Abstract
Identifying the functions of human immunodeficiency virus (HIV)-specific CD8+ T cells that are not merely modulated by the level of virus but clearly distinguish patients with immune control from those without such control is of paramount importance. Features of the HIV-specific CD8+ T-cell response in antiretroviral-treated patients (designated Rx <50) and untreated patients (long-term nonprogressors [LTNP]) matched for very low HIV RNA levels were comprehensively examined. The proliferative capacity of HIV-specific CD8+ T cells was not restored in Rx <50 to the level observed in LTNP, even though HIV-specific CD4+ T-cell proliferation in the two patient groups was comparable. This diminished HIV-specific CD8+ T-cell proliferation in Rx <50 was primarily due to a smaller fraction of antigen-specific cells recruited to divide and not to the numbers of divisions that proliferating cells had undergone. Exogenous interleukin-2 (IL-2) induced proliferating cells to divide further but did not rescue the majority of antigen-specific cells with defective proliferation. In addition, differences in HIV-specific CD8+ T-cell proliferation could not be attributed to differences in cellular subsets bearing a memory phenotype, IL-2 production, or PD-1 expression. Although polyfunctionality of HIV-specific CD8+ T cells in Rx <50 was not restored to the levels observed in LTNP despite prolonged suppression of HIV RNA levels, per-cell cytotoxic capacity was the functional feature that most clearly distinguished the cells of LTNP from those of Rx <50. Taken together, these data suggest that there are selective qualitative abnormalities within the HIV-specific CD8+ T-cell compartment that persist under conditions of low levels of antigen.
Understanding the features of an effective immune response to human immunodeficiency virus (HIV) is among the most important goals for the design of HIV vaccines and immunotherapies. Most HIV-infected patients develop persistent viremia and CD4+ T-cell decline in the absence of antiviral therapy. However, evidence that immunologic control of HIV is possible can be drawn from a small group of rare patients who maintain normal CD4+ T-cell counts and restrict HIV replication to below 50 copies/ml plasma for up to 25 years without antiretroviral therapy (ART) (4, 22, 31, 40). Historically, these unique individuals were included within heterogeneous cohorts referred to as long-term survivors or long-term nonprogressors (LTNP), categorized solely based on their disease-free survival exceeding 7 to 10 years and their stable CD4+ T-cell counts (21). Over time, it became apparent that only a small subset of individuals within these cohorts had truly nonprogressive infection, maintaining good health with nondeclining CD4+ T-cell counts, and these true nonprogressors tended to have HIV type 1 (HIV-1) RNA levels below the lower detection limits of the newly available assays (23, 31). Some investigators have adopted other designations more recently, including elite controllers, elite suppressors, or HIV controllers. These designations vary by institution and, in some cases, rely only upon viral load measurements without a requirement for stable CD4+ T-cell counts (4, 22, 40). However, for our designation of true LTNP, we employ the inclusion criteria of stable health, nondeclining CD4+ T-cell counts, and maintenance of plasma viral RNA levels below 50 copies/ml without ART (29-31).
Several lines of evidence strongly suggest that CD8+ T cells mediate this control of HIV in LTNP. HLA B*5701 is highly overrepresented in these patients, and in B*5701+ patients, the HIV-specific CD8+ T-cell response is largely focused on peptides restricted by the B57 protein (15, 31). In addition, similar control of simian immunodeficiency virus replication has been described in rhesus macaques carrying the Mamu B*08 or B*17 allele (25, 49). In these macaques, CD8+ T-cell depletion studies have strongly suggested that control of viral replication is mediated by CD8+ T cells (14). Although these results support the idea that CD8+ T cells are responsible for immunologic control, the mechanism remains incompletely understood.
Several lines of evidence suggest that immunologic control in LTNP is not simply due to differences in autologous virus recognition by CD8+ T cells. The frequencies of CD8+ T cells specific for HIV or individual HIV-encoded gene products in the peripheral blood are not different in LTNP and untreated progressors (reviewed in reference 32). Putative “escape” mutations are found in viruses of both HLAB*57+ LTNP and HLA-matched progressors (4, 6, 28, 33, 34). In addition, comparable frequencies of CD8+ T cells of LTNP and progressors recognize autologous CD4+ T cells infected with the autologous virus (12, 28). Similar observations have recently been made in the rhesus macaque model (26). Collectively, these observations strongly suggest that features of the CD8+ T-cell response associated with immunologic control are not due to quantitative differences in the numbers of HIV-specific cells or to differential abilities of the autologous virus gene products to be recognized between patient groups.
Several qualitative features in the HIV-specific CD8+ T-cell response have been associated with immunologic control in LTNP. LTNP have been found to have higher frequencies of “polyfunctional” CD8+ T cells, named for their ability to degranulate and produce multiple cytokines, including interleukin-2 (IL-2) (2, 5, 51). However, these cells comprise an extremely small proportion of the HIV-specific CD8+ T-cell response. In addition, there is considerable overlap between patient groups, and many LTNP have few or no such cells. Compared to those of progressors, HIV-specific CD8+ T cells of LTNP have a dramatically higher proliferative capacity, a greater ability to upregulate granzyme B (GrB) and perforin production, and a greater cytolytic capacity against autologous HIV-infected CD4+ T cells (3, 17, 24, 29, 30). Increased HIV-specific CD8+ T-cell proliferative capacity in LTNP compared to progressors has also been associated with lower PD-1 expression or IL-2 production by HIV-specific CD4+ or CD8+ T cells (11, 24, 48, 51).
Considerable controversy exists over the cause-and-effect relationships between these qualitative differences in the CD8+ T-cell response and HIV viremia between patient groups. High levels of antigen can have potent effects on diverse cell types in humans and in animal models. For HIV, lowering the level of viremia through ART has been observed to increase the function of CD4+ and CD8+ T cells, NK cells, monocytes, and plasmacytoid dendritic cells (16, 18, 20, 37, 41, 45-47, 50). However, the vast majority of treated progressors will not control HIV replication when ART is interrupted (7, 9, 35), suggesting that many of the qualitative differences in the CD4+ or CD8+ T-cell response between LTNP and untreated progressors are not the cause of control over HIV but rather are likely an effect of viremia. In some but not all studies, ART was sufficient to restore the proliferative capacity, phenotype, and cytokine production by CD4+ T cells to levels similar to responses to other viruses or to the HIV-specific response of LTNP (13, 16, 18, 20, 37, 46, 50). Because better IL-2 production or function of HIV-specific CD4+ T cells has been associated with increased CD8+ T-cell proliferative capacity (24), it has also been suggested that diminished proliferative capacity of progressor CD8+ T cells may be an effect of viremia during the chronic phase of infection. In some studies, ART is sufficient to increase the frequency of polyfunctional HIV-specific CD8+ T cells or to decrease PD-1 expression (30, 41). However, the interpretations of the observations within these studies have relied on extrapolations between studies based upon cohorts with differing levels and durations of viral suppression or on examination of a limited number of functions or subsets in either CD4+ or CD8+ T cells.
In the present study, we extended our earlier work and comprehensively examined a broad array of functions of HIV-specific T cells derived from two large patient groups, LTNP and progressors on ART, who possess comparable levels of HIV viremia as determined by a sensitive single-copy assay. In response to autologous HIV-infected CD4+ T cells, HIV-specific CD8+ T-cell proliferative capacity, IL-2 responsiveness, surface phenotype, PD-1 expression, polyfunctionality, and cytotoxic capacity were measured in considerable detail. We observe that although ART results in restoration of many of these functions, HIV-specific CD8+ T-cell polyfunctionality and proliferative and killing capacities are not restored to levels observed in LTNP.
MATERIALS AND METHODS
Study subjects.
Subjects were recruited from the Clinical Research Center, National Institutes of Health (Bethesda, MD), and signed National Institute of Allergy and Infectious Diseases Investigational Review Board-approved protocol informed-consent documents. HIV infection was documented by HIV-1/2 immunoassay. LTNP criteria included the following: clinically healthy, negative history for opportunistic diseases, stable T-cell counts, set point HIV-1 RNA levels of <50 copies/ml (bDNA-based Versant HIV-1 RNA assay version 3.0; Bayer Diagnostics, Tarrytown, NY), and no ongoing ART (Table 1). Treated progressors received continuous ART and had HIV RNA levels suppressed to <50 copies/ml for a median of 5 years (range, 0.75 to 11 years) (members of this group are refered to as Rx <50). Median durations of HIV infection for LTNP and Rx <50 were 18 (range, 3 to 25) and 14 (range, 3 to 24) years, respectively, and were not significantly different (P = 0.17). Median CD4+ T-cell counts, which were 992 (range, 277 to 1,746) and 587 (range, 204 to 1,409) cells/ml, respectively, differed significantly between these two groups (P < 0.001). The patients in our study were infected in areas where clade B is the predominant strain. Although sequence data are not available for all patients, autologous viruses sequenced from a subset of individuals have been consistent with clade B infection (28). Peripheral blood mononuclear cells (PBMC) were obtained as described previously (29). HLA class I/II typing was performed by sequence-specific hybridization as described previously (31).
TABLE 1.
