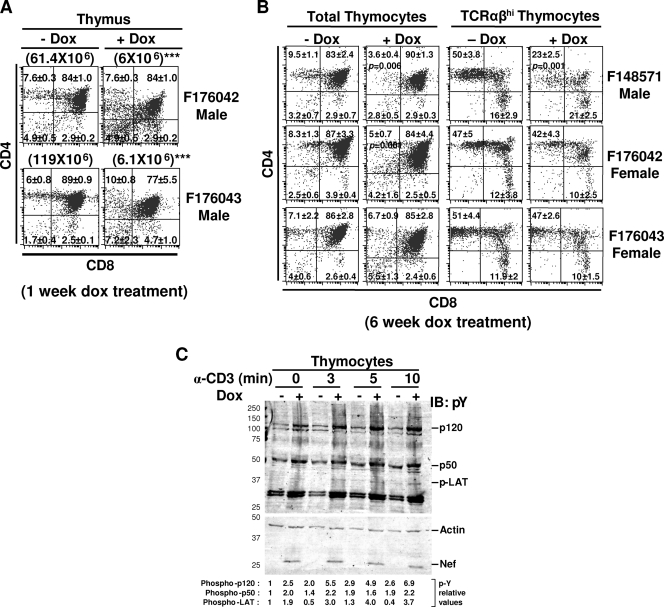
Thymocytes are depleted and hyperresponsive to T-cell receptor (TCR) stimulation in adult Tg mice expressing Nef.
Early expression of Nef in the thymus of CD4C/HIVNef Tg mice leads to cell surface CD4 downmodulation and depletion of thymocytes (18). To determine whether these phenotypes can be induced by Nef expression later in life, 4- to 6-week-old (TRE/HIVNef × CD4C/rtTA) and (TRE/HIVNef × CD4C/rtTA2S-M2) DTg mice were treated with DOX for 1 or 6 weeks and thymocyte population profiles were analyzed by FACS. CD4 expression was found to be downmodulated on CD4+ CD8− SP and CD4+ CD8+ DP thymocytes of all Nef-expressing DTg mice treated with DOX (Fig. 4A and B and Table 2). Nef expression also resulted in significant depletion of thymocytes in mice from all of the tansactivator founder lines (Fig. 4A and B and Table 2). In male Tg mice from F176042 and F176043 transactivator founder lines, severe thymocyte depletion occurred rapidly after only 1 week of DOX treatment even at a low dose of DOX (0.5 mg/ml) (Fig. 4A and Table 2). In these DTg mice, thymus cellularity was over 10-fold lower than in the untreated control DTg mice. This loss was reflected in all thymocyte populations. In male DTg mice from the F148571 line and in female DTg mice from the F176042 and F176043 transactivator founder lines, depletion of thymocytes was not observed after 1 week of DOX induction but was significant after 6 weeks of DOX treatment (2 mg/ml) (Fig. 4B and Table 2).
FIG. 4.
Flow cytometric analysis and Western blot of phosphoproteins of thymocytes in adult DOX-treated DTg mice. (A) FACS analysis of thymocyte populations of male DTg mice from the F176042 and F176043 transactivator founder lines treated or not with 0.5 mg/ml DOX in drinking water for 1 week. The percentages of cells and standard deviations are indicated in each quadrant. Total cell numbers for each thymus are indicated in parentheses. (B) FACS analysis of thymocyte cell populations of male DTg mice from F148571 and female DTg mice from the F176042 and F176043 transactivator founder lines treated or not with 2 mg/ml DOX in drinking water for 6 weeks. The percentages of cells and standard deviations are indicated in each quadrant. (C) Western blot analysis of pY proteins detected with anti-pY MAb (4G10) in thymocyte lysates from DOX-treated and untreated DTg male mice from the F148571 founder line after stimulation with anti-CD3 antibody. The membrane was washed and reblotted with antiactin and anti-Nef antibodies to control loading and to detect Tg expression, respectively. Quantitation was performed as described in Materials and Methods. Data are expressed relative to uninduced non-Tg cells (assigned value of 1). Statistical analysis was performed with Student's t test, and P values are shown in panel B; *** in panel A, P < 0.001.
TABLE 2.
Thymic cell subsets in adult DOX-treated and untreated DTg micea
| Mouse and founder line | Sex | Total DOX treatment (wks)b | No. of cells (106) |
CD4/CD8 ratio | DP CD4 MFI (%)c | |||
|---|---|---|---|---|---|---|---|---|
| Total | CD4+ CD8− | CD8+ CD4− | CD4+ CD8+ | |||||
| CD4C/rtTA × TRE/HIV | ||||||||
| F148571 | Male | None | 70.66 ± 10 | 4.74 ± 0.73 | 1.61 ± 0.62 | 58.8 ± 6.87 | 2.94 | 100 |
| 6 | 48 ± 3.26* | 0.73 ± 0.18** | 0.66 ± 0.08* | 44.8 ± 2.58* | 1.1 | 67.4 ± 4* | ||
| CD4C/rtTA2S-M2 × TRE/HIV | ||||||||
| F176042 | Male | None | 61.4 ± 17.4 | 2.72 ± 0.78 | 0.92 ± 0.31 | 51.8 ± 14.8 | 2.94 | 100 |
| 1 | 6 ± 2.2** | 0.59 ± 0.21** | 0.18 ± 0.08* | 2.8 ± 1.08** | 3.22 | 52.4 ± 20.4 | ||
| Female | None | 109 ± 13.8 | 4.99 ± 1.8 | 1.25 ± 0.53 | 92.6 ± 13.2 | 3.98 | 100 | |
| 6 | 80.5 ± 25.6* | 1.72 ± 0.29** | 0.42 ± 0.14* | 68.5 ± 23.6* | 4.02 | 66.1 ± 6.4*** | ||
| F176043 | Male | None | 119 ± 31 | 5.37 ± 2.03 | 2.07 ± 0.64 | 106 ± 27.9 | 2.58 | 100 |
| 1 | 6.75 ± 3.22** | 0.27 ± 0.09* | 0.08 ± 0.04** | 5.35 ± 2.73** | 3.15 | 42.1 ± 9.7*** | ||
| Female | None | 210 ± 11.5 | 10.7 ± 2.68 | 2.4 ± 0.15 | 180 ± 21.7 | 4.46 | 100 | |
| 6 | 202 ± 26.2 | 5.82 ± 1.74* | 1.21 ± 0.18** | 172 ± 21.7 | 4.79 | 67.7 ± 10.6* | ||
*, P < 0.05; **, P < 0.005; ***, P < 0.0005 (by Student's t test).
DOX-treated animals received a dose of 2 mg/ml for the indicated time period.
MFI, mean fluorescence intensity.
Thymocyte depletion in these mice was reflected by depletion of CD4+ CD8+ DP as well as of both mature TCRαβhi CD4+ CD8− SP and TCRαβhi CD8+ CD4− SP thymocytes (Table 2). The CD4/CD8 ratio was affected in mice from F148571, indicating a faster depletion of CD4+ CD8− SP compared to CD8+ CD4− SP thymocytes. In female DTg mice from the F176043 transactivator founder line, a modest but significant loss of TCRαβhi CD4+ CD8− SP and TCRαβhi CD8+ CD4− SP was observed despite a normal thymic cellularity and an absence of DP thymocyte depletion (Table 2). In DOX-treated female DTg from the F148571 line, the only phenotype observed was a downmodulation of cell surface CD4 on DP thymocytes (see Fig. S2 in the supplemental material).
