Abstract
Hendra virus and Nipah virus, two zoonotic paramyxoviruses in the genus Henipavirus, have recently emerged and continue to cause sporadic disease outbreaks in humans and animals. Mortality rates of up to 75% have been reported in humans, but there are presently no clinically licensed therapeutics for treating henipavirus-induced disease. A recent report indicated that chloroquine, used in malaria therapy for over 70 years, prevented infection with Nipah virus in vitro. Chloroquine was assessed using a ferret model of lethal Nipah virus infection and found to be ineffective against Nipah virus infection in vivo.
Hendra virus (HeV) and Nipah virus (NiV) are the sole members of the genus Henipavirus (8, 23), for which the natural reservoir is several species of flying foxes. NiV was first isolated during an outbreak of disease in humans and pigs in Malaysia in 1998 (5). The virus was transmitted from pigs to humans and was associated with a high level of mortality—265 human cases with 105 deaths. Subsequently, multiple NiV outbreaks have been reported in people in Bangladesh and India, with respiratory and neurological symptoms recorded and mortality rates of up to 75% observed. There was also strong epidemiological evidence of human-to-human transmission in at least one of these outbreaks (1, 11). HeV was first isolated in Queensland, Australia, in 1994, where it led to the deaths of 13 horses and one horse trainer (16). Eleven subsequent spillover events resulted in four human infections, including two fatalities (9), with both respiratory and neurological symptoms recorded. In each case, transmission of HeV to humans appears to have been via infected horses rather than from contact with natural reservoirs (10, 24).
Together, the henipaviruses present a continued potential threat to people in close contact with infected animals, and human-to-human transmission remains a possibility. HeV and NiV are classified as select agents with the potential for causing significant morbidity and mortality in humans and major economic and public health impacts. Therefore, work with live virus requires biosafety level 4 containment. There are currently no prophylactic or therapeutic treatments available.
Empirical success was reported with ribavirin during one outbreak of NiV, with a 36% reduction in mortality observed (4); however, the trial was not blinded or randomized. Although monoclonal antibodies and subunit vaccine candidates are currently under development (2), none are yet licensed for use in humans.
Chloroquine is a 9-aminoquinoline used widely in the mid-20th century as an antimalarial agent until resistance emerged in the malaria parasite. A recent report showed that chloroquine at a concentration of 1 μM or greater inhibited infection with live HeV and NiV in vitro (20). In this study, we report on the efficacy of chloroquine in preventing or moderating infection and disease in vivo in a ferret model of NiV (2) (2a).
Ferrets aged 12 to 18 months received 25 mg/kg/day of chloroquine (Sigma Aldrich, NSW, Australia) in 500 μl of 20% sucrose. This dose was chosen based on a previous study of chloroquine treatment in humans suffering from acute malaria (3) and is twice the dose used in a study to determine the effect of chloroquine on influenza infection in ferrets (22). Ferrets 1 to 3 received a loading dose of chloroquine 24 h before viral challenge, and ferrets 4 to 6 received their first dose of chloroquine 10 h after viral challenge. Control ferrets 7 and 8 were given mock doses of 20% sucrose, one at 24 h before and one at 10 h after challenge. The challenge virus used was a low passage of the NiV Malaysia isolate EUKK 19817 (13) administered oronasally at 5,000 50% tissue culture infective doses per animal (∼10 times the 50% median infective dose). All procedures involving live virus were conducted under biosafety level 4 containment.
Daily clinical observations were recorded, and body temperature was assessed continuously via implanted temperature transponders. Nasal washes, rectal and oral swabs, and urine, serum, and EDTA blood samples were collected from animals anesthetized with ketamine (Ketamil; Troy Laboratories, NSW, Australia) and medetomidine (Domitor; Pfizer Animal Health, NSW, Australia). Samples were collected prior to the first administration of chloroquine, at days 6 and 8 postchallenge, and at euthanasia. At postmortem examination, the tissues listed in Table 1 were collected for virus isolation, viral genome detection, histology, and immunohistology.
TABLE 1.