Characteristics of patients
| Group and patient no. | Diagnosis yr | T-cell count (cells/ml) |
HIV-1 RNA (copies/ml) | HLA Class I |
HIV-1 RNA (copies/ml) determined by single-copy assay | Time (yr) with viral load of <50 copies/ml | |||
|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | A | B | C | |||||
| LTNP | |||||||||
| 4 | 1985 | 1,063 | 1,088 | <50 | 1, 31 | 8, 57 | 6, 7 | 8 | |
| 7 | 1985 | 277 | 385 | <50 | 1, 2 | 57 | 6 | 4 | |
| 8 | 1985 | 664 | 1,120 | <50-930 | 11, 23 | 44, 57 | 4, 6 | ||
| 33 | 1995 | 955 | 881 | <50 | 2, 30 | 13, 57 | 6 | <1 | |
| 34 | 1989 | 1,746 | 1,164 | <50 | 1, 2 | 8, 57 | 6, 7 | 48 | |
| 37 | 1998 | 1,616 | 707 | <50 | 30 | 42, 57 | 17, 18 | 16.3 | |
| 38 | 1990 | 1,329 | 1,243 | <50 | 2, 24 | 44, 57 | 5, 6 | 1 | |
| 47 | 1994 | 500 | 1,284 | <50 | 1, 74 | 57, 81 | 7, 18 | 4 | |
| 58 | 1989 | 485 | 277 | <50 | 30, 74 | 15, 57 | 3, 8 | <1 | |
| 59 | 1986 | 833 | 549 | <50 | 23, 30 | 7, 57 | 7, 15 | ||
| 65 | 1993 | 865 | 388 | <50 | 30, 74 | 14, 57 | 2, 8 | 4 | |
| 66 | 1992 | 1,488 | 713 | <50 | 2, 30 | 13, 57 | 6 | <1 | |
| 68 | 1986 | 1,362 | 1,055 | <50 | 3, 29 | 57, 81 | 18 | 5 | |
| 71 | 1996 | 1,300 | 816 | <50 | 1, 24 | 38, 57 | 6, 12 | <1 | |
| 79 | 1998 | 520 | 780 | <50 | 1, 30 | 42, 57 | 7, 17 | <1 | |
| 17 | 1985 | 1,073 | 1,051 | <50 | 2, 26 | 27, 38 | 1, 12 | 1.5 | |
| 32 | 1994 | 785 | 328 | <50 | 1, 32 | 8, 27 | 1, 7 | 8.3 | |
| 48 | 1989 | 834 | 417 | <50 | 1, 33 | 8, 53 | 1, 4 | 19.3 | |
| 49 | 1992 | 1,084 | 784 | <50 | 3, 68 | 40, 53 | 2, 4 | 23 | |
| 60 | 1985 | 992 | 1,110 | <50 | 3, 11 | 35, 51 | 4, 15 | 9.6 | |
| 61 | 2004 | 751 | 594 | <50 | 2, 29 | 44, 49 | 7, 16 | 1.7 | |
| 62 | 1985 | 1,452 | 807 | <50 | 1, 32 | 35, 73 | 4, 15 | <1 | |
| 67 | 1982 | 1,308 | 880 | <50 | 32 | 27, 44 | 1, 5 | 43.4 | |
| Rx <50a | |||||||||
| 27 | 1993 | 466 | 519 | <50 | 2, 36 | 15, 42 | 3, 17 | <1 | 8 |
| 101 | 1986 | 632 | 902 | <50 | 1, 31 | 51, 57 | 6, 15 | 2.3 | 3 |
| 113 | 1991 | 510 | 1,021 | <50 | 1, 2 | 45, 57 | 7, 16 | 1.4 | 2 |
| 126 | 1991 | 829 | 1,525 | <50 | 1, 68 | 8, 57 | 7, 18 | <1 | 4 |
| 129 | 1988 | 445 | 783 | <50 | 2, 32 | 15, 27 | 1, 2 | 3 | 9 |
| 132 | 1995 | 1,409 | 479 | <50 | 30, 66 | 40, 57 | 3, 7 | <1 | 10 |
| 134 | 2003 | 737 | 590 | <50 | 68 | 15, 27 | 3 | <1 | 1.5 |
| 141 | 2001 | 408 | 553 | <50 | 2, 24 | 35, 49 | 3, 7 | 13.8 | 5 |
| 142 | 1994 | 759 | 821 | <50 | 2, 68 | 27, 40 | 1, 3 | 0.75 | |
| 143 | 1985 | 498 | 581 | <50 | 2, 11 | 15, 27 | 1, 4 | 3 | |
| 144 | 1986 | 741 | 412 | <50 | 3, 26 | 7, 57 | 6, 7 | 10 | |
| 151 | 1994 | 515 | 1,245 | <50 | 2, 23 | 45, 57 | 16, 18 | <1 | 6 |
| 152 | 1988 | 476 | 1,664 | <50 | 2 | 44, 57 | 5, 6 | 6 | 6 |
| 153 | 1993 | 968 | 1,338 | <50 | 2 | 15, 57 | 3, 6 | 1 | 5 |
| 156 | 1987 | 204 | 1,151 | <50 | 1, 24 | 35, 57 | 6, 12 | <1 | 3 |
| 157 | 1987 | 626 | 1,065 | <50 | 2, 24 | 52, 57 | 6, 12 | <1 | 8 |
| 158 | 2001 | 959 | 750 | <50 | 1, 32 | 8, 35 | 4, 7 | 13 | 5 |
| 159 | 1997 | 781 | 521 | <50 | 11 | 15 | 8 | <1 | 9 |
| 160 | 1996 | 922 | 820 | <50 | 26, 68 | 15, 18 | 4, 5 | <1 | 4 |
| 161 | 2001 | 521 | 695 | <50 | 11, 68 | 27 | 2, 7 | 1 | 6 |
| 162 | 1997 | 659 | 678 | <50 | 3 | 14, 35 | 4, 8 | <1 | 5 |
| 163 | 1987 | 668 | 789 | <50 | 24, 30 | 18, 49 | 7, 12 | 14 | 6 |
| 164 | 1987 | 1,004 | 938 | <50 | 24, 31 | 7, 27 | 2, 7 | 5 | 3 |
| 165 | 2001 | 604 | 935 | <50 | 11, 24 | 35 | 4 | 2 | 3 |
| 166 | 1994 | 247 | 447 | <50 | 2, 11 | 8, 15 | 3, 8 | 4 | 2 |
| 167 | 1986 | 649 | 508 | <50 | 2, 3 | 14, 15 | 3, 8 | <1 | 5 |
| 168 | 1990 | 1,167 | 1,228 | <50 | 3, 32 | 14 | 8 | <1 | 4 |
| 169 | 1989 | 476 | 987 | <50 | 2, 3 | 7, 14 | 7, 8 | <1 | 6 |
| 170 | 1994 | 561 | 546 | <50 | 23, 68 | 7, 53 | 4, 7 | 29 | 5 |
| 171 | 1995 | 589 | 828 | <50 | 25, 26 | 8, 49 | 7 | 1 | 7 |
| 172 | 1988 | 729 | 1,200 | <50 | 2 | 13, 35 | 3 | <1 | 11 |
| 173 | 1988 | 599 | 889 | <50 | 2 | 15, 58 | 3 | <1 | 2 |
| 174 | 1992 | 675 | 459 | <50 | 33, 74 | 42, 53 | 4, 17 | <1 | 7 |
| 175 | 1994 | 532 | 627 | <50 | 11, 24 | 35, 40 | 3, 4 | <1 | 6 |
| 176 | 2000 | 382 | 665 | <50 | 2, 23 | 45, 58 | 6 | 1 | 2 |
| 177 | 1985 | 555 | 750 | <50 | 1, 2 | 7, 40 | 3, 7 | 4 | 4 |
| 178 | 2002 | 498 | 227 | <50 | 2, 30 | 45, 50 | 4, 16 | <1 | 5 |
| 179 | 1990 | 501 | 866 | <50 | 2, 25 | 7, 44 | 5, 7 | 10 | 9 |
| 185 | 1991 | 512 | 668 | <50 | 34, 36 | 7, 44 | 7 | 4 | 6 |
| 187 | 1998 | 307 | 247 | <50 | 2, 30 | 18, 58 | 5, 6 | <1 | 4 |
| 188 | 1990 | 642 | 708 | <50 | 1, 24 | 7, 8 | 7 | 2.6 | 8 |
| 189 | 1986 | 800 | 990 | <50 | 1 | 3 | |||
| 190 | 1995 | 729 | 496 | <50 | 2, 68 | 35, 44 | 4, 5 | <1 | 4 |
| 191 | 2004 | 562 | 1,002 | <50 | 29 | 13, 51 | 1, 6 | <1 | 2 |
| 192 | 1997 | 568 | 1,237 | <50 | 2, 74 | 7, 58 | 3, 7 | 53 | 4 |
| 199 | 2002 | 687 | 563 | <50 | 2, 24 | 14, 15 | 1, 8 | 1.2 | 2.5 |
| 250 | 1990 | 400 | 991 | <50 | 2, 32 | 13, 14 | 6, 8 | 12.2 | 10 |
| 251 | 1987 | 351 | 532 | <50 | 1 | 5.75 | |||
| 252 | 2002 | 434 | 337 | <50 | <1 | 3 | |||
| 253 | 2000 | 505 | 949 | <50 | 2, 68 | 40, 58 | 3 | <1 | 5 |
| 254 | 1984 | 481 | 1,213 | <50 | 3, 25 | 14, 18 | 8, 12 | <1 | 3 |
| 255 | 1998 | 507 | 583 | <50 | 2, 3 | 13, 35 | 4, 6 | <1 | 2 |
| 256 | 2003 | 587 | 871 | <50 | 2, 29 | 49 | 7 | <1 | 1.75 |
| 257 | 1991 | 915 | 1,548 | <50 | 2 | 37, 39 | 6, 7 | <1 | 10 |
| 258 | 1983 | 641 | 1,654 | <50 | 1, 30 | 15, 44 | 3, 4 | 4 | 7.25 |
Treated progressors with a viral load of <50 copies/ml.