We previously reported that thymocytes of CD4C/HIVNef Tg mice, expressing Nef early, are hyperresponsive to TCR stimulation (18). Western blot analysis also revealed higher steady-state levels of phosphotyrosine (pY) proteins in thymocytes from DOX-treated adult male DTg mice (F148571) compared to the untreated control mice (Fig. 4C). Stimulation of thymocytes with anti-CD3 resulted in a further increase in the level of pY proteins in DOX-treated mice but not in untreated control mice (Fig. 4C). Hence, expression of Nef in the thymus of adult mice results in thymocyte depletion and downmodulation of surface CD4 expression and induces a state of activation and hyperresponsiveness to TCR stimulation.
Impaired SP thymocyte maturation in Nef-expressing adult mice.
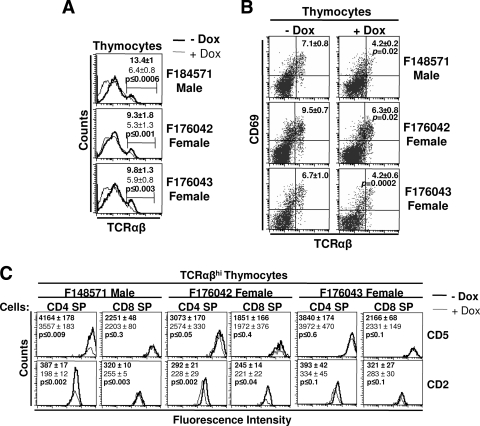
Thymocytes undergo selection and maturation events in the thymus to form immunocompetent CD4+ and CD8+ T cells (39). Thymocyte maturation was studied in DOX-treated DTg mice (male F148571 and female F176042 and F176043 lines) by FACS analysis using different markers (TCRαβ, CD69, CD2, and CD5). This analysis revealed a significantly lower percentage of TCRαβhi thymocytes and the appearance of a TCRαβlow thymocyte population in DOX-treated DTg mice of all founder lines studied (Fig. 5A). In addition, DOX-treated DTg mice also showed a significantly lower percentage of TCRαβhi CD69+ thymocytes (Fig. 5B), indicating that a lower proportion of thymocytes are selected for further maturation. The developmental markers CD2 and CD5 were significantly downmodulated on a higher proportion of TCRαβhi CD4+ CD8− SP thymocytes in DTg mice generated with F148571 and F176042 transactivator mice (Fig. 5C). In contrast, TCRαβhi CD8+ CD4− SP thymocytes exhibited downmodulation of cell surface CD2, but not CD5 (Fig. 5C). In F176043 female DTg mice which maintained thymus cellularity after DOX treatment (Table 2), expression levels of CD2 and CD5 were not downmodulated (Fig. 5C). Together, these results demonstrate that Nef expression can prevent maturation of T cells in adult mice, as it does when expressed earlier (P. Chrobak et al., unpublished data).
FIG. 5.
Analysis of thymocyte maturation in adult DOX-treated mice. (A) Expression of TCRαβ on thymocytes of DTg mice from three transactivator founder lines treated (line) or not (bold) with 2 mg/ml DOX in drinking water for 6 weeks. Percentages of TCRαβhi cells are shown. (B) Expression of TCRαβ and CD69 in thymuses of DOX-treated and untreated control mice. Percentages and standard deviations for TCRαβhiCD69hi cells are indicated. (C) Analysis of maturation markers (CD2 and CD5) on TCRαβhi CD4 SP and CD8 SP thymocytes. Mean fluorescence intensity of each marker is indicated in bold for the untreated control mice and in regular font for DOX-treated mice. Statistical analysis was performed with Student's t test, and P values are shown.
Downregulation of surface CD4 and CD4+ T-cell loss in peripheral lymphoid tissues of inducible adult Tg mice.
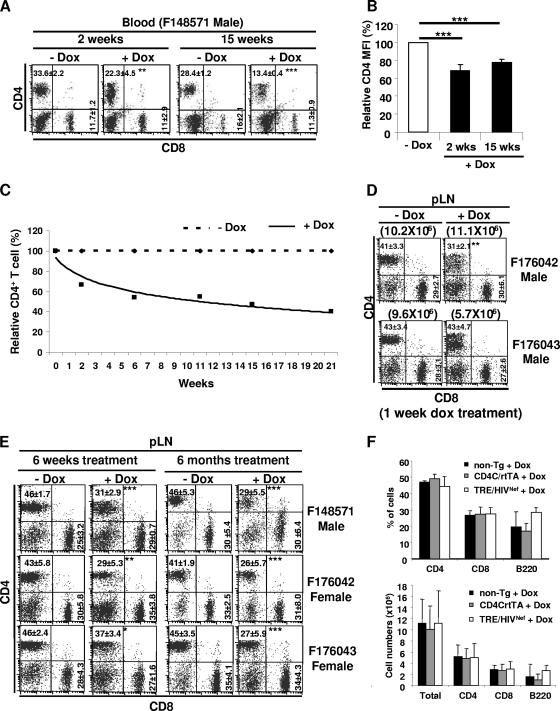
Constitutive expression of Nef starting early in life of CD4C/HIVNef Tg mice results in downregulation of CD4 cell surface expression and depletion of peripheral CD4+ T cells (18). We monitored these two parameters in blood and lymphoid tissues of adult DTg mice treated or not with DOX. CD4 surface expression, monitored by FACS, was found to be downregulated, relative to non-Tg mice, even in TRE/HIVNef STg mice, by ∼40% in peripheral lymph nodes (pLN; P = 0.03) and to a lesser extent in thymocytes (10 to 15%), although these mice expressed no detectable HIV-1 RNA or Nef by Northern or Western blot analysis, respectively (Fig. 1). The degree of this CD4 downmodulation in TRE/HIVNef STg thymocytes was much lower than that observed previously in CD4/HIVNef Tg mice, in which it was found to play a role in disrupting CD4+ SP thymocyte differentiation (Chrobak et al., unpublished). This strongly suggests a very low leakiness of Nef expression in TRE/HIVNef STg mice, consistent with the data with the GFP reporter mice (Fig. 1F). CD4 expression was further downregulated in blood (Fig. 6A and B) and LN (Fig. 6D) as early as 7 days postinduction in male DTg mice from all founder lines. In a number of DTg mice, we noticed, at some point after DOX induction, the presence of two distinct CD4+ T-cell subsets (CD4low and CD4high) (Fig. 6A and D). These two CD4+ T-cell subsets were previously observed in CD4C/HIVNef Tg mice and were shown to represent populations expressing high and very low levels of the HIV transgene, respectively (53). In DOX-treated Tg mice generated with the three transactivator lines, depletion of CD4+ T cells was observed both in blood (Fig. 6A and C) and in LN (Fig. 6D and E) after only a few weeks of Nef expression. This was reflected in lower percentages of cells, lower absolute cell numbers, and in some lines decreased CD4/CD8 ratios (Fig. 6A and C to E and Table 3; see also Tables S1 and S2 in the supplemental material). The severity of this depletion correlated with levels of expression, being more severe in males than in females of the three transactivator founder lines. However, such depletion of CD4+ T cells was not observed in DOX-treated TRE/HIVNef STg mice (Fig. 6F), indicating that the very low leaky Nef expression in these mice was insufficient to deplete these cells. Interestingly, in some DTg mice treated for a longer time with DOX, most of the remaining CD4+ T cells were CD4high (Fig. 6E), suggesting that these cells were not expressing Nef at high levels and thus may have not been depleted. Similar loss of CD4+ T cells was also observed in spleen and mesenteric LN of DOX-treated DTg mice (data not shown).