Relative quantification of NiV RNA levels among different types of tissue in each animal
| Type of tissue | Relative quantification of NiV RNA levelb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group 1, treated with chloroquine 24 h before NiV challenge |
Group 2, treated with chloroquine 10 h after NiV challenge |
Group 3, untreated control, NiV challenge only |
||||||
| Ferret 1 (F) | Ferret 2 (F) | Ferret 3 (M) | Ferret 4 (M) | Ferret 5 (F) | Ferret 6 (F) | Ferret 7 (M) | Ferret 8 (M) | |
| Adrenal gland | 43,884.35 | 0.65 | 0.20 | 1,122.61 | 191.37 | 277.31 | 21.81 | 2,194.99 |
| Bladder | 77.68 | 1.43 | 14.07 | 0.25 | 222.86 | 2.82 | 0.06 | 222.86 |
| Brain—occipital lobea | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Olfactory pole | 1.42 | 0.75 | 0.60 | 2.53 | 2.00 | 1.22 | 3.92 | 5.66 |
| Heart | 37.28 | 1.23 | 0.77 | 0.64 | 4.29 | 1.74 | 0.23 | 13,307.94 |
| Kidney | 1.05 | 2.49 | 1.37 | 2.42 | 3.25 | 337.32 | 96.60 | 1,176.27 |
| Liver | 74.64 | 1.63 | 22.75 | 260.00 | 90.51 | 24.38 | 3.37 | 9.19 |
| Lymph node—bronchial | 1.70 | 1.45 | 0.37 | 309.91 | 778.57 | 338.32 | 173.02 | 512.00 |
| Lymph node—retropharyngeal | 1,433.92 | 2.99 | 115.86 | 1,466.07 | 1,063.96 | 1,499.34 | 475.70 | 2,352.53 |
| Lung—apical lobe | 1,170.29 | 1.60 | 109.93 | 744.18 | 1,480.49 | 1,241.45 | 310.52 | 1,552.09 |
| Lung—diaphragmatic lobe | 75.51 | 2.15 | 17.70 | 331.56 | 1,057.63 | 512.68 | 928.25 | 1,260.69 |
| Ovary | 90.09 | 2.36 | N/A | N/A | 57.79 | 38.00 | N/A | N/A |
| Spleen | 80.46 | 3.03 | 0.12 | 380.03 | 242.61 | 1,511.86 | 598.24 | 4,705.07 |
| Uterine horn | 136.10 | 5.97 | N/A | N/A | 0.87 | 21.07 | N/A | N/A |
| Testis | N/A | N/A | 60.24 | 821.61 | N/A | N/A | 891.44 | 548.75 |
The values obtained for the tissue from the occipital lobe of the brain are used as the calibrator, and quantification is relative to this tissue type in each animal.
Tissues that were also positive by virus isolation are indicated in boldface. N/A, results obtained for sex organs for half of the animals only. The gender of each animal is indicated by the single letter in parentheses after the animal number (F, female; M, male).
Swabs were collected into 1 ml of phosphate-buffered saline. Tissue samples were either collected in 1 ml of phosphate-buffered saline for virus isolation, submerged in 800 μl MagMAX buffer (Ambion, Victoria, Australia) for genome extraction, or fixed in 10% neutral buffered formalin for 48 h prior to routine processing for histology. RNA was extracted using the MagMAX-96 viral RNA isolation kit (Ambion). TaqMan real-time PCR was carried out using the AgPath-ID one-step reverse transcription-PCR kit (Applied Biosystems, Victoria, Australia), targeting the N gene of NiV using primers FOR (5′-TCAGCAGGAAGGCAAGAGAGTAA-3′), REV (5′-CCCCTTCATCGATATCTTGATCA-3′), and probe (5′-6-carboxyfluorescein-CCTCCAATGAGCACACCTCCTGCAG-6- carboxytetramethylrhodamine-3′). Positive results were defined by a cycle threshold (CT) value of ≤40. For virus isolation, supernatants from homogenized tissues positive for the NiV genome were incubated on Vero cell monolayers and scored positive if syncytia were present after 6 days.