HIV-1 RNA determination.
Single-copy assays were performed as described previously (38). The limit of quantification is a function of the amount of plasma used for the assay; for these experiments using stored plasma, a limit of one copy of HIV-1 RNA/ml of plasma was employed as described previously (30).
HIVSF162-infected autologous CD4+ T-cell targets.
CD4+ T cells were positively selected from cryopreserved PBMC by magnetic automated cell sorting (AutoMACS; Miltenyi Biotec, Germany) and polyclonally stimulated prior to infection as previously described (29). CD4+ lymphoblasts were infected over 24 to 36 h as recently described (30, 42). Briefly, concentrated HIVSF162 was bound to ViroMag beads (OZ Biosciences, Marseille, France). CD4+ lymphoblasts were resuspended in warmed medium containing IL-2 (Roche Diagnostics, Manheim, Germany) at 40 IU/ml and plated at 5 × 105 cells/50 μl/well in 96-well flat-bottom tissue culture plates. Bead-labeled virus or medium was added to “infected” or “uninfected” control wells, respectively. The plates were centrifuged at 1.600 rpm for 2 min prior to 15 min of incubation on a magnet (OZ Biosciences). The volume was raised to 210 μl with IL-2 medium, and the plates were incubated at 37°C for 24 h prior to use as targets in cytotoxicity assays or to stimulate PBMC. The percent infection of CD4+ T-cell targets was confirmed in all cases by flow cytometry and was not significantly different between LTNP and Rx <50 (medians of 51.9 and 54.2%, respectively; P > 0.5).
CFSE proliferation assays.
PBMC were labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) and incubated with medium containing anti-CD3 (Orthoclone OKT3, 1 μg/ml; Ortho Biotech, Bridgewater, NJ) and anti-CD28 (1 μg/ml) antibodies or uninfected or HIVSF162-infected autologous CD4+ T-cell targets in 96-well, deep-well culture plates (PGC Scientifics, Frederick, MD) at a density of 106 PBMC/well/ml for 6 days as previously described (29).
CD8+ T-cell stimulation assays for intracellular protein detection.
In experiments measuring polyfunctionality, PBMC were thawed and rested overnight in 10% human AB (HAB) (Gem Cell Gemini Bio-Products, Sacramento, CA) medium. On the following day, cells were counted, pelleted, resuspended in fresh 10% HAB medium containing monensin (Golgi Stop, 0.7 μg/ml; BD Biosciences) and anti-CD107a (Pacific Blue, BD PharMingen), and incubated with medium, phorbol myristate acetate (6.5 nM; Calbiochem, Darmstadt, Germany) plus ionomycin (0.2 μM; Sigma Aldrich, St. Louis, MO), uninfected or HIVSF162-infected autologous CD4+ T-cell targets, or pooled HIV-1 Gag peptides plus costimulatory antibodies (anti-CD28 and anti-CD49d, 1 μg/ml; BD Biosciences) at 37°C for 6 h as previously described (29). For experiments using Gag peptides, 123 15-mers overlapping by 10 amino acids were used at a 2-μg/ml final concentration of each peptide (NIH AIDS Research and Reference Reagent Program). In experiments using cell targets, autologous CD4+ T cells were labeled with a Live/Dead Fixable Aqua Dead Cell Stain kit (Invitrogen Molecular Probes, Eugene, OR) per the manufacturer's instructions prior to incubating with effector cells at an effector-to-target cell (E:T) ratio of 1:1. At 6 h, the cells were washed and stained with surface antibodies prior to fixation, permeabilization, and intracellular staining as described previously (29).
In experiments using CD4+ T-cell targets to measure the total frequency of virus-specific CD8+ T cells, negatively selected CD8+ T cells (rested overnight or incubated with HIVSF162-infected targets for 6 days) were coincubated with uninfected or HIVSF162-infected autologous CD4+ T-cell targets at an E:T ratio of 1:1 as described previously (29). (This E:T ratio differs from the 25:1 E:T ratio used in replicates to measure cytotoxicity because the goal in this case was to maximize the stimulation conditions to a point of saturation in order to accurately enumerate all of the HIV-specific effectors present. An E:T ratio of 1:1 was found to be optimal in preliminary experiments.) At 6 h, the cells were stained for surface markers prior to fixation, permeabilization, and intracellular gamma interferon (IFN-γ) staining as described previously (30, 31).
GrB cytotoxicity assay and infected CD4+ T-cell elimination (ICE).
Day 0 cells and day 6 cells (incubated with infected targets) were labeled with immunomagnetic beads (CD8+ T-cell isolation kit II; Miltenyi Biotec) prior to negative selection of CD8+ T cells by magnetic automated cell sorting as described previously (30). HIVSF162-infected and uninfected targets were washed with HAB medium and labeled with a Live/Dead Fixable Violet Stain kit (Invitrogen Molecular Probes) per the manufacturer's instructions. Target cells were washed and gently resuspended in 10% HAB medium, mixed with day 0 or day 6 enriched CD8+ T cells at an E:T ratio of 25:1, centrifuged, resuspended in 75 μl of GrB substrate (GranToxiLux; OncoImmunin, Inc., Gaithersburg, MD) (36) diluted fourfold, plated in 96-well round-bottom plates, and incubated at 37°C for 1 h in the dark. An E:T ratio of 25:1 and coincubation times of 1 to 6 h provided optimal signal-to-noise ratios (data not shown). After 1 h, cells were washed, gently resuspended in phosphate-buffered saline-1% bovine serum albumin, placed on ice, and analyzed immediately by flow cytometry.
Following analysis by flow cytometry, cells (including targets not incubated with effectors) were transferred to new V-bottom tubes, centrifuged, fixed, and permeabilized with Cytofix/Cytoperm (BD PharMingen, San Diego, CA) prior to staining with allophycocyanin (APC)-conjugated anti-CD4 (BD Biosciences, San Jose, CA) and anti-p24 (Kc57 RDI; Beckman Coulter, Inc., Fullerton, CA) antibodies to confirm infection and to measure elimination of p24-expressing cells. The E:T ratios were corrected as follows: the true effector numbers were adjusted based on the frequencies of IFN-γ-positive CD8+ T cells detected in parallel replicates after a 6-hour incubation (see above), and the true target numbers were corrected based on the total percentages of HIVSF162-infected (p24+) cells as determined by the sum of the percentages of the upper quadrants in plots containing only infected targets. ICE was calculated as follows: [(% p24 expression of infected targets − % p24 expression of infected targets mixed with day 0 or day 6 cells)/% p24 expression of infected targets] × 100. Regarding these ICE calculations, variation in replicate cultures, as expressed by the mean of the standard deviations for each patient in a subset of individuals, was determined in preliminary experiments to be 3.6%.
HLA class I tetramers.
Nineteen HLA class I tetramers conjugated to either phycoerythrin (PE) or APC (Beckman Coulter, Inc.) were used to label epitope-specific CD8+ T cells as previously described (19, 29).
Flow cytometry.
Multiparameter flow cytometry was performed according to standard protocols. Surface and/or intracellular staining was done using the following antibodies, which were from BD Biosciences unless otherwise noted: fluorescein isothiocyanate-conjugated anti-CD3, purified murine anti-human CCR7 primary antibody added at 37°C followed by Alexa 488-conjugated goat anti-mouse secondary antibody (Invitrogen Molecular Probes) added at 4°C, peridinine chlorophyll protein-conjugated anti-CD3 and anti-CD45RA, APC-conjugated anti-IFN-γ, PE-conjugated anti-CD8 and anti-IL-2, PE Cy7-conjugated anti-tumor necrosis factor alpha (TNF-α), PE-Texas Red-conjugated anti-CD4 (Caltag Laboratories, Inc., Burlingame, CA), PE-Alexa 700-conjugated anti-CD3 (Caltag Laboratories, Inc.), Alexa 700-conjugated anti-CD8 (Caltag Laboratories, Inc.), and APC-Cy7-conjugated anti-MIP-1β. PD-1 staining was performed using a biotinylated anti-human PD-1 affinity-purified goat immunoglobulin G primary stain (R&D Systems, Inc., Minneapolis, MN) and a Qdot 655 streptavidin conjugate as secondary stain (Invitrogen Molecular Probes). Unless otherwise specified, all staining was performed at 4°C for 30 min. In flow cytometry experiments for surface phenotype or polyfunctionality, 1 × 106 to 2 × 106 live lymphocyte events were collected. In cytotoxicity experiments, gates were drawn on labeled CD4+ T-cell targets and 5,000 to 8,000 events were collected. Samples were analyzed on a FACSAria multilaser cytometer (Becton-Dickinson) with FACSDiva software. Color compensations were performed using single-stained samples for each of the fluorochromes used. Data were analyzed using FlowJo software (TreeStar, San Carlos, CA). The FlowJo Proliferation Platform was used to obtain detailed information about the division characteristics of gated tetramer-positive CD8+ T cells as described previously (19). In polyfunctional analyses, the FlowJo Boolean Gate Platform was used to create 32 response patterns for the five tested functions. Data were plotted and further analyzed with SPICE software. Background values were subtracted from test values. CD8+ T-cell responses were considered positive if the frequencies of responding cells were ≥2 times the frequency measured against medium alone and if the values for a given function were ≥0.03% responding cells among CD8+ T cells.
Statistical analysis.