FIG. 6.
FACS analysis of peripheral T-cell subsets in adult DOX-treated mice. (A to E) FACS analysis of blood (A to C) or pLN (D and E) T-cell populations of the indicated DTg mice treated or not with DOX (2 mg/ml) for the indicated number of weeks. (B) Bar graph showing relative CD4 mean fluorescence intensity (MFI) in blood of the same DOX-treated and untreated DTg mice described for panel A. (C) Graph showing relative loss of CD4+ T cells in blood of DTg mice (F148571) over time after DOX treatment (solid line) or no treatment (dashed line). (D) FACS analysis of pLN cell populations of male DTg mice from founder lines F176042 and F176043 treated or not with 0.5 mg/ml DOX for 1 week. (E) FACS analysis of pLN cell populations of DTg mice of the three founder lines treated or not with DOX (2 mg/ml) for 6 weeks or 6 months. (F) Bar graphs showing percentages (upper panel) and cell numbers (lower panel) of pLN T and B cells from non-Tg, CD4C/rtTA STg, and TRE/HIVNef STg mice. For panels A, D, and E, the percentages of cells and standard deviations are indicated in each quadrant. For panel D, total pLN cell number is indicated in parentheses. Statistical analysis was performed with Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 3.
pLN cell subsets in adult DOX-treated and untreated DTg micea
| Mouse and founder line | Sex | DOX treatmentb (wks) | No. of cells (106) |
CD4/CD8 ratio | CD4 MFIc (%) | |||
|---|---|---|---|---|---|---|---|---|
| Total | CD4+ T | CD8+ T | B | |||||
| CD4C/rtTA × TRE/HIV | Male | None | 17.8 ± 2.02 | 8.16 ± 0.71 | 4.521 ± 0.32 | 4.55 ± 1.15 | 1.8 | 100 |
| F148571 | 6 | 5.05 ± 1.1** | 1.57 ± 0.46*** | 1.48 ± 0.31*** | 1.65 ± 0.33* | 1.06 | 87.8 ± 3.7 | |
| CD4C/rtTA2S-M2 × TRE/HIV | Male | None | 8.8 ± 2.28 | 3.29 ± 0.79 | 2.53 ± 0.77 | 3.47 ± 1.67 | 1.29 | 100 |
| F176042 | 2-6 | 0.52 ± 0.56*** | 0.17 ± 0.18*** | 0.13 ± 0.13*** | 0.14 ± 0.16*** | 1.28 | 87.1 ± 31.9 | |
| Female | None | 18.2 ± 3.8 | 7.38 ± 1.04 | 5.49 ± 1.17 | 2.77 ± 1.23 | 1.34 | 112 | |
| 7 | 13.1 ± 1.66* | 4.02 ± 0.97*** | 4.61 ± 0.75 | 3.56 ± 0.92 | 0.87 | 118 ± 13.1 | ||
| F176043 | Female | None | 19.7 ± 2.06 | 9.16 ± 1.38 | 5.48 ± 0.82 | 4.69 ± 0.97 | 1.67 | 100 |
| 6 | 8.1 ± 2.99** | 3.51 ± 0.8** | 2.22 ± 0.77** | 2.27 ± 1.16* | 1.58 | 103 ± 21 | ||
*, P < 0.05; **, P <0.005; ***, P < 0.0005 (by Student's t test).
DOX-treated animals received 2 mg/ml for the indicated time period.
MFI, mean fluorescence intensity.
There was also loss of CD8+ T cells in pLN of mice from all founder lines (Table 3) and of B cells in DTg males from two lines after shorter induction (Table 3). None of the above-mentioned phenotypes was observed in single-Tg or non-Tg mice treated with the same dose of DOX (data not shown), ruling out the possible detrimental effects of long-term DOX treatment on these cell populations.
These results show that Nef expression causes downmodulation of surface CD4 expression and depletion of T lymphocytes in peripheral lymphoid organs of adult mice.
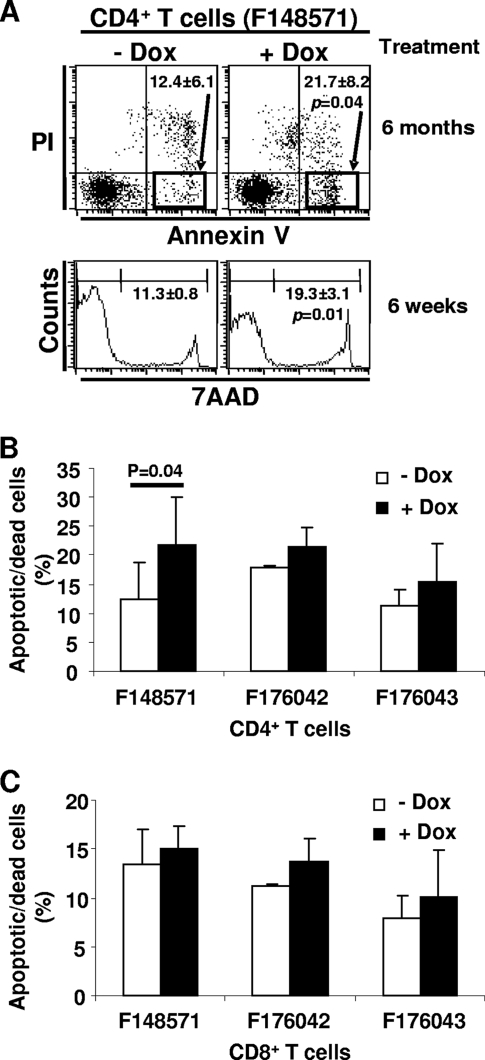
Peripheral CD4+ T cells undergo increased apoptosis/cell death in DOX-treated adult Tg mice.
We previously found that the CD4+ and CD8+ T cells from CD4C/HIVNef Tg mice show a higher level of apoptosis/cell death than their non-Tg littermates (37). To determine whether T-cell depletion in DOX-treated mice was associated with apoptotic cell death, we measured apoptosis/cell death in cells of lymphoid organs by annexinV/PI or 7-AAD staining and FACS analysis. In pLN (Fig. 7A and B) and in mesenteric LN and spleens (data not shown), an increased proportion of CD4+ T cells was positive for annexinV/PI or 7-AAD in all DTg mice treated with DOX compared to the untreated control mice but reached statistical significance only in mice from the F148571 transactivator founder line. No significant increase of apoptotic/dead cells was observed among the CD8+ T cells (Fig. 7C).
FIG. 7.
CD4+ T-cell apoptosis/death in adult DOX-treated mice. Apoptotic/dead cells were analyzed by FACS after staining cells with anti-CD4 and anti-CD8 MAbs and 7-AAD or annexin V/PI. (A) FACS analysis of apoptotic and dead cells among the CD4+ T cells of DTg male mice (F148571) treated or not with DOX (2 mg/ml) for 6 weeks or 6 months. The percentages and standard deviations of apoptotic/dead annexin V+ PI− or 7-AAD+ cells are indicated. (B and C) Bar graphs showing percentages of apoptotic/dead cells among the pLN CD4+ (B) and CD8+ (C) T cells from DTg mice treated with DOX for 6 months. Statistical analysis was performed with Student's t test, and P values are shown when significant.