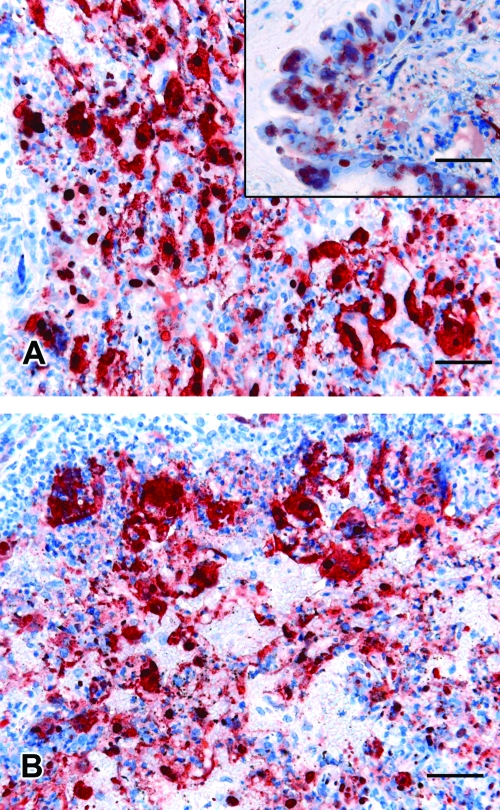
Ferrets were euthanized if they reached a predetermined humane end point based on experience with NiV infection in cats (14, 15). End points were a surrogate for lethality and included fever plus signs of rapidly progressing clinical illness. All ferrets were febrile (>40°C) by day 6 postinfection and deteriorated rapidly thereafter. Euthanasia was carried out on day 7 or 8 postinfection with ferrets that were severely depressed or obtunded and showing various combinations of hind limb paresis, tremor, myoclonus, urinary incontinence, subcutaneous edema of the neck and throat, petechial hemorrhages in the skin, and blood in oral secretions. There were no clinical differences noted between treatment and control animals. Similarly, at postmortem examination, abnormalities detected included pinpoint hemorrhagic nodules throughout the lung, enlarged hemorrhagic, edematous submandibular, and retropharyngeal lymph nodes, and petechial hemorrhages of viscera. Histological examination confirmed systemic vasculitis in each ferret, with prominent endothelial syncytia in the spleen, kidney, lung, lymph node, and meninges. Severe bronchointerstitial pneumonia and glomerulonephritis with syncytial formation were also identified. No differences in lesion severity or viral antigen load (assessed using a rabbit polyclonal antiserum against recombinant NiV nucleoprotein) between treatment and control animals were observed (Fig. 1).
FIG. 1.
Immunohistochemical detection of NiV antigen in lung tissue from a ferret treated with chloroquine after NiV challenge (A) or before NiV challenge (A, inset) compared to that of a control ferret (B). Bar = 50 μm.
Viral RNA was detected in all fluids and tissues from each ferret. Relative quantification of viral RNA levels in the tissues of each animal relative to the occipital lobe of the brain was performed using the comparative CT or ΔΔCT method (12). Viral RNA was detected most abundantly in the retropharyngeal lymph node, apical node of the lung, spleen, reproductive organs, and adrenal gland (Table 1). These tissues were also among those from which virus was most consistently isolated (Table 1). Furthermore, virus distribution in vivo did not vary between treatment and control animals apart from the additional abundant virus detection and isolation from the heart of one control ferret (ferret 8).
Quantification of viral RNA levels in selected tissues among ferrets was carried out relative to those of a control ferret (ferret 7). NiV RNA distribution was random, with treated ferrets sometimes showing higher relative RNA levels than those of control animals and vice versa (Fig. 2). Nevertheless, the failure to show consistently higher levels of viral RNA in control animals than those in treated animals indicated that virus replication had not been reduced in animals receiving chloroquine.
FIG. 2.
Relative quantification of NiV RNA in selected tissues among different ferrets. Quantification is relative to control ferret 7 (labeled). For each type of tissue, the first six bars (left to right) represent relative NiV RNA levels in ferrets 1 to 3 (treated with chloroquine 24 h prechallenge) and ferrets 4 to 6 (treated with chloroquine 10 h postchallenge). The last two bars represent control ferrets 7 and 8 (no chloroquine).
As the pharmacokinetics of chloroquine in ferrets are unknown, we used mass spectrometry to estimate chloroquine concentrations in serum samples. Based on a published method (21), aliquots of sera (20 μl) were precipitated with methanol (180 μl), vortexed for 20 s, and centrifuged at 12,000 × g for 10 min. A 100-μl aliquot of supernatant was diluted 1:1 with 0.4% (vol/vol) formic acid to give a final solvent composition of 50% methanol-0.2% formic acid. Diluted samples were directly infused into an LCQ ion trap mass spectrometer (Thermo, San Jose, CA). Data collected from selected reaction monitoring using the precursor-to-product ion transition of m/z 320 to 247 was used to estimate chloroquine concentrations in serum samples. Serum samples were taken from all eight ferrets prior to any treatment, pooled, spiked with chloroquine, and then used to generate a standard curve from which chloroquine concentrations in the sera of experimental animals at euthanasia were estimated. While no chloroquine was detected in the serum samples of the two control ferrets, the six ferrets that received the drug had estimated serum concentrations ranging from 1.6 to 16.8 μM, at least equivalent to the reported effective in vitro dose of 1 μM (20) and also within a plasma concentration range similar to that (1.6 to 12.5 μM) detected in humans receiving chloroquine treatment (7).