The Wilcoxon signed rank test was used to compare paired data. Independent groups were compared by the Wilcoxon two-sample test. Correlation was determined by the Spearman rank method. The Bonferroni method was used to adjust P values for multiple testing. Regression analysis and analysis of covariance were used to quantify the difference in GrB target cell activity and ICE in LTNP and Rx <50 over the range of logged E:T ratios and at the median log E:T ratio of the combined groups.
RESULTS
HIV-specific CD8+ T-cell proliferation is not restored in treated progressors despite restoration of CD4+ T-cell proliferation.
HIV-specific CD8+ T cells of LTNP maintain a greater proliferative capacity than those of progressors (3, 17, 24, 29). These differences in in vitro proliferation were recently shown to be associated with increases in cytotoxic granule content and granule exocytosis-mediated cytotoxicity of HIV-infected targets by LTNP-derived CD8+ T cells (29, 30). We also previously observed that the proliferative capacity and cytotoxic responses of HIV-specific CD8+ T cells in a subset of treated progressors with antiretroviral-induced suppression of HIV viremia (Rx <50) were significantly lower than those measured in LTNP (29, 30). However, it remained unclear whether increases in CD4+ T-cell proliferation or IL-2 production observed in treated patients in cross-sectional and longitudinal cohorts would translate into increased CD8+ T-cell proliferative or cytotoxic capacities (16, 18, 20, 24, 37, 46, 50). In the present study, we recruited a larger number of Rx <50, with HIV RNA levels suppressed to <50 copies/ml for a median of 5 years, to determine the impact of reduced antigen load and immune activation on HIV-specific CD4+ and CD8+ T-cell function.
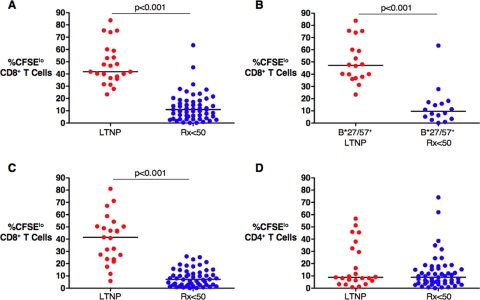
Using autologous HIVSF162-infected CD4+ T-cell targets derived from 23 LTNP and 55 Rx <50, HIV-specific CD8+ T-cell proliferation was significantly lower in the Rx <50 group than in LTNP (medians of 10.9% and 41.9% CFSElo CD8+ T cells, respectively; P < 0.001) (Fig. 1A), consistent with prior observations (29). Similar results were obtained when the analysis was limited to 34 B*27/57+ patients (medians of 9.6% and 47.3%, respectively; P < 0.001) (Fig. 1B). In the 74 patients in whom HIV RNA levels were quantitated using an ultrasensitive assay with a lower detection limit of 1 copy/ml plasma, HIV RNA levels were similar, but they tended to be higher in LTNP than in Rx <50 (4 versus 0.9 copies/ml, respectively; P = 0.05). However, even when we repeated these analyses with LTNP (n = 15) and Rx <50 (n = 25) with detectable viremia above 1 copy/ml (eight versus four copies/ml, respectively; P = 0.2), we found that the differences in HIV-specific CD8+ T-cell proliferation between the groups remained significant (medians of 41.9% and 10.9%, respectively; P < 0.001) (data not shown). In either case, HIV-specific CD8+ T-cell proliferation did not correlate with HIV RNA levels (P > 0.5 for all comparisons; data not shown). HIV-specific CD8+ T-cell proliferation also did not correlate with duration of viral load containment (estimated as the length of time from diagnosis for LTNP) or absolute CD4 counts within either the LTNP or Rx <50 group (P > 0.5 for all comparisons; data not shown).
FIG. 1.
Virus-specific CD8+ T-cell proliferation in response to HIVSF162-infected autologous CD4+ T-cell targets or Gag peptides. (A) Summary of the percentages of CFSElo CD8+ T cells following 6 days of stimulation with autologous CD4+ T-cell targets for 23 LTNP (red circles) and 55 antiretroviral recipients with HIV RNA levels suppressed below 50 copies/ml (Rx <50) (blue circles). (B) Summary of the percentages of CFSElo CD8+ T cells for HLA B*27/57+ patients, 18 LTNP and 16 Rx <50. (C and D) Summary of the percentages of CFSElo CD8+ (C) and CD4+ (D) T cells following 6 days of stimulation with Gag peptides for 23 LTNP and 53 Rx <50. Horizontal lines represent median values. Comparisons were made using the Wilcoxon two-sample test. Only significant P values are shown.
The relationship between HIV-specific CD4+ and CD8+ T-cell proliferation was then examined. Peptide pools spanning the Gag gene product were used to examine HIV Gag-specific T-cell responses for the same individuals in the same experimental tube. Consistent with prior results, Gag-specific CD8+ T-cell proliferation was significantly different between 23 LTNP and 53 Rx <50 (medians of 41.5% and 7.2% CFSElo CD8+ T cells, respectively; P < 0.001) (Fig. 1C). In contrast, Gag-specific CD4+ T-cell proliferation was equivalent between the patient groups (medians of 8.8% and 8.9%, respectively; P > 0.5) (Fig. 1D) as measured in the same culture. These results suggest that defective HIV-specific CD8+ T-cell proliferation in patients with progressive infection is not readily restored by ART-induced reductions in HIV RNA levels and immune activation, even in individuals with HIV-specific CD4+ T-cell proliferation comparable to that in LTNP.
Defective HIV-specific CD8+ T-cell proliferation in Rx <50 is a consequence of a smaller fraction of cells proceeding through the cell cycle than in LTNP.
It remained unclear whether the greater percentages of CFSElo CD8+ T cells observed in LTNP than in Rx <50 in response to HIV-infected CD4+ T cells were a consequence of a greater number of divisions, a greater numbers of cells entering the proliferating pool, or both. Increases in the number of divisions could potentially be secondary to autocrine or paracrine IL-2 production similar to that recently described (24, 51). To examine virus-specific CD8+ T-cell proliferation in greater detail, tetramer staining and proliferation analysis software were used to measure the proportion of antigen-specific cells entering the proliferating pool (percent divided) and changes in the number of divisions that cells had undergone (proliferation index). Autologous uninfected or HIVSF162-infected CD4+ T-cell targets with or without IL-2 were used to stimulate HIV-specific CD8+ T cells. A pool of 15-mer peptides spanning the pp65 gene product of cytomegalovirus (CMV) was also used for comparison. The baseline frequencies of CMV tetramer-positive CD8+ T cells were similar in Rx <50 and LTNP (medians of 0.94% and 1.03%, respectively; P > 0.5), whereas the frequencies of HIV tetramer-positive CD8+ T cells were significantly lower in the Rx <50 group than in LTNP prior to stimulation (medians of 0.45% and 1.05%, respectively; P = 0.01) (data not shown), as reported previously (19, 29, 39). Such differences should not influence the interpretation of our more detailed analysis, since the indices generated by the proliferation software are independent of the starting frequencies of virus-specific CD8+ T cells.
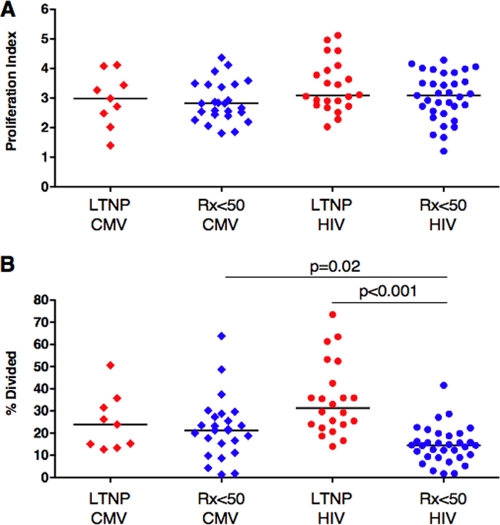
The numbers of divisions (proliferation index) that CMV or HIV tetramer-positive CD8+ T cells underwent in response to the CMV pp65 peptide pool or HIVSF162-infected CD4+ T-cell targets, respectively, did not differ for different virus specificities or patient groups (Fig. 2A). In addition, the proportion of CMV-specific CD8+ T cells induced to divide (percent divided) was similar in the two patient groups (medians of 21.2% and 23.9%, respectively; P > 0.5) (Fig. 2B). In contrast, the fraction of HIV-specific CD8+ T cells of Rx <50 that entered the proliferating pool was significantly less than that of LTNP (medians of 14.5% and 31.3%, respectively; P < 0.001). Within the Rx <50 group, the percentage of HIV-specific CD8+ T cells recruited to divide was significantly less than the percentage of CMV-specific CD8+ T cells (medians of 14.5% and 21.2%, respectively; P = 0.02) (Fig. 2B). These results, which are consistent with responses measured using CMV and HIV optimal epitopes (19), suggest that the defective CD8+ T-cell proliferation of progressors is specific to HIV, relates not to fewer divisions of proliferating cells but rather to a smaller fraction of cells entering and proceeding through cell cycle, and is not corrected by ART.
FIG. 2.
Proliferation analysis of virus-specific CD8+ T cells. (A and B) Summary of the proliferation index (A) and percent divided (B) for CMV (diamonds, n = 34 stainings) and HIV (circles, n = 55 stainings) tetramer-positive CD8+ T cells of LTNP (red symbols) and Rx <50 (blue symbols). Following stimulation for 6 days with either CMV pp65 peptides or HIV-infected autologous CD4+ T-cell targets, CD8+ T cells were stained with CMV or HIV tetramers, respectively, and analyzed with proliferation software to generate measures of the numbers of divisions that dividing cells had undergone (proliferation index) and the percentage of the original population of cells that had divided (percent divided). Horizontal lines represent median values. Comparisons were made using the Wilcoxon two-sample test. Only significant P values are shown.