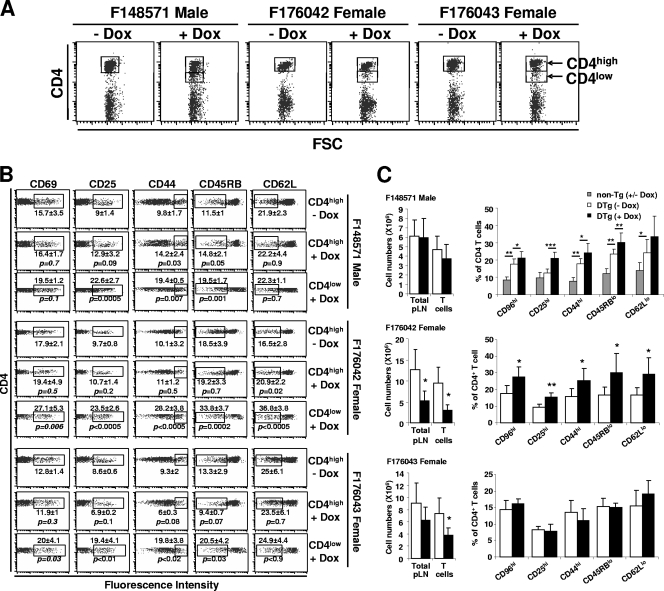
Peripheral CD4+ and CD8+ T cells exhibit an effector/memory-like phenotype in DOX-treated adult Tg mice.
We previously reported that CD4+ T cells from CD4C/HIVNef Tg mice exhibit an effector/memory-like phenotype, being CD25+, CD44+, CD45RBlow, CD62Llow, and CD69+ (53). To determine whether Nef-expressing adult CD4+ T cells would show the same phenotype, both CD4hi and CD4low LN cell subsets (Fig. 8A) from the same DTg mice studied above, and treated for 1, 6, or 24 weeks with DOX, were analyzed by FACS after labeling with different antibodies against activation and developmental markers. Results from this experiment revealed that untreated DTg mice already showed an enhanced activated phenotype compared to non-Tg mice (Fig. 8C), suggesting that the very low leaky Nef expression in these mice was sufficient to elicit an effector/memory-like phenotype. We observed that DOX-induction for 1 week was insufficient to significantly further activate CD4+ or CD8+ T cells, despite being sufficient to document CD4 downregulation (data not shown). After longer induction (6 and 24 weeks), we could observe that a larger proportion of CD4+ T cells from DOX-treated DTg mice expressed CD25 and CD69 and were CD44high, CD45RBlow, and CD62Llow compared to the untreated control mice (Fig. 8B and C). This was especially evident in the CD4low T-cell subset. A similar FACS analysis of CD8+ T cells from the same mice showed signs of activation in the DTg of two transactivator lines (see Fig. S3 in the supplemental material). This analysis could not be performed in males from founder lines F176042 and F176043 due to the massive atrophy of the pLNs and the severe depletion of T cells in these DOX-treated DTg mice.
FIG. 8.
Immunophenotype of pLN CD4+ T cells from adult DOX-treated mice. (A) FACS dot plots showing gates on CD4+ T cells with high and low levels of CD4 expression in DTg mice treated or not with DOX (2 mg/ml) for 6 weeks. (B and C) FACS analysis of activation/developmental markers (CD25, CD44, CD45RB, CD62L, and CD69) on CD4high and CD4low subsets (B) or on total (C) CD4+ T cells of DTg mice induced with DOX for 6 weeks (B) or 6 months (C). In panel C, the non-Tg controls shown for the F48571 mice were also used for mice from the other founder lines (not illustrated). Percentages and standard deviations for cells enclosed in the box are indicated. Statistical analysis was performed with Student's t test, and P values are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The effector/memory-like phenotype of CD4+ T cells is cell autonomous.
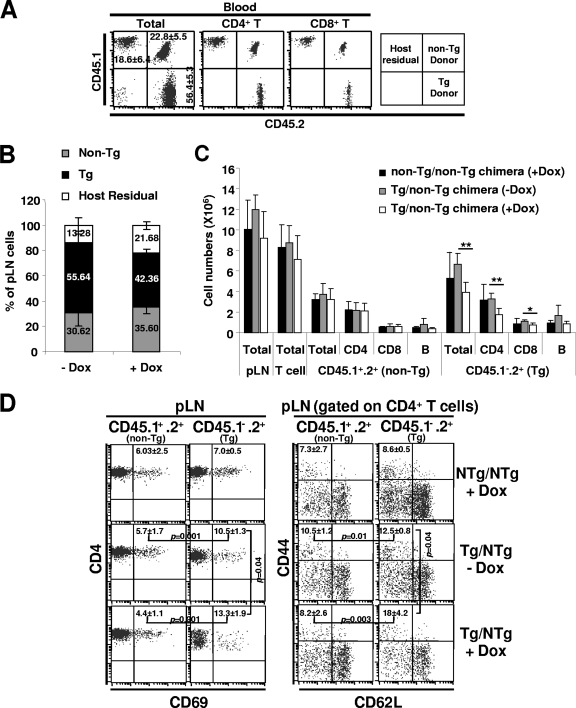
To determine whether Nef expression in T cells is sufficient to cause T-cell activation, we generated mixed BM chimeras by cotransfer of T-cell-depleted BM cells from inducible Nef Tg and non-Tg mice into lethally irradiated CD45.1+ congenic C3H host mice. The donor and host immune cells could be distinguished by the CD45 allele expression. Nef Tg donor cells were CD45.1− CD45.2+, non-Tg donor cells were CD45.1+ CD45.2+, and host cells were CD45.1+ CD45.2−. Two months after BM transfer, FACS analysis of blood cells revealed efficient reconstitution of host with donor-derived cells, ∼56% of which were of Nef Tg origin (Fig. 9A). To induce Nef expression, mice were treated with DOX (2 mg/ml) in drinking water for a period of 2 months. Following this induction period, the mice were sacrificed and pLN were analyzed by flow cytometry. Chimeric mice which were not treated with DOX were used as controls. Staining with antibody against CD45.1 and CD45.2 showed chimerism in pLN cell populations, the Tg cells constituting approximately 55% of cells in these organs (Fig. 9B). As expected, the proportion of Tg cells decreased after treatment, due to Nef-induced loss of T cells (Fig. 9B). Further analysis showed that the total cellularity and total T-cell number of pLN were maintained in DOX-treated chimeras (Fig. 9C). However, as expected, the number of Tg CD4+ and to a lesser extent of CD8+ T cells was reduced in the pLN from DOX-treated mixed chimeras compared to the untreated control chimeras, while the non-Tg T-cell numbers were unaffected (Fig. 9C). These results demonstrate that the Nef-induced loss of Tg CD4+ T cells is cell autonomous and shows no bystander loss of non-Tg T cells.
FIG. 9.
Peripheral CD4+ T-cell depletion and activation in mixed bone marrow chimeras. Lethally irradiated normal mice (CD45.1+ CD45.2−) were reconstituted with mixed BM cells from male Tg (CD45.1− CD45.2+) (F148571) and non-Tg (CD45.1+ CD45.2+) mice. Two months after reconstitution, they were treated with DOX (2 mg/ml) for 2 months and sacrificed for analysis. (A) FACS analysis of T cells from blood of mice reconstituted for 2 months and before DOX treatment. Percentages and standard deviations for cells are shown in each quadrant. (B) Bar graphs showing chimerism in pLN from DOX-treated and untreated chimeric mice. Average percentages are indicated. (C) Bar graph showing cellularity of the pLNs in the DOX-treated and untreated chimeras. (D) FACS analysis of activation markers (CD69, CD44, and CD62L) on non-Tg (CD45.1+ CD45.2+) and Nef Tg (CD45.1− CD45.2+) peripheral LN CD4+ T cells. Percentages and standard deviations for cells with an effector/memory phenotype (CD69+ and CD44+ CD62L−) are indicated. Student's t test was performed, and P values are shown where applicable.