To confirm that chloroquine was active against NiV infection in our study, we carried out an in vitro assay based on that of Porotto et al. (20). Chloroquine at 0.4, 0.2, and 0.1 μM prevented the spread of NiV infection beyond single cells, with no syncytial formation observed (data not shown). This is consistent with observations made using pseudotyped vesicular stomatitis virus expressing HeV fusion (F) and attachment (G) glycoproteins (20).
Viral spread via F-mediated cell-cell fusion requires that the henipavirus F protein is cleaved to an active form by cathepsin L in the endosomes, and this cleavage is pH dependent (6, 17, 18, 19). Reduced henipavirus spread observed in the presence of chloroquine led to speculation that chloroquine prevents proteolytic cleavage of henipavirus F, either by directly inhibiting cathepsin L or by having an effect on endosomal pH, and without this step, virions are not infectious (20). Obviously, there is a difference between the in vivo and the in vitro results achieved with chloroquine treatment of NiV infection, but the reason for the discrepancy is unknown. However, a similar outcome has been reported for influenza treatment with chloroquine (22).
Although chloroquine was effective in preventing the spread of NiV infection in vitro, it did not prevent the spread of NiV infection in vivo when used as either a prophylactic or a postexposure therapeutic. By a range of measures employed in the present study, NiV replication and pathology were unaltered between the treatment and control groups of animals. We confirmed that chloroquine was active against NiV in vitro and established that all ferrets at the time of euthanasia had estimated serum concentrations of chloroquine greater than that required for anti NiV activity in vitro. From these observations, we conclude that chloroquine is not likely to be a useful drug in the treatment of henipavirus infections.
Acknowledgments
This work was supported by NIH grant 1 U01 AI077995-01.
Footnotes
Published ahead of print on 16 September 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Blum, L. S., R. Khan, N. Nahar, and R. F. Breiman. 2009. In-depth assessment of an outbreak of Nipah encephalitis with person-to-person transmission in Bangladesh: implications for prevention and control strategies. Am. J. Trop. Med. Hyg. 80:96-102. [PubMed] [Google Scholar]
- 2.Bossart, K. N., J. Bingham, and D. Middleton. 2007. Targeted strategies for henipavirus therapeutics. Open Virol. J. 1:14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bossart, K. N., Z. Zhu, D. Middleton, J. Klippel, G. Crameri, J. Bingham, J. A. McEachern, D. Green, T. J. Hancock, Y.-P. Chan, A. C. Hickey, D. S. Dimitrov, L.-F. Wang, and C. C. Broder. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog., in press. [DOI] [PMC free article] [PubMed]
- 3.Charmot, G., and J. P. Coulaud. 1990. Treatment of Plasmodium falciparum malaria in Africa (except cerebral malaria). Med. Trop. 50:103-108. [PubMed] [Google Scholar]
- 4.Chong, H.-T., A. Kamarulzaman, C.-T. Tan, K.-J. Goh, T. Thayaparan, S. R. Kunjapan, N.-K. Chew, K.-B. Chua, and S.-K. Lam. 2001. Treatment of acute Nipah encephalitis with ribavirin. Ann. Neurol. 49:810-813. [DOI] [PubMed] [Google Scholar]
- 5.Chua, K. B., W. J. Bellini, P. A. Rota, B. H. Harcourt, A. Tamin, S. K. Lam, T. G. Ksiazek, P. E. Rollin, S. R. Zaki, W.-J. Shieh, C. S. Goldsmith, D. J. Gubler, J. T. Roehrig, B. Eaton, A. R. Gould, J. Olson, H. Field, P. Daniels, A. E. Ling, C. J. Peters, L. J. Anderson, and B. W. J. Mahy. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288:1432-1435. [DOI] [PubMed] [Google Scholar]
- 6.Diederich, S., M. Moll, H-D. Klenk, and A. Maisner. 2005. The Nipah virus fusion protein is cleaved within the endosomal compartment. J. Biol. Chem. 280:29899-29903. [DOI] [PubMed] [Google Scholar]
- 7.Ducharme, J., and R. Farinotti. 1996. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin. Pharmacokinet. 31:257-274. [DOI] [PubMed] [Google Scholar]
- 8.Eaton, B. T., J. S. Mackenzie, and L.-F. Wang. 2007. Henipaviruses, p. 1587-1600. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA.