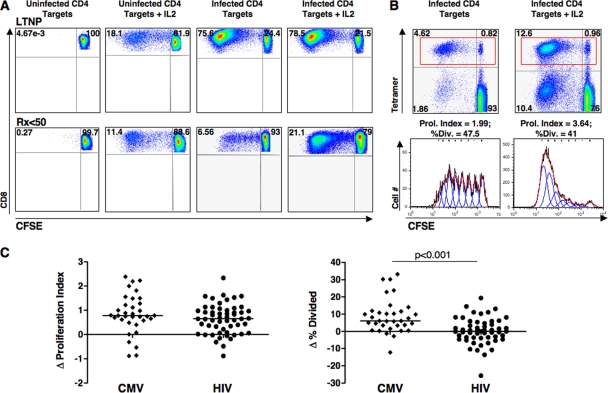
Exogenous IL-2 can induce higher frequencies of CFSElo CD8+ T cells but does not rescue nonproliferating HIV-specific CD8+ T cells.
It has been suggested that exogenous IL-2 or autocrine or paracrine IL-2-secreting CD4+ or CD8+ T cells can cause proliferation of HIV-specific CD8+ T cells of progressors (24, 51). IL-2 can produce increases in the fraction of CFSElo CD8+ T cells when added to cultures, as shown in Fig. 3A and reported previously (24, 51). However, it has remained unclear whether IL-2 causes increases in CFSElo HIV-specific cells by driving more cells to proceed through cell cycle and divide, causes an increase in the number of divisions of cells already in the proliferating pool, or both. To address these possibilities, proliferation analysis software was next used to examine the effects of IL-2 on virus-specific CD8+ T-cell proliferation in greater detail. Proliferation was examined in the presence or absence of exogenous IL-2 in response to autologous HIV-infected CD4+ T cells or in response to a CMVpp65 overlapping peptide pool. Because IL-2 can cause nonspecific proliferation even at low concentrations (Fig. 3A), HLA class I tetramers were used to analyze the percent divided cells and proliferation index (Fig. 3B). For the CMV tetramer-positive CD8+ T cells of 34 patients (LTNP and Rx <50), the average number of divisions that proliferating cells underwent was significantly higher when cells were stimulated with peptides and IL-2 than when they were stimulated with peptides alone (3.7 versus 2.8, respectively; P < 0.001). The percent divided was also increased by the addition of IL-2 compared to stimulation with peptides alone (28.3% versus 21.5%, respectively; P < 0.001) (data not shown). In contrast, the addition of IL-2 to the HIV-specific CD8+ T cells of 55 patients increased only the number of divisions of cells already in cycle (3.8 versus 3.1, respectively; P < 0.001) and had no effect on the percentage of cells recruited to divide (18.8% versus 18.8%, respectively; P > 0.5) (data not shown). The effect of IL-2 on virus-specific CD8+ T cells was similar within the individual LTNP and Rx <50 groups and when the analysis was limited to B*27/57+ patients (data not shown).
FIG. 3.
Effects of exogenous IL-2 on virus-specific CD8+ T-cell proliferation. (A) Flow plots gated on the CD8+ T cells of two representative patients, an LTNP (top row) and a Rx <50 patient (bottom row), following stimulation with uninfected or HIV-infected CD4+ T-cell targets with or without IL-2 for 6 days. IL-2 induced nonspecific increases in the percentages of CFSElo cells. Quadrant numbers indicate percentages of gated CD8+ T cells. (B) Gating on the HIV tetramer-positive CD8+ T cells (red rectangles) of the Rx <50 patient shown in panel A following 6-day stimulation with HIV-infected CD4+ T-cell targets alone (left column) or in combination with IL-2 (right column); the resulting CFSE histograms are fit with models separating the populations into individual generations (bottom row). Proliferation index, percent divided, delta proliferation index, and delta percent divided are defined in the text. In this example, exogenous IL-2 induced 1.6 more divisions of HIV-specific cells (3.64 versus 1.99) without increasing the numbers of cells recruited to divide (41% versus 47.5%). (C) The net effects of IL-2 on virus-specific CD8+ T-cell proliferation, assessed by the delta proliferation index (left) and delta percent divided (right), are compared between CMV (diamonds, n = 34 patients) and HIV (circles, n = 55) tetramer-positive CD8+ T cells. Horizontal lines represent median values. Comparisons were made using the Wilcoxon two-sample test. Only significant P values are shown.
The differential effects of IL-2 on CMV- and HIV-specific CD8+ T-cell proliferation were compared through analyses of the delta proliferation index (proliferation index with IL-2 minus proliferation index without IL-2) and the delta percent divided cells (percent divided with IL-2 minus percent divided without IL-2) (Fig. 3C). The delta proliferation index was not different for CMV- and HIV-specific CD8+ T cells (medians of 0.8 and 0.7, respectively; P = 0.33) (Fig. 3C, left panel). In contrast, the delta percent divided was significantly greater for CMV-specific CD8+ T cells than for HIV-specific CD8+ T cells (medians of 6.1% and 0.1%, respectively; P < 0.001) (Fig. 3C, right panel). In summary, unlike the situation with CMV-specific CD8+ T cells, the effects of IL-2 on HIV-specific CD8+ T-cell proliferation were limited to increasing the number of divisions of proliferating cells without significantly increasing the fraction of HIV-specific CD8+ T cells able to proceed through cell cycle. These results suggest that the pathogenesis of impaired HIV-specific CD8+ T-cell proliferation likely involves mechanisms not readily overcome by ART or IL-2.
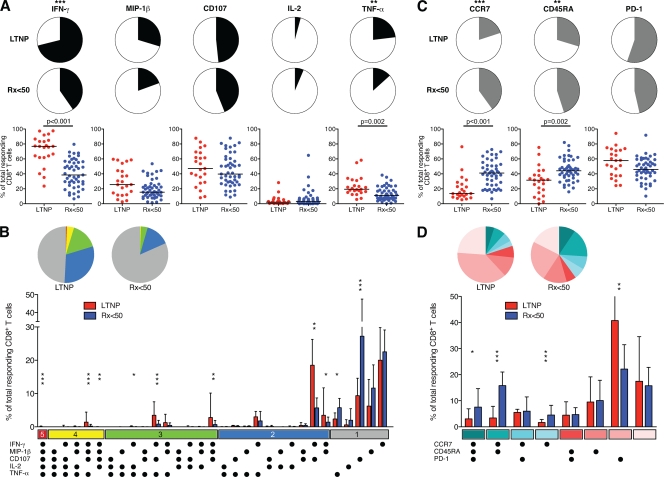
Polyfunctional HIV-specific CD8+ T cells in Rx <50 are not restored to the levels observed in LTNP, although there is considerable overlap.
Increased frequencies of HIV-specific CD8+ T cells of LTNP that are capable of degranulation and the secretion of several cytokines (IFN-γ, TNF-α, and IL-2) and chemokines (MIP-1β) have been reported in comparisons made with viremic progressors (2, 5). In these studies, “polyfunctional” cells comprised an extremely small fraction of the total HIV-specific response and were not detected in all LTNP. Differences in polyfunctionality have not been well characterized in large cohorts of LTNP and treated progressors matched for viral load. In addition, diminished proliferation of HIV-specific CD8+ T cells has been associated with surface marker phenotype or increased PD-1 expression (8, 11, 48). The effects of ART on these subsets have been studied in some recent work, with disparate results (30, 41, 43).
To examine whether differences in polyfunctionality are present in cohorts matched for viral load, we assessed the capacity of HIV-specific CD8+ T cells derived from 23 LTNP and 47 Rx <50 with equally low levels of HIV RNA to carry out multiple functions in response to autologous HIVSF162-infected CD4+ T-cell targets (Fig. 4A and B). Considering the contribution of each individual function to the total number of responding cells, a greater percentage of HIV-specific CD8+ T cells of LTNP made IFN-γ (medians of 76.8% and 38.5%, respectively; P < 0.001) and TNF-α (median 19.4% and 11%, respectively; P = 0.002) than did the responding cells of Rx <50 (Fig. 4A). No significant differences were observed in their ability to degranulate or secrete MIP-1β or IL-2 (Fig. 4A). An analysis of the ability of HIV-specific CD8+ T cells to execute multiple effector functions simultaneously revealed differences that achieved statistical significance in some of the individual functional subsets between LTNP and Rx <50; however, considerable overlap was observed between the patient groups (Fig. 4B, bar graph). The fraction of responding HIV-specific CD8+ T cells of LTNP exhibiting more than one function (2 to 5+) was significantly higher than those of Rx <50 (P < 0.001) (Fig. 4B, pie charts). Although our findings suggest that polyfunctionality in the HIV-specific CD8+ T cells of Rx <50 is not restored to the level observed in LTNP despite very prolonged ART-induced suppression of HIV RNA levels, these differences were not robust and do not clearly distinguish the HIV-specific CD8+ T cells of LTNP from those of Rx <50.
FIG. 4.