Taking advantage of this favorable context of absence of T-cell lymphopenia, the activation status of Tg and non-Tg CD4+ T cells in the pLN was next investigated by staining for CD69, CD44, and CD62L. Unexpectedly, we noticed that, even in the absence of DOX treatment, Nef Tg CD4+ T cells expressed lower levels of cell surface CD4 and that a higher proportion of them were CD69+ and CD44+ CD62L− (effector/memory phenotype), compared to non-Tg CD4+ T cells (Fig. 9D). This phenomenon is specific to Tg cells and was not observed in non-Tg CD4+ T cells in the same mice. This result is consistent with previous data showing activation of CD4+ T cells even in untreated DTg mice (Fig. 8C). Upon DOX treatment, a further higher number of Tg CD4low T cells acquired an effector/memory phenotype relative to control untreated mixed chimeras (Fig. 9D). In contrast, the proportion of non-Tg CD4+ T cells showing a CD69+ and CD44+ CD62L− phenotype was equal in both DOX-treated and untreated mixed chimera groups (Fig. 9D). Similar results were obtained in a second experiment with the same F148571 DTg mice and with recipient mice transplanted with BM cells from F176042 DTg mice (data not shown). These results indicate that Nef-expressing CD4+ T cells can develop an effector/memory phenotype in the absence of pLN T cell lymphopenia, in a cell autonomous manner. A summary of all data is presented in Table 4.
TABLE 4.
Summary of phenotypes in inducible Nef DTg mice
| Mouse and founder line | Sex | Exptb | Phenotypea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 downregulation |
Thymocyte loss |
pLN total cell loss |
Effector/memory | Proteinuria | Nonlymphoid organ diseasesc | |||||||||
| Thymus | pLN | Total | CD4+ CD8− | CD8+ CD4− | CD4+ CD8+ | Total | CD4+ | CD8+ | ||||||
| CD4C/rtTA × TRE/HIVNef | ||||||||||||||
| F148571 | Maled | M | ++ | + | + | ++ | + | + | ++ | +++ | + | ++ | − | − |
| Female | L | + | − | − | − | − | − | − | − | − | − | − | − | |
| CD4C/rtTA2S-M2 × TRE/HIVNef | ||||||||||||||
| F176042 | Maled | H | ++ | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | NDe | +++ | +++ |
| Female | M | ++ | − | + | + | + | + | + | ++ | + | +++ | ++ | ++ | |
| F176043 | Maled | H | ++ | ++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | NDe | +++ | +++ |
| Female | M | ++ | − | − | + | + | − | ++ | ++ | ++ | ++ | ++ | + | |
The most severe phenotype shown after DOX induction for each DTg line.
Nef expression: H, high; M, moderate; L, low.
Scored at 6 months after DOX induction, except for males of F176042 and F176043 lines, which were scored after 1 and 6 weeks.
All phenotypes seen within 6 weeks of DOX induction.
ND, not determined; the number of pLN T cells was too low to determine this phenotype.
DISCUSSION
We describe here the development of a novel inducible mouse model of AIDS using the tetracycline-inducible system comprising the inducible TRE/HIVNef and the rtTA or rtTA2S-M2 transgenes, the latter acting as transactivators in the presence of DOX. Since Nef has previously been shown to be sufficient and necessary to induce an AIDS-like disease in Tg mice (18), the TRE/HIVNef mice were constructed to harbor a transgene with the capacity to code for only Nef among the known HIV gene products. The TRE/HIVNef STg or uninduced (TRE/HIVNef × CD4/rtTA or CD4C/rtTA2S-M2) DTg mice show very little leakiness, as assessed by HIV RNA or Nef expression and most importantly by the absence of CD4+ T-cell depletion and of organ diseases, even in older mice. However, this low leaky Nef expression was sufficient to induce some CD4 downregulation, mostly in peripheral T cells, and to elicit an effector/memory phenotype, indicating that these latter phenotypes can be triggered with very low levels of Nef. The less severe CD4 downregulation on thymocytes of uninduced TRE/HIVNef STg mice relative to that on thymocytes of CD4/HIVNef Tg mice is likely to explain the apparently normal generation of CD4+ T cells in the thymus of these TRE/HIVNef STg mice and the lack of depletion of their peripheral CD4+ T cells. As expected for such a system, the DTg mice exhibited dose-dependent induction, to levels sufficient to cause very severe phenotypes in a relatively short period of time. The immune defects and organ diseases developing after induction of Nef expression in these DTg mice were observed in males of one CD4C/rtTA line and in males and females of two CD4C/rtTA2S-M2 founder lines, thus ruling out induction of phenotypes caused by disruption of the site of transgene DNA integration itself. Therefore, Nef is most likely causing these phenotypes, as reported before with studies on various HIV mutants (18).
A specific feature of this new inducible model is that female DTg mice develop less severe phenotypes and express lower levels of Nef than male DTg mice, as expected. This characteristic most likely reflects the X chromosome inactivation of the TRE/HIVNef DNA integrated at the HPRT locus. Indeed, this differential expression of the transgene was not observed in DTg mice generated with the same CD4C/rtTA or CD4C/rtTA2S-M2 inducer lines and the tetO/GFP reporter line. Therefore, female DTg mice are mosaic for Nef expression, offering an opportunity to study the effects of Nef in a different context with the same mouse line. We have previously reported that mosaic mice, generated by injecting ES cells harboring the CD4C/HIVwt DNA into non-Tg blastocysts, develop the same AIDS-like disease as CD4C/HIVwt Tg mice themselves (17). These results were confirmed here. In addition, this mosaicism is likely to decrease the number of Nef-expressing cells in the thymus below the expected 50%, as Nef-expressing cells are outcompeted by cells not expressing Nef during thymic differentiation, as we recently documented in hematopoietic chimeric mice generated by transplantation of mixed Nef Tg/non-Tg fetal liver cells into lethally irradiated hosts (Chrobak et al., unpublished). This phenomenon could explain part of the less severe depletion of thymic and peripheral CD4+ T cells observed in female relative to male DTg mice (Tables 2 and 3).
In addition, for females of the F148571 line, other factors may prevent the depletion of CD4+ T cells, despite expressing Nef at levels equivalent to those in females of the other lines. First, males from this founder also show, for an unknown reason, a more modest loss of DP thymocytes than in the other founders, despite expressing Nef at levels as high as those of the other founders. Since the F148571 females express Nef at lower levels than males, they are expected to show an even more modest (undetectable) depletion. Second, although the expression of Nef in total thymocytes of F148571 females seems to be similar to that of females from the other founders, the proportion and the identity of thymic cells expressing the transgene (as assessed by GFP expression) in this founder (F148571) were clearly different from those in F176042 and F176043. Indeed, a very small proportion of F148571 DN thymocytes express GFP, while most DN thymocytes (83% and 91%) of the other two founders (F176042 and F176043) express GFP (Fig. 1F). Since Nef blocks the DN→DP differentiation, as recently shown in CD4C/HIVNef Tg mice (Chrobak et al., unpublished), the much lower Nef expression in F148571 DN thymocytes may be insufficient to participate in the depletion of thymic T cells. Third, males and females of the F148571 founder line do not develop organ diseases. We have recently proposed that Nef expression in a yet-unidentified cell population is responsible for the development of organ diseases in Tg mice (19). This population or another one (not yet identified either) may be involved in the Nef-induced depletion of thymic T cells and may not express Nef either in this F148571 founder line. Again, this would help in maintaining the thymocyte number intact.