- 9.Field, H. 2009. Hendra virus re-visited. Virol. Sin. 24:105-109. [Google Scholar]
- 10.Field, H., P. Young, J. M. Yob, J. Mills, L. Hall, and J. Mackenzie. 2001. The natural history of Hendra and Nipah viruses. Microbes Infect. 3:307-314. [DOI] [PubMed] [Google Scholar]
- 11.Gurley, E. S., J. M. Montgomery, M. J. Hossain, M. Bell, A. K. Azad, M. R. Islam, M. A. R. Molla, D. S. Carroll, T. G. Ksiazek, P. A. Rota, L. Lowe, J. A. Comer, P. Rollin, M. Czub, A. Grolla, H. Feldmann, S. P. Luby, J. L. Woodward, and R. F. Breiman. 2007. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg. Infect. Dis. 13:1031-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 13.Middleton, D. J., C. J. Morrissy, B. M. van der Heide, G. M. Russell, M. A. Braun, H. A. Westbury, K. Halpin, and P. W. Daniels. 2007. Experimental Nipah virus infection in Pteropid bats (Pteropus poliocephalus). J. Comp. Pathol. 136:266-272. [DOI] [PubMed] [Google Scholar]
- 14.Middleton, D. J., H. A. Westbury, C. J. Morrissy, B. M. van der Heide, G. M. Russell, M. A. Braun, and A. D. Hyatt. 2002. Experimental Nipah virus infection in pigs and cats. J. Comp. Pathol. 126:124-136. [DOI] [PubMed] [Google Scholar]
- 15.Mungall, B. A., D. Middleton, G. Crameri, J. Bingham, K. Halpin, G. Russell, D. Green, J. McEachern, L. I. Pritchard, B. T. Eaton, L. F. Wang, K. N. Bossart, and C. C. Broder. 2006. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based vaccine. J. Virol. 80:12293-12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray, K., P. Selleck, P. Hooper, A. Hyatt, A. Gould, L. Gleeson, H. Westbury, L. Hiley, L. Selvey, B. Rodwell, and P. Ketterer. 1995. A morbillivirus that caused fatal disease in horses and humans. Science 268:94-97. [DOI] [PubMed] [Google Scholar]
- 17.Pager, C. T., W. W. Craft, Jr., J. Patch, and R. E. Dutch. 2006. A mature and fusogenic form of the Nipah virus fusion protein requires proteolytic processing by cathepsin L. Virology 346:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pager, C. T., and R. E. Dutch. 2005. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 79:12714-12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pager, C. T., M. A. Wurth, and R. E. Dutch. 2004. Subcellular localization and calcium and pH requirements for proteolytic processing of the Hendra virus fusion protein. J. Virol. 78:9154-9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porotto, M., G. Orefice, C. C. Yokoyama, B. A. Mungall, R. Realubit, M. L. Sganga, M. Aljofan, M. Whitt, F. Glickman, and A. Moscona. 2009. Simulating henipavirus multicycle replication in a screening assay leads to identification of a promising candidate for therapy. J. Virol. 83:5148-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singhal, P., A. Gaur, V. Behl, A. Gautam, B. Varshney, J. Paliwal, and V. Batra. 2007. Sensitive and rapid liquid chromatography/tandem mass spectrometric assay for the quantification of chloroquine in dog plasma. J. Chromatogr. B 852:293-299. [DOI] [PubMed] [Google Scholar]
- 22.Vigerust, D. J., and J. A. McCullers. 2008. Chloroquine is effective against influenza A virus in vitro but not in vivo. Influenza Other Respir. Viruses 1:189-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, L.-F., M. Yu, E. Hansson, I. Pritchard, B. Shiell, W. P. Michalski, and B. T. Eaton. 2000. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J. Virol. 74:9972-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young, P. L., K. Halpin, P. W. Selleck, H. Field, J. L. Gravel, M. A. Kelly, and J. S. Mackenzie. 1996. Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg. Infect. Dis. 2:239-240. [DOI] [PMC free article] [PubMed] [Google Scholar]