Polyfunctionality and phenotype of HIV-specific CD8+ T cells following stimulation with HIV-infected CD4+ T-cell targets for 6 h. (A) Summary pie charts show median percentages of total responding CD8+ T cells that express IFN-γ, MIP-1β, CD107, IL-2, or TNF-α in LTNP (n = 23, first row) and Rx <50 (n = 47, second row). Scatter plots (third row) depict the percent expression of each function for each LTNP and Rx <50. Horizontal lines represent median values. Comparisons were made using the Wilcoxon two-sample test. Only significant P values are shown. (B) Summary pie charts (top row) show median percentages of responding CD8+ T cells grouped according to the number of simultaneous functions executed: five functions, red; four, yellow; three, green; two, blue; and one, black. Individual functional subsets are shown in the summary bar graph (bottom row) for LTNP (red) and Rx <50 (blue). Background responses to uninfected targets have been subtracted. A line extends from the median to the upper interquartile range. Asterisks indicate significant differences between response pairs: ***, P < 0.001; **, P < 0.01; and *, P < 0.05. (C) Summary pie charts (first and second rows) and scatter plots (third row) show the fraction of the total responding CD8+ T cells expressing CCR7, CD45RA, or PD-1 for the same patients as in panel A. (D) Summary pie charts and bar graph show median percentages of responding CD8+ T cells expressing various combinations of the three phenotypic markers. CCR7+ subsets are shown in shades of teal, and CCR7− subsets are shown in shades of pink.
We also examined the surface expression of CCR7, CD45RA, and PD-1 on responding HIV-specific CD8+ T cells (Fig. 4C and D). In Rx <50, the frequency of HIV-specific CD8+ T cells expressing CCR7 or CD45RA was significantly higher than that in LTNP (CCR7 medians of 41.1% in Rx <50 and 13.7% in LTNP [P < 0.001]; CD45RA medians of 44.2% in Rx <50 and 31.5% in LTNP [P = 0.002]), which is consistent with published data demonstrating a shift away from a CCR7+ CD45RA+ phenotype on the HIV-specific CD8+ T cells or CD4+ T cells of patients undergoing an interruption of ART (19, 46). PD-1 expression was similar between these patient groups (45.9% in Rx <50 and 57.8% in LTNP; P = 0.13) (Fig. 4C). The latter finding is consistent with previous observations of reduced PD-1 percent expression and mean fluorescence intensity on total and HIV tetramer-positive CD8+ T cells in the setting of suppressed HIV RNA levels (30, 41). Furthermore, PD-1 expression of unstimulated HIV tetramer-positive CD8+ T cells of 13 of these LTNP as reported previously was strikingly similar to its percent expression on responding CD8+ T cells following stimulation in the present study (medians of 48.1% and 51.8%, respectively; P > 0.5) (data not shown), suggesting that expression of this surface marker at least is not significantly altered by brief in vitro activation (30). Upon examination of combinations of the phenotypic markers, subsets of responding cells expressing CCR7 were present at significantly higher frequencies in Rx <50 than in LTNP (Fig. 4D), similar to the analysis of each individual marker (Fig. 4C). These results suggest that the HIV-specific CD8+ T cells of LTNP compared with those of Rx <50 do not exhibit a classic central memory or terminally differentiated effector phenotype even though they likely exert effective control over HIV, supporting the idea that functional capacity cannot be reliably assigned based upon surface phenotype.
The per-cell cytotoxic capacities of HIV-specific CD8+ T cells are significantly different in LTNP and Rx <50.
Using assays measuring the delivery of cytotoxic proteins to, and elimination of, HIV-infected CD4+ T-cell targets, we recently reported that the HIV-specific CD8+ T cells of LTNP possess an extraordinary cytotoxic capacity on a per-cell basis, which is an effector mechanism clearly distinguishing these individuals from those without immune-mediated control of HIV (30). This difference was most pronounced with effector cells that had been exposed to HIV-infected CD4+ T-cell targets for 6 days prior to coincubation of the same effector and target cells in a 1-hour assay, suggesting that lytic granule loading of memory cells is a critical determinant of cytotoxicity that must be induced for maximal per-cell killing capacity (30).
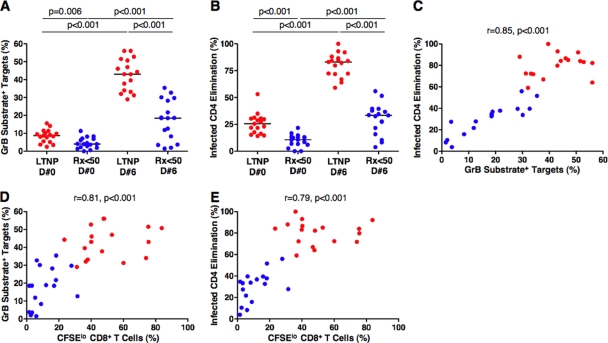
We next examined GrB target cell activity and ICE mediated by the HIV-specific CD8+ T cells of 17 LTNP and 17 Rx <50 with equally low HIV-1 RNA levels (P = 0.08) to measure the impact of reduced viremia and decreased immune system activation on the recovery of cytotoxic capacity. Using day 0 CD8+ T cells (at a ratio of 25 total CD8+ T cells to 1 target cell), the cytotoxic responses measured by GrB activity or ICE were significantly different for LTNP and Rx <50 (medians of 8.7% and 4%, respectively [P = 0.006] for GrB [Fig. 5A]; medians of 25.6% and 10.7%, respectively [P < 0.001] for ICE [Fig. 5B]). Although cytotoxicity measured by either technique increased significantly within the LTNP and Rx <50 patient groups when day 6 cells were used as effectors compared with day 0 cells (P < 0.001 for all comparisons), the median 31.9% increase in GrB activity and 57.5% increase in ICE in cells of LTNP were significantly greater than the 14.5% (P < 0.001) and 24.7% (P < 0.001) changes observed in these measurements, respectively, in the cells of Rx <50 (Fig. 5A and B), consistent with previous findings (30). In these 34 patients, GrB activity and ICE had a weaker correlation when day 0 cells served as effectors (r = 0.53, P = 0.003) but strongly correlated with the more robust responses resulting from day 6 cells (r = 0.85, P < 0.001), as recently reported (30). We have previously associated CD8+ T-cell proliferation with upregulation of the cytotoxic proteins contained within lytic granules (29, 30). In the present study, CD8+ T-cell proliferation correlated strongly with the functional readouts of cytotoxicity, GrB activity (r = 0.81, P < 0.001) and ICE (r = 0.79, P < 0.001), using day 6 CD8+ T cells (Fig. 5D and E). In analyses within each patient group, the only correlations reaching statistical significance were between GrB activity and ICE (r = 0.92, P < 0.001) and between CD8+ T-cell proliferation and ICE (r = 0.57, P = 0.03) with day 6 cells derived from Rx <50 (Fig. 5C and D and E and data not shown). Similar correlations with CD8+ T cells of LTNP did not achieve statistical significance, which was likely due to the fact that measurements of GrB activity and ICE approached the theoretical maximum for LTNP in these assays (30).
FIG. 5.
The cytotoxic capacity of HIV-specific CD8+ T cells of LTNP is significantly greater than that of HIV-specific CD8+ T cells of Rx <50. (A and B) Summary data for the total cytotoxic responses measured by GrB activity (A) or ICE (B) with day 0 or day 6 CD8+ T cells derived from LTNP (red symbols, n = 17) and Rx <50 (blue symbols, n = 17). Horizontal lines indicate median values. Comparisons were made using the Wilcoxon two-sample or signed rank test. (C) Using day 6 CD8+ T cells, GrB target cell activity correlates directly with ICE (n = 34). (D and E) With day 6 CD8+ T cells, HIV-specific CD8+ T-cell proliferation directly correlates with both GrB target cell activity (D) and ICE (E). Statistical analyses were performed using the Spearman correlation.
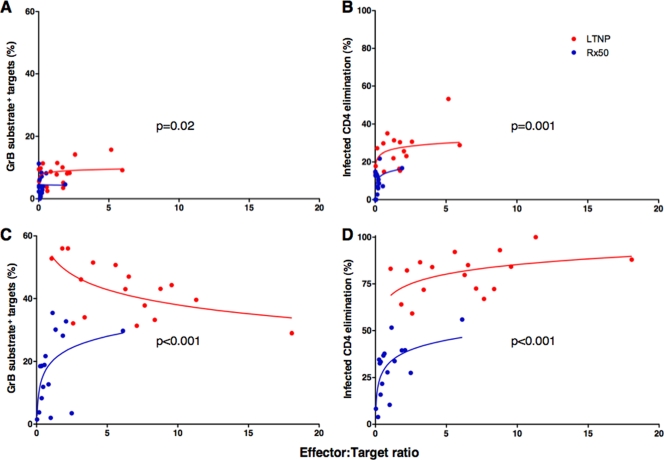
To determine whether differences in cytotoxic activity between LTNP and Rx <50 were due to differences in proliferation in vitro or to persistent qualitative defects in the cytotoxic capacity of each cell in Rx <50, we also analyzed GrB activity and ICE on a per-cell basis. By measuring the percentage of total HIV-specific CD8+ T cells present in each sample by IFN-γ production in response to the autologous targets and the fraction of HIV p24-expressing CD4+ T-cell targets, we determined the true E:T ratios (Fig. 6). In prior work, minimal overlap of the measured E:T ratios between Rx <50 and other groups prevented meaningful comparisons (30), which was not the case with the responses measured in the cohorts in the present study matched for low levels of antigen. With day 0 HIV-specific CD8+ T cells, target cell GrB activity and ICE were greater in LTNP than in Rx <50 by small, constant, but significant amounts of 3.4% (P = 0.02) and 11.4% (P = 0.001), respectively, over the range of shared logged E:T ratios (Fig. 6A and B). In contrast to results with day 0 cells, cytotoxicity measured by either method with day 6 cells was significantly greater for LTNP than Rx <50, with minimal overlap in these responses observed between the groups (Fig. 6C and D). Regarding GrB activity mediated by day 6 cells, the relationship between killing and E:T ratio differed between the groups (P = 0.001), with a decreasing relationship for LTNP (P = 0.02) and an increasing relationship for Rx <50 at the lower range of E:T ratios (P = 0.01) (Fig. 6C). This most likely reflects maximum or near-maximum responses occurring at lower E:T ratios in LTNP but peak responses not being reached even at relatively high E:T ratios in Rx <50. For this reason, GrB target cell activity using day 6 cells was compared at the median log E:T ratio of 2.2, whereby cells of LTNP exhibited 25.2% significantly greater GrB activity than the cells of Rx <50 (P < 0.001) (Fig. 6C). In the ICE assay, cytotoxicity mediated by day 6 cells was greater in LTNP than in Rx <50 by a constant and significant 35.2% over the range of shared logged E:T ratios (P < 0.001) (Fig. 6D). The observation that the per-cell cytotoxic capacities of these cells are not substantially restored in patients with ART-induced suppression of viremia supports the idea that the diminished cytotoxicity of untreated progressors' cells is not simply a consequence of large amounts of antigen in the chronic phase of infection. Defects in proliferative and cytotoxic capacities persist in patients with prolonged ART-induced suppression of viremia. This was true despite levels of PD-1 expression of HIV-specific CD8+ T cells and HIV-specific CD4+ T-cell proliferation that were equivalent to those of LTNP.