To validate the model, we initially recapitulated the conditions of Nef induction mimicking those present in the CD4C/HIVNef Tg mice, i.e., exhibiting a constitutive expression of Nef starting early in life. Nef was induced early, beginning during late embryogenesis of DTg mice, and maintained until weaning and then continuously during their adult life. Under these early induction conditions, DOX-induced DTg mice developed an AIDS-like disease which was a phenocopy of that previously described in CD4C/HIVNef Tg mice (18, 37, 53), as expected. In particular, these DTg mice showed downmodulation of surface CD4, depletion of thymic and peripheral CD4+ T cells, increased apoptotic/cell death, and activation of CD4+ T cells, as well as organ diseases. When Nef expression was induced in adult DTg mice, an AIDS-like disease very similar to that observed after early induction or in CD4C/HIVNef Tg mice also developed. In particular, we could document not only depletion of DP, SP CD4+, and at a later stage SP CD8+ thymocytes, and organ diseases, as reported previously (18, 37, 53), but also impaired maturation of SP CD4+ thymocytes, as recently shown in CD4C/HIVNef Tg mice (Chrobak et al., unpublished). The organ disease arising in these adult Nef Tg mice is unlikely to result from the expression of Nef in T cells, macrophages, or dendritic cells but is most probably caused by expression of Nef in a nontransplantable cell subset, not yet identified, as recently shown for CD4C/HIVNef Tg mice (19).
In addition, in two founder lines (F176042 and F176043), SP CD8+ thymocytes and peripheral CD8+ T cells were depleted as severely as CD4+ T cells. This novel phenotype, not present in CD4C/HIVNef Tg mice, has been observed in other Tg mice expressing Nef with different T-cell-restricted promoters (CD2, TcRβ) (3, 30), as well as in a proportion of HIV-1-infected individuals (6, 7, 28, 31, 40). Loss of CD8+ T cells is likely to result from the expression of Nef in this mouse population and may reflect a situation encountered in some HIV-infected individuals. Indeed, frequent infection of peripheral CD8+ T cells has been reported in human AIDS and found to correlate with depletion of CD8+ T cells (6, 7, 31, 40). These results indicate that the severe impairment caused by Nef in different cell populations and/or tissues is not dependent on underlying aberrant developmental problems arising during embryogenesis or in the newborn period.
Interestingly, our study of the effects of short-term induction of Nef on DP cells of these DTg mice seems to have shed some light on the mechanism for their depletion. In male DTg mice generated with two inducer founder lines (F176042 and F176043) and treated with DOX for only 1 week, a severe loss (>90%) of DP thymocytes caused by high levels of Tg expression was detected. This depletion could originate from a block of maturation of DN to DP thymocytes, a phenotype that we have documented in CD4C/HIVNef Tg mice (Chrobak et al., unpublished) or from a direct or indirect (through stroma) action of Nef on DP cells themselves. By comparing the extent of depletion of Nef-expressing DP cells with that of DP cells in inducible Rag-deficient mice (induced to delete Rag during adulthood) (1), it appears that DP cells are depleted to a larger extent in Nef-expressing DTg mice than in the absence of Rag (∼80% versus ∼50% at 1 week). Since Rag deficiency induces a DN→DP differentiation block, this comparative result strongly suggests that disruption of thymic differentiation can account by itself only partially for the rapid and severe loss of DP thymocytes in (TRE/HIVNef × CD4C/rtTA2S-M2) DTg mice. Additional mechanisms, such as DP thymocyte death caused by the direct action of Nef in DP cells or by its indirect action on thymic stromal cells, are likely to contribute to the rapid and severe depletion of DP thymocytes. The availability of the present Nef inducible system has been instrumental in recognizing the contribution of these direct or indirect effects of Nef on DP thymocytes.
This inducible Tg system which allows induction of Nef for different periods of time, in the same mouse founder and in adult CD4+ T cells, has also led to a better appreciation of the homeostasis of peripheral CD4+ T cells. Short induction periods of Nef (1 or 2 weeks) were indeed found to induce a significant proportion of CD4low T cells (Fig. 7A and D), as expected, since Nef downregulates CD4 cell surface expression. However, in most DTg mice induced for longer periods (6 weeks or 6 months), the CD4low T-cell population was surprisingly less abundant, with most CD4+ T cells being CD4high (Fig. 7E and 8A). The most likely explanation for this phenomenon is that the CD4high T cells, which express Nef at much lower levels, as shown before (53), are conserved and possibly expanding, while the Nef-expressing CD4low T cells are depleted with time.
We took advantage of this novel biological system to reassess the role of Nef in CD4+ T-cell activation. We found that CD4+ T cells from uninduced adult Tg mice were activated, most likely as a result of low Nef expression leakiness. These cells were further activated after DOX treatment, indicating that this activation is positively correlated with Nef expression levels, as previously reported in CD4C/HIVNef mice (53) and with the duration of Nef expression, remaining at basal levels when induction was limited to 1 week. This latter characteristic is intriguing, as it suggests that an additional yet-unknown factor(s), other than the simple high expression of Nef and CD4 downregulation (already present after 1 week of DOX treatment), is (are) involved in the DOX-induced activation phenotype. These could represent cytokines/lymphokines produced by other Nef-expressing cells or through their action. Some time may be necessary to build up sufficiently high levels of these factors to activate Nef-expressing Tg CD4+ T cells. Alternatively, Nef-expressing CD4+ T cells may need to interact with other cells to be further activated; delayed trafficking or other factors may retard activation. Whatever the identity of the yet-unknown factor(s) contributing to activation of Nef-expressing CD4+ T cells, it does not appear to be lymphopenia, as others have recently suggested (26).
Indeed, in uninduced DTg mice as well as in induced mixed chimeras generated from non-Tg and inducible Nef Tg BM cells, we documented that Tg CD4+ T cells (but not non-Tg ones) exhibit an activated phenotype in the absence of lymphophenia. These results contrast with the study by Koenen and coworkers, who reported no T-cell activation in similar mixed chimeric mice reconstituted with BM cells from wild-type and CD2/Nef Tg mice, expressing Nef in both CD4 and CD8 T cells (26). However, in these latter chimeras, the Nef Tg T cells constituted only 1 to 2% of the total peripheral LN T-cell compartment where activation was measured (26). Moreover, the authors did not document that these residual pLN Tg T cells expressed Nef. They rather relied on the downregulation of CD4 as a surrogate for Nef expression. The Tg T cells were outcompeted by wild-type T cells and may have been selected aberrantly in the thymus and/or may represent a small proportion of T cells not expressing the transgene. In contrast, in the experiment presented here (Fig. 9), we were able to overcome the problem of low Tg T-cell number in mixed BM chimeras by using the inducible Nef Tg mice, in which Tg cells constituted ∼55% of total pLN cells before DOX treatment. In these induced mixed chimeras, Nef Tg, but not non-Tg, CD4+ T cells showed an effector/memory phenotype in the absence of lymphophenia. Moreover, we could rule out that the specific postirradiation/BM transplantation environment contributes in a major way to the Nef-mediated CD4+ T-cell activation by documenting this same phenotype (although to a lesser extent), again in uninduced DTg mice not subjected to experimental manipulations. Therefore, Nef expression seems to be the most likely mediator of these effects and appears to be required to cause T-cell activation in Tg mice. If this model of CD4+ T-cell activation reflects the immune T-cell activation documented in HIV-1-infected individuals (11, 14, 23), it would predict that possibly part of this human CD4+ T-cell activation may also be caused by very low expression of Nef in these non-productively infected human cells.
Although the present inducible Nef Tg model represents an improvement relative to previous mouse models of AIDS, it has its own limitations. First, since the mutated Tg HIV DNA can code only for Nef, the contribution of other HIV-1 genes, alone or in collaboration with Nef, in the pathogenesis of AIDS cannot be assessed. Second, the contribution of Nef to the pathogenesis of AIDS is studied in this model in the absence of virus replication and of reverse transcription, thus preventing investigation on the role of Nef on virus replication and on the early phase of the virus cycle. Third, total absence of Nef expression could not be achieved in uninduced Tg mice, and the low leaky Nef expression, although too low to induce CD4+ T-cell depletion and organ diseases, could be sufficient to prevent some studies, such as the induction of an immune response against Nef.
In summary, despite some limitations of the model, we have shown that expression of Nef in adult mice induces an AIDS-like disease very similar to the disease reported previously (18, 37, 53) in Tg mice expressing Nef early in life. The severity of the disease depends on the levels of Nef expression. The inducible Nef Tg mice described here represent a unique model to study Nef functions and effects in vivo in relation to the levels and time of expression of Nef and in vitro in primary immune cells. Moreover, these mice should be useful for the study of immune reconstitution, since Nef expression can be turned off after DOX withdrawal, thus offering the possibility of following the fate of cell populations and/or tissues once exposed to Nef, an experimental condition mimicking the clinical situation after antiviral therapy. To our knowledge, these Tg mice represent the first model expressing Nef in an inducible manner in target cells relevant for AIDS pathogenesis.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, HIV/AIDS Research Program, from CANFAR, from NHLBI (NIH), and from the FRSQ-SIDA Network to P.J.
We are grateful to Alan Peterson, McGill University, for providing the HPRT targeting vector and the BK4 ES cells and for helpful discussions and to W. Hillen and Bujard for giving us the rtTA2S-M2 DNA clone. We thank Benoît Laganière, Ginette Massé, and Isabelle Corbin for their excellent technical assistance. We are grateful to the personnel of our core facilities for their excellent assistance: Martine Dupuis and Eric Massicotte with flow cytometry, Annie Lavallée with histology, and Michel Robillard and Quinzhang Zhu for generating Tg and chimeric mice. We thank Kate Ng for typing the manuscript.
Footnotes
Published ahead of print on 9 September 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bourgeois, C., Z. Hao, K. Rajewsky, A. J. Potocnik, and B. Stockinger. 2008. Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen. Proc. Natl. Acad. Sci. USA 105:8691-8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady, H. J., D. J. Abraham, D. J. Pennington, C. G. Miles, S. Jenkins, and E. A. Dzierzak. 1995. Altered cytokine expression in T lymphocytes from human immunodeficiency virus Tat transgenic mice. J. Virol. 69:7622-7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady, H. J., D. J. Pennington, C. G. Miles, and E. A. Dzierzak. 1993. CD4 cell surface downregulation in HIV-1 Nef transgenic mice is a consequence of intracellular sequestration. EMBO J. 12:4923-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 5.Bronson, S. K., E. G. Plaehn, K. D. Kluckman, J. R. Hagaman, N. Maeda, and O. Smithies. 1996. Single-copy transgenic mice with chosen-site integration. Proc. Natl. Acad. Sci. USA 93:9067-9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochrane, A., S. Imlach, C. Leen, G. Scott, D. Kennedy, and P. Simmonds. 2004. High levels of human immunodeficiency virus infection of CD8 lymphocytes expressing CD4 in vivo. J. Virol. 78:9862-9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullen, B. R. 2001. A new entry route for HIV. Nat. Med. 7:20-21. [DOI] [PubMed] [Google Scholar]
- 8.Dickie, P. 2000. Nef modulation of HIV type 1 gene expression and cytopathicity in tissues of HIV transgenic mice. AIDS Res. Hum. Retrovir. 16:777-790. [DOI] [PubMed] [Google Scholar]
- 9.Dickie, P., F. Ramsdell, A. L. Notkins, and S. Venkatesan. 1993. Spontaneous and inducible epidermal hyperplasia in transgenic mice expressing HIV-1 Nef. Virology 197:431-438. [DOI] [PubMed] [Google Scholar]
- 10.Djordjevic, J. T., S. D. Schibeci, G. J. Stewart, and P. Williamson. 2004. HIV type 1 Nef increases the association of T cell receptor (TCR)-signaling molecules with T cell rafts and promotes activation-induced raft fusion. AIDS Res. Hum. Retrovir. 20:547-555. [DOI] [PubMed] [Google Scholar]
- 11.Fahey, J. L., J. M. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. 1998. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS 12:1581-1590. [DOI] [PubMed] [Google Scholar]
- 12.Fenard, D., W. Yonemoto, C. De Noronha, M. Cavrois, S. A. Williams, and W. C. Greene. 2005. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J. Immunol. 175:6050-6057. [DOI] [PubMed] [Google Scholar]
- 13.Furth, P. A., L. St Onge, H. Boger, P. Gruss, M. Gossen, A. Kistner, and H. L. Bujard. 1994. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. USA 91:9302-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859-870. [DOI] [PubMed] [Google Scholar]
- 15.Gossen, M., S. Freundlieb, G. Bender, G. Muller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766-1769. [DOI] [PubMed] [Google Scholar]
- 16.Goudreau, G., S. Carpenter, N. Beaulieu, and P. Jolicoeur. 1996. Vacuolar myelopathy in transgenic mice expressing human immunodefiency virus type 1 proteins under the regulation of the myelin basic protein gene promoter. Nat. Med. 2:655-661. [DOI] [PubMed] [Google Scholar]
- 17.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 19.Hanna, Z., E. Priceputu, P. Chrobak, C. Hu, V. Dugas, M. Goupil, M. Marquis, L. de Repentigny, and P. Jolicoeur. 2009. Selective expression of human immunodeficiency virus Nef in specific immune cell populations of transgenic mice is associated with distinct AIDS-like phenotypes. J. Virol. 83:9743-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna, Z., E. Priceputu, C. Hu, P. Vincent, and P. Jolicoeur. 2006. HIV-1 Nef mutations abrogating downregulation of CD4 affect other Nef functions and show reduced pathogenicity in transgenic mice. Virology 346:40-52. [DOI] [PubMed] [Google Scholar]
- 21.Hanna, Z., N. Rebai, J. Poudrier, and P. Jolicoeur. 2001. Distinct regulatory elements are required for faithful expression of human CD4 in T-cells, macrophages and dendritic cells of transgenic mice. Blood 98:2275-2278. [DOI] [PubMed] [Google Scholar]