FIG. 6.
Day 6 HIV-specific CD8+ T cells of LTNP mediate greater cytotoxicity of HIV-infected CD4+ T-cell targets on a per-cell basis than cells of Rx <50. GrB activity (A and C) and ICE (B and D) using day 0 (A and B) or day 6 (C and D) cells plotted against true E:T ratios based on measurements of IFN-γ-secreting CD8+ T cells and p24-expressing targets, respectively. Curves represent trends for LTNP (red) and Rx <50 (blue). Regression analysis and analysis of covariance were used to quantify the difference in GrB target cell activity and ICE in LTNP and Rx <50 over the range of logged E:T ratios and at the median log E:T ratio of the combined groups.
DISCUSSION
The results of the present study provide several important insights regarding features of the HIV-specific CD8+ T-cell response in patients with and without immunologic control under conditions of similarly low levels of viral antigen. A comprehensive array of qualitative features of the HIV-specific CD8+ T-cell response in well-characterized cohorts of LTNP and Rx <50 were investigated. Consistent with prior work, but here demonstrated with a larger cohort and in greater detail, the proliferative capacity of HIV-specific CD8+ T cells was not restored in patients on ART (29, 30). Diminished proliferation of HIV-specific CD8+ T cells was not simply a result of fewer divisions but rather was primarily a result of diminished numbers of antigen-specific cells proceeding through the cell cycle. These differences were not readily attributable to differences in memory subsets, CD4+ T-cell function, IL-2 production, or PD-1 expression. Perhaps most importantly, the per-cell cytotoxic capacity of HIV-specific CD8+ T cells was not restored in patients on ART. Taken together, these data suggest that there are selective qualitative abnormalities within the HIV-specific CD8+ T-cell compartment that persist under conditions of low levels of antigen.
These results have some implications for the cause-and-effect relationship between levels of HIV antigen in vivo during the chronic phase of infection and measurement of CD8+ T-cell function in vitro. The association between CD8+ T-cell function and lower viral load may be a cause, an effect, or both. Because nearly all HIV-infected patients do not control HIV replication upon discontinuation of ART (7, 9, 35), functions appearing to be restored in the majority of treated patients would not predict immunologic control and are most likely an effect of suppressed viremia. Diminished in vitro function during HIV viremia and restoration during ART have now been described for a wide array of immune system cells and functions. These include proliferation, IL-2 production, and CCR7 expression of HIV-specific CD4+ T cells (16, 18, 20, 37, 46, 50). In one recent longitudinal study, restoration of multiple functions of the HIV-specific CD8+ T cells of chronically infected progressors was also observed after 26 weeks of ART (41). However, in the present study we detected significant differences in the percentage of cells with greater than three functions between LTNP and Rx <50, consistent with some prior work involving cells from viremic progressors (5). Although these observations may seem disparate at initial inspection, it should be noted that there was no comparison made with LTNP in the longitudinal study, considerable overlap of the individual functional subsets was observed between patient groups, and some of the disparities in functionality between studies may be simply technical. They may be related to sensitivity based on the method of stimulation (e.g., peptides versus autologous HIV-infected CD4+ T-cell targets), number of events analyzed, or fluorochromes employed. In addition, differences were primarily limited to decreases in IFN-γ- and TNF-α-secreting subsets of HIV-specific CD8+ T cells in Rx <50 compared to LTNP. This decrease and prior descriptions of diminished nuclear translocation of NFAT are reminiscent of some prior reports of split exhaustion of virus-specific cells following prolonged high-level exposure to antigen in other systems (30, 44). Despite differences in the number of functions of HIV-specific CD8+ T cells between LTNP and Rx <50, the frequencies of polyfunctional cells constituted an extremely small fraction of the total HIV-specific CD8+ T-cell response and most of the functional subsets overlapped substantially between the patient groups, supporting the idea that CD8+ T-cell polyfunctionality is not a robust determinant of immune control over HIV. In addition, HIV-specific CD8+ T-cell production of IL-2 and PD-1 expression and HIV-specific CD4+ T-cell proliferation were not significantly different between Rx <50 and LTNP, consistent with some prior studies (2, 16, 18, 20, 30, 37, 46, 50). Taken together, these results suggest that the functions that are similar in LTNP and Rx <50 are likely not reliable predictors of immunologic restriction of HIV and that decreases in many of these functions in patients off ART are likely an effect of viremia.
Those functions not restored by ART, such as proliferative and cytotoxic capacities, do not appear to be an effect of viremia during the chronic phase of infection. However, it should be noted that these results do not provide formal proof that the proliferative or cytotoxic capacities of CD8+ T cells measured in these assays cause control of HIV replication. It remains possible that host or viral factors may increase peak viremia during acute infection, resulting in disruption of proliferative or cytotoxic functions. However, this is a related but separate issue. The goal of the present study was to determine those functions of the HIV-specific CD8+ T-cell response that remain disrupted during the chronic phase of infection despite reduced levels of antigen during ART. A formal demonstration that the proliferative and cytotoxic capacities of LTNP-derived HIV-specific CD8+ T cells cause immunologic restriction of HIV would require passive transfer studies with humans or experimental animals.
These results also have implications for the potential associations between HIV-specific CD4+ T-cell functions, or autocrine or paracrine IL-2 production, and proliferative or cytotoxic capacities of HIV-specific CD8+ T cells. Although several functions of HIV-specific CD4+ and CD8+ T cells of treated patients were similar to those of LTNP, this was not the case for proliferative or killing capacities of HIV-specific CD8+ T cells. In some prior work, the addition of HIV-specific CD4+ T cells or exogenous IL-2 led to increased proliferation of HIV-specific CD8+ T cells based upon the percent CFSElo cells (24, 51). This has been interpreted to suggest that CD8+ T-cell proliferation and potentially other functions are a consequence of differences in CD4+ T-cell function or autocrine or paracrine IL-2 production. However, several lines of evidence suggest that the case is considerably more complex. We have not observed restoration of HIV-specific CD8+ T-cell proliferation or cytotoxic capacity even after addition of autologous CD4 blasts or addition of anti-CD3 and anti-CD28; either condition supplies large amounts of paracrine IL-2 (29, 30). In the present study, HIV-specific CD8+ T-cell proliferation and cytotoxic capacities were dramatically different in LTNP and Rx <50, although HIV-specific CD4+ T-cell proliferation, measured in the same tube, was the same in these patient groups. Exogenous IL-2 led to increases in the percentage of CFSElo cells, consistent with prior observations; however, its effect was primarily to increase the number of divisions that proliferating cells underwent and not to increase the number of cells recruited to divide. Taken together, these results suggest that defects in CD8+ T-cell proliferative and cytotoxic capacities persist even in the presence of a functional CD4+ T-cell response and in the presence of exogenous IL-2. They are also consistent with the observation that IL-2 administration to patients neither increases HIV-specific CD8+ T-cell proliferative capacity ex vivo nor decreases viral load (1, 10).
These results provide a subset of functions of CD8+ T cells that are not restored by ART. Formal demonstrations that they are predictive of immunologic control would require therapy interruption studies or testing in vaccinees. Thus far, preliminary studies of polyfunctionality of HIV-specific CD8+ T cells have not predicted immunologic restriction in recent HIV vaccine trials (27). Proliferative and cytotoxic capacities have not yet been tested. Given that it is not certain which of the functions that correlate with immunologic control during chronic infection will be predictive of control following vaccination, detailed testing of each of these functions is most likely warranted.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Abrams, D. I., J. D. Bebchuk, E. T. Denning, R. T. Davey, L. Fox, H. C. Lane, J. Sampson, R. Verheggen, D. Zeh, and N. P. Markowitz. 2002. Randomized, open-label study of the impact of two doses of subcutaneous recombinant interleukin-2 on viral burden in patients with HIV-1 infection and CD4+ cell counts of ≥300/mm3: CPCRA 059. J. Acquir. Immune Defic. Syndr. 29:221-231. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrode, G., J. S. Finke, H. Zebroski, F. P. Siegal, and R. M. Steinman. 2005. CD8+ T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses. Eur. J. Immunol. 35:159-170. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 203:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankson, J. N., J. R. Bailey, S. Thayil, H. C. Yang, K. Lassen, J. Lai, S. K. Gandhi, J. D. Siliciano, T. M. Williams, and R. F. Siliciano. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 81:2508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casazza, J. P., M. R. Betts, L. J. Picker, and R. A. Koup. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 75:6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 9.Davey, R. T., Jr., N. Bhat, C. Yoder, T. W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey, R. T., Jr., R. L. Murphy, F. M. Graziano, S. L. Boswell, A. T. Pavia, M. Cancio, J. P. Nadler, D. G. Chaitt, R. L. Dewar, D. K. Sahner, A. M. Duliege, W. B. Capra, W. P. Leong, M. A. Giedlin, H. C. Lane, and J. O. Kahn. 2000. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA 284:183-189. [DOI] [PubMed] [Google Scholar]
- 11.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 12.Draenert, R., C. L. Verrill, Y. Tang, T. M. Allen, A. G. Wurcel, M. Boczanowski, A. Lechner, A. Y. Kim, T. Suscovich, N. V. Brown, M. M. Addo, and B. D. Walker. 2004. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J. Virol. 78:630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emu, B., E. Sinclair, H. Hatano, A. Ferre, B. Shacklett, J. N. Martin, J. M. McCune, and S. G. Deeks. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 82:5398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich, T. C., L. E. Valentine, L. J. Yant, E. G. Rakasz, S. M. Piaskowski, J. R. Furlott, K. L. Weisgrau, B. Burwitz, G. E. May, E. J. Leon, T. Soma, G. Napoe, S. V. Capuano III, N. A. Wilson, and D. I. Watkins. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81:3465-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulder, P. J., M. Bunce, P. Krausa, K. McIntyre, S. Crowley, B. Morgan, A. Edwards, P. Giangrande, R. E. Phillips, and A. J. McMichael. 1996. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res. Hum. Retroviruses 12:1691-1698. [DOI] [PubMed] [Google Scholar]
- 16.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 17.Horton, H., I. Frank, R. Baydo, E. Jalbert, J. Penn, S. Wilson, J. P. McNevin, M. D. McSweyn, D. Lee, Y. Huang, S. C. De Rosa, and M. J. McElrath. 2006. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J. Immunol. 177:7406-7415. [DOI] [PubMed] [Google Scholar]
- 18.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagannathan, P., C. M. Osborne, C. Royce, M. M. Manion, J. C. Tilton, L. Li, S. Fischer, C. W. Hallahan, J. A. Metcalf, M. McLaughlin, M. Pipeling, J. F. McDyer, T. J. Manley, J. L. Meier, J. D. Altman, L. Hertel, R. T. Davey, Jr., M. Connors, and S. A. Migueles. 2009. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J. Virol. 83:2728-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen, C. A., I. M. De Cuyper, R. Steingrover, S. Jurriaans, S. U. Sankatsing, J. M. Prins, J. M. Lange, D. van Baarle, and F. Miedema. 2005. Analysis of the effect of highly active antiretroviral therapy during acute HIV-1 infection on HIV-specific CD4 T cell functions. AIDS 19:1145-1154. [DOI] [PubMed] [Google Scholar]
- 21.Klein, M. R., and F. Miedema. 1995. Long-term survivors of HIV-1 infection. Trends Microbiol. 3:386-391. [DOI] [PubMed] [Google Scholar]
- 22.Lambotte, O., F. Boufassa, Y. Madec, A. Nguyen, C. Goujard, L. Meyer, C. Rouzioux, A. Venet, and J. F. Delfraissy. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053-1056. [DOI] [PubMed] [Google Scholar]
- 23.Lefrere, J. J., L. Morand-Joubert, M. Mariotti, H. Bludau, B. Burghoffer, J. C. Petit, and F. Roudot-Thoraval. 1997. Even individuals considered as long-term nonprogressors show biological signs of progression after 10 years of human immunodeficiency virus infection. Blood 90:1133-1140. [PubMed] [Google Scholar]
- 24.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loffredo, J. T., J. Maxwell, Y. Qi, C. E. Glidden, G. J. Borchardt, T. Soma, A. T. Bean, D. R. Beal, N. A. Wilson, W. M. Rehrauer, J. D. Lifson, M. Carrington, and D. I. Watkins. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81:8827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maness, N. J., L. J. Yant, C. Chung, J. T. Loffredo, T. C. Friedrich, S. M. Piaskowski, J. Furlott, G. E. May, T. Soma, E. J. Leon, N. A. Wilson, H. Piontkivska, A. L. Hughes, J. Sidney, A. Sette, and D. I. Watkins. 2008. Comprehensive immunological evaluation reveals surprisingly few differences between elite controller and progressor Mamu-B*17-positive simian immunodeficiency virus-infected rhesus macaques. J. Virol. 82:5245-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migueles, S. A., A. C. Laborico, H. Imamichi, W. L. Shupert, C. Royce, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, C. W. Hallahan, and M. Connors. 2003. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral Gag sequences. J. Virol. 77:6889-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 30.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 97:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migueles, S. A., J. C. Tilton, and M. Connors. 2004. Advances in understanding immunologic control of HIV infection. Curr. HIV/AIDS Rep. 1:12-17. [DOI] [PubMed] [Google Scholar]
- 33.Miura, T., M. A. Brockman, A. Schneidewind, M. Lobritz, F. Pereyra, A. Rathod, B. L. Block, Z. L. Brumme, C. J. Brumme, B. Baker, A. C. Rothchild, B. Li, A. Trocha, E. Cutrell, N. Frahm, C. Brander, I. Toth, E. J. Arts, T. M. Allen, and B. D. Walker. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare Gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphotye recognition. J. Virol. 83:2743-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navis, M., I. Schellens, D. van Baarle, J. Borghans, P. van Swieten, F. Miedema, N. Kootstra, and H. Schuitemaker. 2007. Viral replication capacity as a correlate of HLA B57/B5801-associated nonprogressive HIV-1 infection. J. Immunol. 179:3133-3143. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz, G. M., M. Wellons, J. Brancato, H. T. Vo, R. L. Zinn, D. E. Clarkson, K. Van Loon, S. Bonhoeffer, G. D. Miralles, D. Montefiori, J. A. Bartlett, and D. F. Nixon. 2001. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc. Natl. Acad. Sci. USA 98:13288-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Packard, B. Z., W. G. Telford, A. Komoriya, and P. A. Henkart. 2007. Granzyme B activity in target cells detects attack by cytotoxic lymphocytes. J. Immunol. 179:3812-3820. [DOI] [PubMed] [Google Scholar]
- 37.Palmer, B. E., E. Boritz, N. Blyveis, and C. C. Wilson. 2002. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4+ T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J. Virol. 76:5925-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer, S., A. P. Wiegand, F. Maldarelli, H. Bazmi, J. M. Mican, M. Polis, R. L. Dewar, A. Planta, S. Liu, J. A. Metcalf, J. W. Mellors, and J. M. Coffin. 2003. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papagno, L., V. Appay, J. Sutton, T. Rostron, G. M. Gillespie, G. S. Ogg, A. King, A. T. Makadzanhge, A. Waters, C. Balotta, A. Vyakarnam, P. J. Easterbrook, and S. L. Rowland-Jones. 2002. Comparison between HIV- and CMV-specific T cell responses in long-term HIV infected donors. Clin. Exp. Immunol. 130:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563-571. [DOI] [PubMed] [Google Scholar]
- 41.Rehr, M., J. Cahenzli, A. Haas, D. A. Price, E. Gostick, M. Huber, U. Karrer, and A. Oxenius. 2008. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J. Virol. 82:3391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacha, J. B., C. Chung, E. G. Rakasz, S. P. Spencer, A. K. Jonas, A. T. Bean, W. Lee, B. J. Burwitz, J. J. Stephany, J. T. Loffredo, D. B. Allison, S. Adnan, A. Hoji, N. A. Wilson, T. C. Friedrich, J. D. Lifson, O. O. Yang, and D. I. Watkins. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 178:2746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundrud, M. S., and A. Rao. 2007. New twists of T cell fate: control of T cell activation and tolerance by TGF-beta and NFAT. Curr. Opin. Immunol. 19:287-293. [DOI] [PubMed] [Google Scholar]
- 45.Tilton, J. C., A. J. Johnson, M. R. Luskin, M. M. Manion, J. Yang, J. W. Adelsberger, R. A. Lempicki, C. W. Hallahan, M. McLaughlin, J. M. Mican, J. A. Metcalf, C. Iyasere, and M. Connors. 2006. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J. Virol. 80:11486-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilton, J. C., M. R. Luskin, A. J. Johnson, M. Manion, C. W. Hallahan, J. A. Metcalf, M. McLaughlin, R. T. Davey, Jr., and M. Connors. 2007. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 81:2713-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilton, J. C., M. M. Manion, M. R. Luskin, A. J. Johnson, A. A. Patamawenu, C. W. Hallahan, N. A. Cogliano-Shutta, J. M. Mican, R. T. Davey, Jr., S. Kottilil, J. D. Lifson, J. A. Metcalf, R. A. Lempicki, and M. Connors. 2008. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J. Virol. 82:3997-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-202. [DOI] [PubMed] [Google Scholar]
- 49.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80:5074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 102:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]