- 22.Hanna, Z., C. Simard, and P. Jolicoeur. 1994. Specific expression of the human CD4 gene in mature CD4+ CD8− and immature CD4+ CD8+ T cells and in macrophages of transgenic mice. Mol. Cell. Biol. 14:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 24.Iwakura, Y., T. Shioda, M. Tosu, E. Yoshida, M. Hayashi, T. Nagata, and H. Shibuta. 1992. The induction of cataracts by HIV-1 in transgenic mice. AIDS 6:1069-1075. [DOI] [PubMed] [Google Scholar]
- 25.Kistner, A., M. Gossen, F. Zimmermann, J. Jerecic, C. Ullmer, H. Lubbert, and H. Bujard. 1996. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA 93:10933-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenen, P. G., F. M. Hofhuis, M. A. Oosterwegel, and K. Tesselaar. 2007. T cell activation and proliferation characteristic for HIV-Nef transgenic mice is lymphopenia induced. J. Immunol. 178:5762-5768. [DOI] [PubMed] [Google Scholar]
- 27.Kopp, J. B., M. E. Klotman, S. H. Adler, L. A. Bruggeman, P. Dickie, N. J. Marinos, M. Eckhaus, J. L. Bryant, A. L. Notkins, and P. E. Klotman. 1992. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc. Natl. Acad. Sci. USA 89:1577-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kourtis, A. P., C. Ibegbu, A. J. Nahmias, F. K. Lee, W. S. Clark, M. K. Sawyer, and S. Nesheim. 1996. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335:1431-1436. [DOI] [PubMed] [Google Scholar]
- 29.Leonard, J. M., J. W. Abramczuk, D. S. Pezen, R. Rutledge, J. H. Belcher, F. S. G. Hakim, L. Lamperth, W. Travis, T. Fredrickson, et al. 1988. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242:1665-1670. [DOI] [PubMed] [Google Scholar]
- 30.Lindemann, D., R. Wilhelm, P. Renard, A. Althage, R. Zinkernagel, and J. Mous. 1994. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J. Exp. Med. 179:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livingstone, W. J., M. Moore, D. Innes, J. E. Bell, P. Simmonds, et al. 1996. Frequent infection of peripheral blood CD8-positive T- lymphocytes with HIV-1. Lancet 348:649-654. [DOI] [PubMed] [Google Scholar]
- 32.Oswald-Richter, K., S. M. Grill, N. Shariat, M. Leelawong, M. S. Sundrud, D. W. Haas, and D. Unutmaz. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennington, D. J., S. A. Jenkins, H. J. Brady, C. G. Miles, E. A. Dzierzak, and D. J. Abraham. 1997. HIV- I Nef severely impairs thymocyte development and peripheral T-cell function by a CD4-independent mechanism. Genes Funct. 1:321-335. [DOI] [PubMed] [Google Scholar]
- 34.Plum, J., M. De Smedt, G. Leclercq, T. Taghon, T. Kerre, and B. Vandekerckhove. 2008. Human intrathymic development: a selective approach. Semin. Immunopathol. 30:411-423. [DOI] [PubMed] [Google Scholar]
- 35.Popov, J., T. McGraw, B. Hofmann, B. Vowels, A. Shum, P. Nishanian, and J. L. Fahey. 1992. Acute lymphoid changes and ongoing immune activation in SIV infection. J. Acquir. Immune Defic. Syndr. 5:391-399. [PubMed] [Google Scholar]
- 36.Priceputu, E., Z. Hanna, C. Hu, M.-C. Simard, P. Vincent, S. Wildum, M. Schindler, F. Kirchhoff, and P. Jolicoeur. 2007. Primary human immunodeficiency virus type 1 Nef alleles show major differences in pathogenicity in transgenic mice. J. Virol. 81:4677-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priceputu, E., I. Rodrigue, P. Chrobak, J. Poudrier, T. W. Mak, Z. Hanna, Hu.C., D. G. Kay, and P. Jolicoeur. 2005. The Nef-mediated AIDS-like disease of CD4C/HIV transgenic mice is associated with increased Fas/FasL expression on T cells and T cell death, but is not prevented in Fas, FasL, TNFR-1 and ICE deficient nor in Bcl2-expressing transgenic mice. J. Virol. 79:6377-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radja, F., D. G. Kay, S. Albrecht, and P. Jolicoeur. 2003. Oligodendrocyte-specific expression of human immunodeficiency virus type 1 Nef in transgenic mice leads to vacuolar myelopathy and alters oligodendrocyte phenotype in vitro. J. Virol. 77:11745-11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenberg, E. V., and T. Taghon. 2005. Molecular genetics of T cell development. Annu. Rev. Immunol. 23:601-649. [DOI] [PubMed] [Google Scholar]
- 40.Saha, K., J. Zhang, and B. Zerhouni. 2001. Evidence of productively infected CD8+ T cells in patients with AIDS: implications for HIV-1 pathogenesis. J. Acquir. Immune Defic. Syndr. 26:199-207. [DOI] [PubMed] [Google Scholar]
- 41.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 42.Simard, M.-C., P. Chrobak, D. G. Kay, Z. Hanna, S. Jothy, and P. Jolicoeur. 2002. Expression of simian immunodeficiency virus Nef in immune cells of transgenic mice leads to a severe AIDS-like disease. J. Virol. 76:3981-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 44.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tinkle, B. T., L. Ngo, P. A. Luciw, T. Maciag, and G. Jay. 1997. Human immunodeficiency virus-associated vasculopathy in transgenic mice. J. Virol. 71:4809-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toggas, S. M., E. Masliah, E. M. Rockenstein, G. F. Rall, C. R. Abraham, and L. Mucke. 1994. Central nervous system damage produced by expression of the HIV- 1 coat protein gp120 in transgenic mice. Nature 367:188-193. [DOI] [PubMed] [Google Scholar]
- 47.Tumbar, T., G. Guasch, V. Greco, C. Blanpain, W. E. Lowry, M. Rendl, and E. Fuchs. 2004. Defining the epithelial stem cell niche in skin. Science 303:359-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urlinger, S., U. Baron, M. Thellmann, M. T. Hasan, H. Bujard, and W. Hillen. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA 97:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vellutini, C., N. Horschowski, V. Philippon, D. Gambarelli, and K. A. F. P. Nave. 1995. Development of lymphoid hyperplasia in transgenic mice expressing the HIV tat gene. AIDS Res. Hum. Retrovir. 11:21-29. [DOI] [PubMed] [Google Scholar]
- 50.Vincent, P., E. Priceputu, D. Kay, K. Saksela, P. Jolicoeur, and Z. Hanna. 2006. Activation of p21-activated kinase 2 and its association with Nef are conserved in murine cells but are not sufficient to induce an AIDS-like disease in CD4C/HIV transgenic mice. J. Biol. Chem. 281:6940-6954. [DOI] [PubMed] [Google Scholar]
- 51.Vogel, J., S. H. Hinrichs, L. A. Napolitano, L. Ngo, and G. Jay. 1991. Liver cancer in transgenic mice carrying the human immunodeficiency virus tat gene. Cancer Res. 51:6686-6690. [PubMed] [Google Scholar]
- 52.Vogel, J., S. H. Hinrichs, R. K. Reynolds, P. A. Luciw, and G. Jay. 1988. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature 335:606-611. [DOI] [PubMed] [Google Scholar]
- 53.Weng, X., E. Priceputu, P. Chrobak, J. Poudrier, D. G. Kay, Z. Hanna, T. W. Mak, and P. Jolicoeur. 2004. CD4 T cells from CD4C/HIV nef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro, but are dispensable for the development of a severe AIDS-like organ disease. J. Virol. 78:5244-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.